Evaluation of a Structured Light Scanner for 3D Facial Imaging: A Comparative Study with Direct Anthropometry
Abstract
:1. Introduction
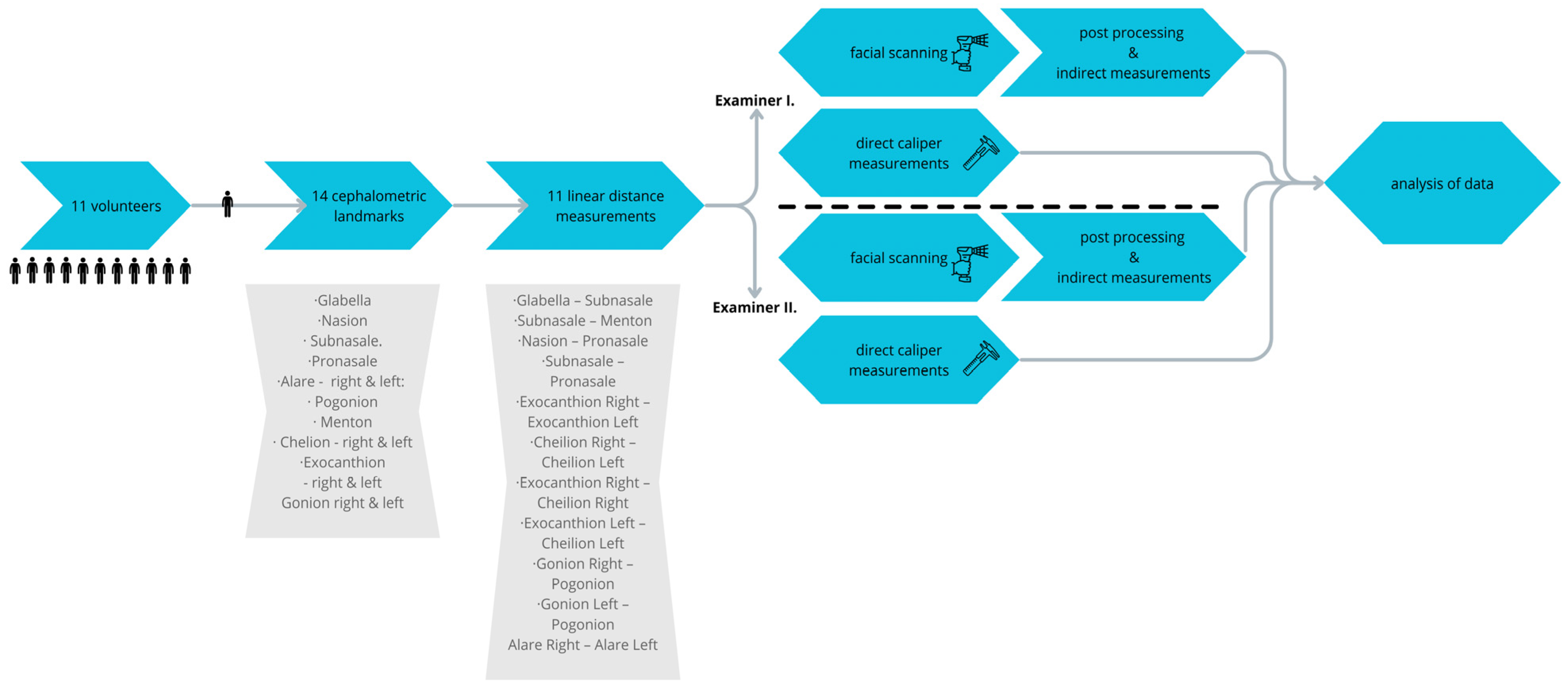
2. Materials and Methods
2.1. Subjects
2.2. Examiners
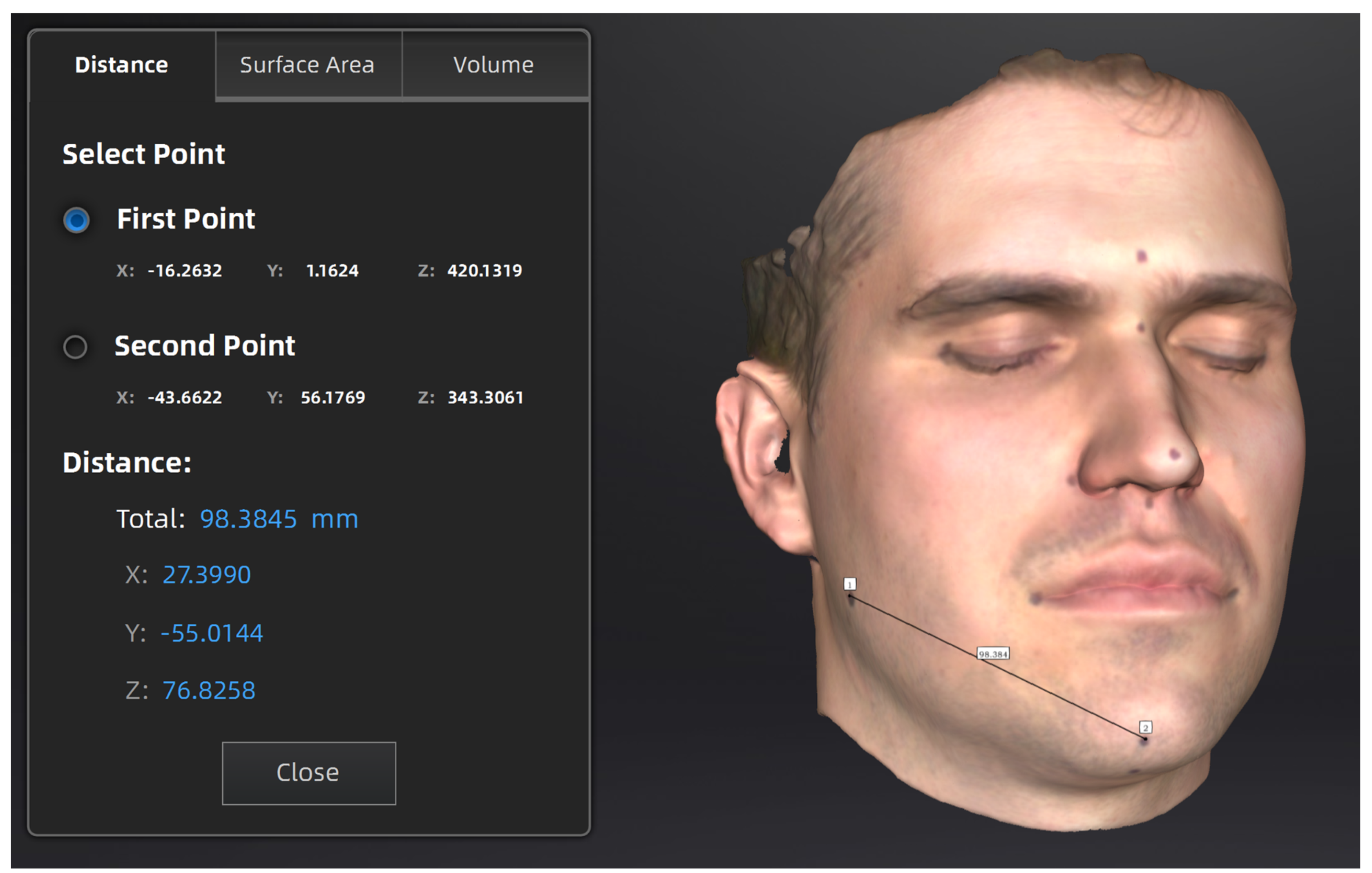
2.3. 3D Imaging and Measurements
- G—Glabella: the most anterior midpoint on the fronto-orbital soft tissue contour;
- N—Nasion: the midpoint on the soft tissue contour of the base of the nasal root, at the level of the frontonasal suture;
- Sn—Subnasale: the midpoint on the nasolabial soft tissue contour between the columella crest and the upper lip;
- Prn—Pronasale: the most anterior midpoint of the nasal tip;
- Al—Alare right (Alr) and left (All) is the most lateral point on each alar contour;
- Pg—Pogonion is the most anterior midpoint of the chin;
- Me—Menton is the most inferior midpoint on the soft tissue contour of the chin;
- Ch—Chelion right (Chr) and left (Chl) is the point located at each labial commissure;
- Ex—Exocanthion right (Exr) and left (Exl) is the soft tissue point located at the outer commissure of each eye fissure;
- Go—Gonion right (Gor) and left (Gol) is the most lateral point on the soft tissue contour of each mandibular angle.
