Reconstruction of the Permittivity of Ex Vivo Animal Tissues in the Frequency Range 1–20 GHz Using a Water-Based Dielectric Model
Abstract
1. Introduction
2. Material and Methods
2.1. Dielectric Properties of Biological Tissues and Water-Based Dielectric Model
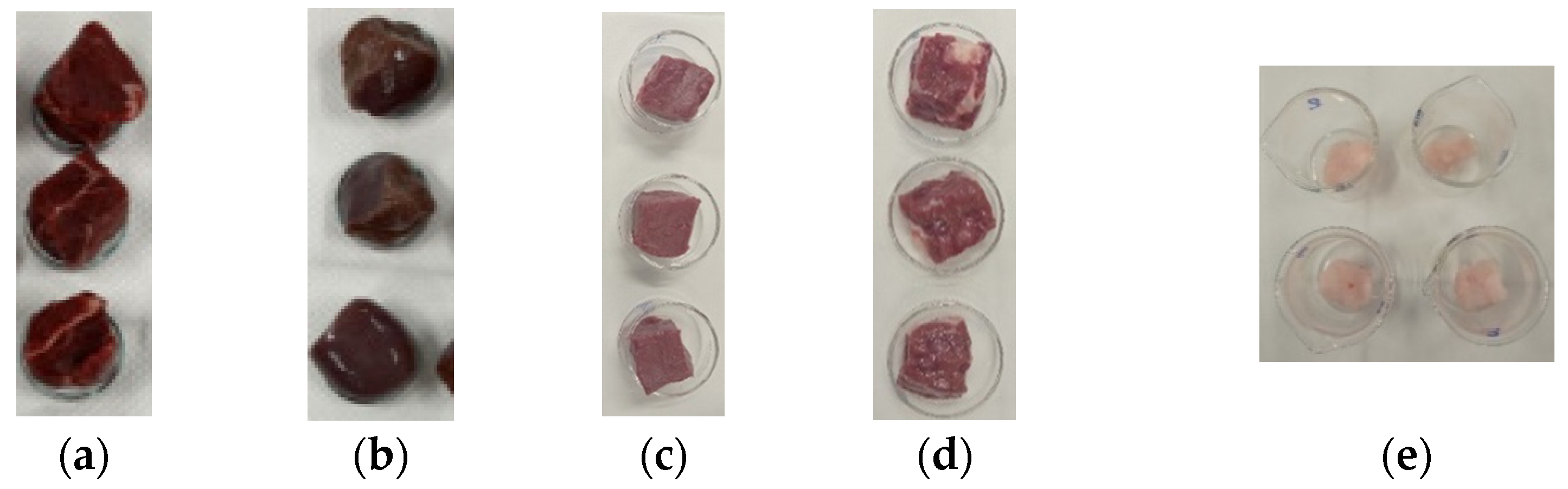