- Glabella—Subnasale;
- Subnasale—Menton;
- Nasion—Pronasale;
- Subnasale—Pronasale;
- Exocanthion Right—Exocanthion Left;
- Cheilion Right—Cheilion Left;
- Exocanthion Right—Cheilion Right;
- Exocanthion Left—Cheilion Left;
- Gonion Right—Pogonion;
- Gonion Left—Pogonion;
- Alare Right—Alare Left.
- Scan mode: portrait scan;
- Alignment: hybrid;
- Resolution: 0.2 mm;
- Data Quality Indicator: on;
- Hair more: off.
2.4. Time Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stan, Z.; Li, A.K.J. Handbook of Face Recognition; Springer: Chem, Switzerland, 2011. [Google Scholar]
- Wersenyi, G.; Scheper, V.; Spagnol, S.; Eixelberger, T.; Wittenberg, T. Cost-effective 3D scanning and printing technologies for outer ear reconstruction: Current status. Head. Face Med. 2023, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Beeler, T.; Hahn, F.; Derek, B.; Bernd, B.; Paul, B.; Craig, G.; Robert, W.; Sumne, M.G. High-quality passive facial performance capture using anchor frames. Assoc. Comput. Mach. 2011, 30, 75. [Google Scholar] [CrossRef]
- Liberton, D.K.; Mishra, R.; Beach, M.; Raznahan, A.; Gahl, W.A.; Manoli, I.; Lee, J.S. Comparison of Three-Dimensional Surface Imaging Systems Using Landmark Analysis. J. Craniofac. Surg. 2019, 30, 1869–1872. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.H.J.; Kraeima, J.; Meesters, A.M.L.; Delli, K.; Vissink, A.; Jansma, J.; Schepers, R.H. Reproducibility of 3D scanning in the periorbital region. Sci. Rep. 2021, 11, 3671. [Google Scholar] [CrossRef] [PubMed]
- Camison, L.; Bykowski, M.; Lee, W.W.; Carlson, J.C.; Roosenboom, J.; Goldstein, J.A.; Losee, J.E.; Weinberg, S.M. Validation of the Vectra H1 portable three-dimensional photogrammetry system for facial imaging. Int. J. Oral Maxillofac. Surg. 2018, 47, 403–410. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, M.; Rosati, R.; Ferrario, V.F.; Sforza, C. Accuracy and reproducibility of a 3-dimensional stereophotogrammetric imaging system. J. Oral Maxillofac. Surg. 2010, 68, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.; Khan, A.; Aldhanhani, A.; Alnaser, H.; Naudi, K.; Ju, X.; Gillgrass, T.; Mossey, P. The Validation of an Innovative Method for 3D Capture and Analysis of the Nasolabial Region in Cleft Cases. Cleft Palate Craniofac. J. 2021, 58, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, Y.N.R.; Salazar-Gamarra, R.; Bohner, L.; De Oliveira, J.I.; Dib, L.L.; Sesma, N. Evaluation of the 3D error of 2 face-scanning systems: An in vitro analysis. J Prosthet. Dent. 2023, 129, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xiao, S. 3D face recognition: A survey. Human-Centric Comput. Inf. Sci. 2018, 8, 35. [Google Scholar] [CrossRef]
- Li, M.; Huang, B.; Tian, G. A comprehensive survey on 3D face recognition methods. Eng. Appl. Artif. Intell. 2022, 110, 104669. [Google Scholar] [CrossRef]
- Adjabi, I.; Ouahabi, A.; Benzaoui, A.; Taleb-Ahmed, A. Past, Present, and Future of Face Recognition: A Review. Electronics 2020, 1188, 52. [Google Scholar] [CrossRef]
- Shamata, A.; Thompson, T. Documentation and analysis of traumatic injuries in clinical forensic medicine involving structured light three-dimensional surface scanning versus photography. J. Forensic Leg. Med. 2018, 58, 93–100. [Google Scholar] [CrossRef]
- Maiese, A.; Manetti, A.C.; Ciallella, C.; Fineschi, V. The Introduction of a New Diagnostic Tool in Forensic Pathology: LiDAR Sensor for 3D Autopsy Documentation. Biosensors 2022, 12, 132. [Google Scholar] [CrossRef]
- Franco de Sa Gomes, C.; Libdy, M.R.; Normando, D. Scan time, reliability and accuracy of craniofacial measurements using a 3D light scanner. J. Oral Biol. Craniofac. Res. 2019, 9, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.F.; Sforza, C.; Dellavia, C.; Tartaglia, G.M.; Colombo, A.; Caru, A. A quantitative three-dimensional assessment of soft tissue facial asymmetry of cleft lip and palate adult patients. J. Craniofac. Surg. 2003, 14, 739–746. [Google Scholar] [CrossRef]
- Modabber, A.; Peters, F.; Kniha, K.; Goloborodko, E.; Ghassemi, A.; Lethaus, B.; Holzle, F.; Mohlhenrich, S.C. Evaluation of the accuracy of a mobile and a stationary system for three-dimensional facial scanning. J. Craniomaxillofac. Surg. 2016, 44, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Lv, L.; Liu, Y.; Liu, Y.; Zhou, Y. Evaluation of the Accuracy, Reliability, and Reproducibility of Two Different 3D Face-Scanning Systems. Int. J. Prosthodont. 2016, 29, 213–218. [Google Scholar] [CrossRef]
- Weinberg, S.M.; Scott, N.M.; Neiswanger, K.; Brandon, C.A.; Marazita, M.L. Digital three-dimensional photogrammetry: Evaluation of anthropometric precision and accuracy using a Genex 3D camera system. Cleft Palate Craniofac. J. 2004, 41, 507–518. [Google Scholar] [CrossRef]
- Andrade, L.M.; Rodrigues da Silva, A.M.B.; Magri, L.V.; Rodrigues da Silva, M.A.M. Repeatability Study of Angular and Linear Measurements on Facial Morphology Analysis by Means of Stereophotogrammetry. J. Craniofac. Surg. 2017, 28, 1107–1111. [Google Scholar] [CrossRef]
- Tzou, C.H.; Frey, M. Evolution of 3D surface imaging systems in facial plastic surgery. Facial Plast. Surg. Clin. N. Am. 2011, 19, 591–602. [Google Scholar] [CrossRef]
- Akan, B.; Akan, E.; Sahan, A.O.; Kalak, M. Evaluation of 3D Face-Scan images obtained by stereophotogrammetry and smartphone camera. Int. Orthod. 2021, 19, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.M.; Kolar, J.C. Three-dimensional surface imaging: Limitations and considerations from the anthropometric perspective. J. Craniofac. Surg. 2005, 16, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Modabber, A.; Rana, M.; Ghassemi, A.; Gerressen, M.; Gellrich, N.C.; Holzle, F.; Rana, M. Three-dimensional evaluation of postoperative swelling in treatment of zygomatic bone fractures using two different cooling therapy methods: A randomized, observer-blind, prospective study. Trials 2013, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.N.; Lee, D.H. Accuracy of Mobile Device-Compatible 3D Scanners for Facial Digitization: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e22228. [Google Scholar] [CrossRef] [PubMed]
- Sforza, C.; de Menezes, M.; Ferrario, V. Soft- and hard-tissue facial anthropometry in three dimensions: What’s new. J. Anthropol. Sci. 2013, 91, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Koban, K.C.; Perko, P.; Li, Z.; Xu, Y.; Giunta, R.E.; Alfertshofer, M.G.; Kohler, L.H.; Freytag, D.L.; Cotofana, S.; Frank, K. 3D Anthropometric Facial Imaging—A comparison of different 3D scanners. Facial Plast. Surg. Clin. N. Am. 2022, 30, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Koban, K.C.; Perko, P.; Etzel, L.; Li, Z.; Schenck, T.L.; Giunta, R.E. Validation of two handheld devices against a non-portable three-dimensional surface scanner and assessment of potential use for intraoperative facial imaging. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Hsung, R.T.; Ajmera, D.H.; Leung, Y.Y.; McGrath, C.; Gu, M. Can smartphones be used for routine dental clinical application? A validation study for using smartphone-generated 3D facial images. J. Dent. 2023, 139, 104775. [Google Scholar] [CrossRef] [PubMed]
- Tzou, C.H.; Artner, N.M.; Pona, I.; Hold, A.; Placheta, E.; Kropatsch, W.G.; Frey, M. Comparison of three-dimensional surface-imaging systems. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, B.R.; Oliveira Junior, O.B.; de Sousa Gomes Costa, J.L.; Zanetti, T.F.; Pretel, H. Cloner 3D photogrammetric facial scanner: Assessment of accuracy in a controlled clinical study. J. Esthet. Restor. Dent. 2023, 35, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.; Alwafi, A.; Bichu, Y.M.; Pliska, B.T.; Mostafa, N.; Zou, B. Validation of three-dimensional facial imaging captured with smartphone-based photogrammetry application in comparison to stereophotogrammetry system. Heliyon 2023, 9, e15834. [Google Scholar] [CrossRef] [PubMed]
- Knoops, P.G.; Beaumont, C.A.; Borghi, A.; Rodriguez-Florez, N.; Breakey, R.W.; Rodgers, W.; Angullia, F.; Jeelani, N.U.; Schievano, S.; Dunaway, D.J. Comparison of three-dimensional scanner systems for craniomaxillofacial imaging. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Gellrich, N.C.; Joos, U.; Piffko, J.; Kater, W. 3D evaluation of postoperative swelling using two different cooling methods following orthognathic surgery: A randomised observer blind prospective pilot study. Int. J. Oral Maxillofac. Surg. 2011, 40, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Aragón Niño, Í.; Del Castillo Pardo de Vera, J.L.; Rodríguez Arias, J.P.; Gutiérrez Venturini, A.; Cebrián Carretero, J.L. 3D surface scanner in Maxillofacial Surgery: State of the art. Adv. Oral Maxillofac. Surg. 2024, 13, 100473. [Google Scholar] [CrossRef]
- Beretta, M.; Federici Canova, F.; Zaffarano, L.; Gianolio, A. Face Scan for Ceph 3D: A green way for diagnosis in children. Eur. J. Paediatr. Dent. 2022, 23, 201–203. [Google Scholar] [CrossRef]
- Andlauer, R.; Wachter, A.; Schaufelberger, M.; Weichel, F.; Kuhle, R.; Freudlsperger, C.; Nahm, W. 3D-Guided Face Manipulation of 2D Images for the Prediction of Post-Operative Outcome After Cranio-Maxillofacial Surgery. IEEE Trans. Image Process 2021, 30, 7349–7363. [Google Scholar] [CrossRef] [PubMed]
- Maal, T.J.; van Loon, B.; Plooij, J.M.; Rangel, F.; Ettema, A.M.; Borstlap, W.A.; Berge, S.J. Registration of 3-dimensional facial photographs for clinical use. J. Oral Maxillofac. Surg. 2010, 68, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.M.; Naidoo, S.; Govier, D.P.; Martin, R.A.; Kane, A.A.; Marazita, M.L. Anthropometric Precision and Accuracy of Digital Three-Dimensional Photogrammetry: Comparing the Genex and 3dMD Imaging Systems with One Another and with Direct Anthropometry. J. Craniofacial Surg. 2006, 17, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, K.; Boyadjiev, S.A.; Capone, G.T.; DeLeon, V.B.; Richtsmeier, J.T. Precision and error of three-dimensional phenotypic measures acquired from 3dMD photogrammetric images. Am. J. Med. Genet. A 2005, 138A, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Metzler, P.; Sun, Y.; Zemann, W.; Bartella, A.; Lehner, M.; Obwegeser, J.A.; Kruse-Gujer, A.L.; Lubbers, H.T. Validity of the 3D VECTRA photogrammetric surface imaging system for cranio-maxillofacial anthropometric measurements. Oral Maxillofac. Surg. 2014, 18, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bahsi, I.; Orhan, M.; Kervancioglu, P.; Karatepe, S.; Sayin, S. Craniofacial Anthropometry of Healthy Turkish Young Adults: Analysis of Head and Face. J. Craniofac. Surg. 2021, 32, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, S.; Fang, C.; Lan, D.; Dong, C. Application of 3-Dimensional White-Light Scanning to Observe the Lip and Nose Morphology of Chinese Children. J. Craniofac. Surg. 2023, 34, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Bannister, J.J.; Wilms, M.; Aponte, J.D.; Katz, D.C.; Klein, O.D.; Bernier, F.P.; Spritz, R.A.; Hallgrimsson, B.; Forkert, N.D. Comparing 2D and 3D representations for face-based genetic syndrome diagnosis. Eur. J. Hum. Genet. 2023, 31, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Hammond, P. The use of 3D face shape modelling in dysmorphology. Arch. Dis. Child. 2007, 92, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Hajeer, M.Y.; Mao, Z.; Millett, D.T.; Ayoub, A.F.; Siebert, J.P. A new three-dimensional method of assessing facial volumetric changes after orthognathic treatment. Cleft Palate Craniofac. J. 2005, 42, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Friscia, M.; Seidita, F.; Committeri, U.; Troise, S.; Abbate, V.; Bonavolonta, P.; Orabona, G.D.; Califano, L. Efficacy of Hilotherapy face mask in improving the trend of edema after orthognathic surgery: A 3D analysis of the face using a facial scan app for iPhone. Oral Maxillofac. Surg. 2022, 26, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Aung, S.C.; Ngim, R.C.K.; Lee, S.T. Evaluation of the laser scanner as a surface measuring tool and its accuracy compared with direct facial anthropometric measurements. Br. J. Plast. Surg. 1995, 48, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Maal, T.J.J.; Verhamme, L.M.; van Loon, B.; Plooij, J.M.; Rangel, F.A.; Kho, A.; Bronkhorst, E.M.; Bergé, S.J. Variation of the face in rest using 3D stereophotogrammetry. Int. J. Oral Maxillofac. Surg. 2011, 40, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bornstein, M.M.; Hsung, R.T.; Ajmera, D.H.; Leung, Y.Y.; Gu, M. Frontiers in Three-Dimensional Surface Imaging Systems for 3D Face Acquisition in Craniofacial Research and Practice: An Updated Literature Review. Diagnostics 2024, 14, 423. [Google Scholar] [CrossRef] [PubMed]


| Scan Mode | Structured Light Scan |
| Point Distance | 0.1 mm~3 mm |
| Light Source | Infrared VCSEL Structured Light |
| Working Distance | Effective Working Distance: 160 mm–1400 mm; Optimal Working Distance: 400 mm |
| Depth of Field | 160 mm–1400 mm |
| Maximum Field of View | 434 mm × 379 mm (under optimal work distance) |
| Scan Speed | 980,000 points/s, up to 14 FPS |
| Align Modes | Feature Alignment, Hybrid Alignment, Texture Alignment, GloLeft Markers |
| Safety | Eye-safe |
| Texture Scan | Yes |
| Outdoor Scanning | Yes |
| Interface | USB2.0 or above |
| Output Formats | OBJ; STL; PLY; P3; 3MF |
| Scanner Size | 220 mm × 46 mm × 55 mm |
| Carrying Case Size | 245 mm × 245 mm × 90 mm |
| Scanner Body Weight | 500 g |
| Operating Temperature Range | 0–40 °C |
| Operating Humidity Range | 10–90% |
| Certification | CE, FCC, ROHS, WEEE, KC |
| Recommended Configuration | OS: Win 10/11, 64 bit; graphics card: NVIDIA GTX1060 (Nvidia, Santa Clara, CA, USA); video memory: ≥6 GB; processor: I7-11800H; memory: ≥32 GB |
| Basic Computer Configuration | OS: Win 10, 64 bit; graphics card: NVIDIA GTX1050 (Nvidia, Santa Clara, CA, USA); video memory: ≥4 GB; processor: I7-7700H; memory: ≥16 GB |
| Examiner 1 (Caliper) | Examiner 2 (Caliper) | Difference of the Means | t-Test | Correlation Coefficient (ICC) | |
|---|---|---|---|---|---|
| Glabella—Subnasale | 66.73 | 66.91 | −0.18 | 0.751 | 0.954 *** |
| Subnasale—Menton | 69.94 | 68.53 | 1.41 | 0.001 | 0.990 *** |
| Nasion—Pronasale | 45.16 | 45.25 | −0.09 | 0.842 | 0.949 *** |
| Subnasale—Pronasale | 20.98 | 21.32 | −0.34 | 0.475 | 0.880 ** |
| Exocanthion—Exocanthion | 95.66 | 96.18 | −0.52 | 0.263 | 0.990 *** |
| Cheilion—Cheilion | 53.38 | 53.28 | 0.1 | 0.796 | 0.967 *** |
| Exocanthion Right—Cheilion Right | 71.31 | 71.04 | 0.27 | 0.603 | 0.963 *** |
| Exocanthion Left—Cheilion Left | 71.08 | 71.09 | −0.01 | 0.984 | 0.960 *** |
| Gonion Left—Pogonion | 86.01 | 85.44 | 0.57 | 0.255 | 0.992 *** |
| Gonion Right—Pogonion | 85.00 | 84.34 | 0.66 | 0.128 | 0.993 *** |
| Alare-Alare | 34.69 | 34.21 | 0.48 | 0.317 | 0.933 *** |
| Examiner 1 (Scanner) | Examiner 2 (Scanner) | Difference of the Means | t-Test | Correlation Coefficient (ICC) | |
|---|---|---|---|---|---|
| Glabella—Subnasale | 67.41 | 67.54 | −0.13 | 0.798 | 0.933 *** |
| Subnasale—Menton | 70.45 | 70.05 | 0.4 | 0.192 | 0.992 *** |
| Nasion—Pronasale | 44.82 | 44.65 | 0.17 | 0.713 | 0.948 *** |
| Subnasale—Pronasale | 21.80 | 21.94 | −0.14 | 0.674 | 0.922 *** |
| Exocanthion—Exocanthion | 95.06 | 95.01 | 0.05 | 0.048 | 0.988 *** |
| Cheilion—Cheilion | 53.06 | 53.13 | −0.07 | 0.948 | 0.791 *** |
| Exocanthion Right—Cheilion Right | 72.23 | 72.26 | −0.03 | 0.924 | 0.986 *** |
| Exocanthion Left—Cheilion Left | 71.26 | 70.69 | 0.57 | 0.177 | 0.974 *** |
| Gonion Left—Pogonion | 86.98 | 86.31 | 0.67 | 0.080 | 0.994 *** |
| Gonion Right—Pogonion | 84.93 | 84.71 | 0.22 | 0.522 | 0.995 *** |
| Alare-Alare | 34.51 | 34.68 | −0.17 | 0.682 | 0.946 *** |
| Examiner 1 (Caliper) | Examiner 1 (Scanner) | Difference of the Means | t-Test | Correlation Coefficient (ICC) | |
|---|---|---|---|---|---|
| Glabella—Subnasale | 66.73 | 67.41 | −0.68 | 0.050 | 0.978 *** |
| Subnasale—Menton | 69.94 | 70.45 | −0.51 | 0.193 | 0.989 *** |
| Nasion—Pronasale | 45.16 | 44.82 | 0.34 | 0.360 | 0.970 *** |
| Subnasale—Pronasale | 20.98 | 21.80 | −0.82 | 0.124 | 0.860 ** |
| Exocanthion—Exocanthion | 95.66 | 95.06 | 0.6 | 0.346 | 0.982 *** |
| Cheilion—Cheilion | 53.38 | 53.06 | 0.32 | 0.341 | 0.320 *** |
| Exocanthion Right—Cheilion Right | 71.31 | 72.23 | −0.92 | 0.048 | 0.970 *** |
| Exocanthion Left—Cheilion Left | 71.08 | 72.23 | −1.15 | 0.703 | 0.965 *** |
| Gonion Left—Pogonion | 86.01 | 86.98 | −0.97 | 0.063 | 0.990 *** |
| Gonion Right—Pogonion | 85.00 | 84.93 | 0.07 | 0.834 | 0.998 *** |
| Alare-Alare | 34.69 | 34.51 | 0.18 | 0.749 | 0.904 *** |
| Examiner 2 (Caliper) | Examiner 2 (Scanner) | Difference of the Means | t-Test | Correlation Coefficient (ICC) | |
|---|---|---|---|---|---|
| Glabella—Subnasale | 66.91 | 67.54 | −0.63 | 0.270 | 0.937 *** |
| Subnasale—Menton | 68.53 | 70.05 | −1.52 | 0.005 | 0.983 *** |
| Nasion—Pronasale | 45.25 | 44.65 | 0.6 | 0.289 | 0.928 *** |
| Subnasale—Pronasale | 21.32 | 21.94 | −0.62 | 0.053 | 0.935 *** |
| Exocanthion—Exocanthion | 96.18 | 95.01 | 1.17 | 0.685 | 0.991 *** |
| Cheilion—Cheilion | 53.28 | 53.13 | 0.15 | 0.878 | 0.800 * |
| Exocanthion Right—Cheilion Right | 71.04 | 72.26 | −1.22 | 0.009 | 0.978 *** |
| Exocanthion Left—Cheilion Left | 71.09 | 70.69 | 0.4 | 0.510 | 0.943 *** |
| Gonion Left—Pogonion | 85.44 | 86.31 | −0.87 | 0.116 | 0.989 *** |
| Gonion Right—Pogonion | 84.34 | 84.71 | −0.37 | 0.426 | 0.991 *** |
| Alare-Alare | 34.21 | 34.68 | −0.47 | 0.281 | 0.928 *** |
| Examiner 1 (caliper) | 02:25 | Examiner 2 (caliper) | 02:53 |
| Examiner 1 (scanner) sum | 08:04 | Examiner 2 (scanner) sum | 08:58 |
| Scanning time | 01:18 | Scanning time | 01:40 |
| Image processing | 05:14 | Image processing | 05:35 |
| Measurement on mesh | 01:30 | Measurement on mesh | 01:42 |
| Measurement with Caliper | 3D Scanning | |
|---|---|---|
| Price of the device | Inexpensive | Higher-priced |
| Method of measurement | Direct | Indirect—on a virtual model |
| Time of the measurement | Time-consuming |
|
| Reproducibility | Presence of the patient required | Repeated measurements on a 3D model are possible |
| Possible causes of measurement error | Tissue distortion during measurement | Motion artifacts |
| Other benefits | - |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Major, M.; Mészáros, B.; Würsching, T.; Polyák, M.; Kammerhofer, G.; Németh, Z.; Szabó, G.; Nagy, K. Evaluation of a Structured Light Scanner for 3D Facial Imaging: A Comparative Study with Direct Anthropometry. Sensors 2024, 24, 5286. https://doi.org/10.3390/s24165286
Major M, Mészáros B, Würsching T, Polyák M, Kammerhofer G, Németh Z, Szabó G, Nagy K. Evaluation of a Structured Light Scanner for 3D Facial Imaging: A Comparative Study with Direct Anthropometry. Sensors. 2024; 24(16):5286. https://doi.org/10.3390/s24165286
Chicago/Turabian StyleMajor, Martin, Bence Mészáros, Tamás Würsching, Melinda Polyák, Gábor Kammerhofer, Zsolt Németh, György Szabó, and Krisztián Nagy. 2024. "Evaluation of a Structured Light Scanner for 3D Facial Imaging: A Comparative Study with Direct Anthropometry" Sensors 24, no. 16: 5286. https://doi.org/10.3390/s24165286
APA StyleMajor, M., Mészáros, B., Würsching, T., Polyák, M., Kammerhofer, G., Németh, Z., Szabó, G., & Nagy, K. (2024). Evaluation of a Structured Light Scanner for 3D Facial Imaging: A Comparative Study with Direct Anthropometry. Sensors, 24(16), 5286. https://doi.org/10.3390/s24165286







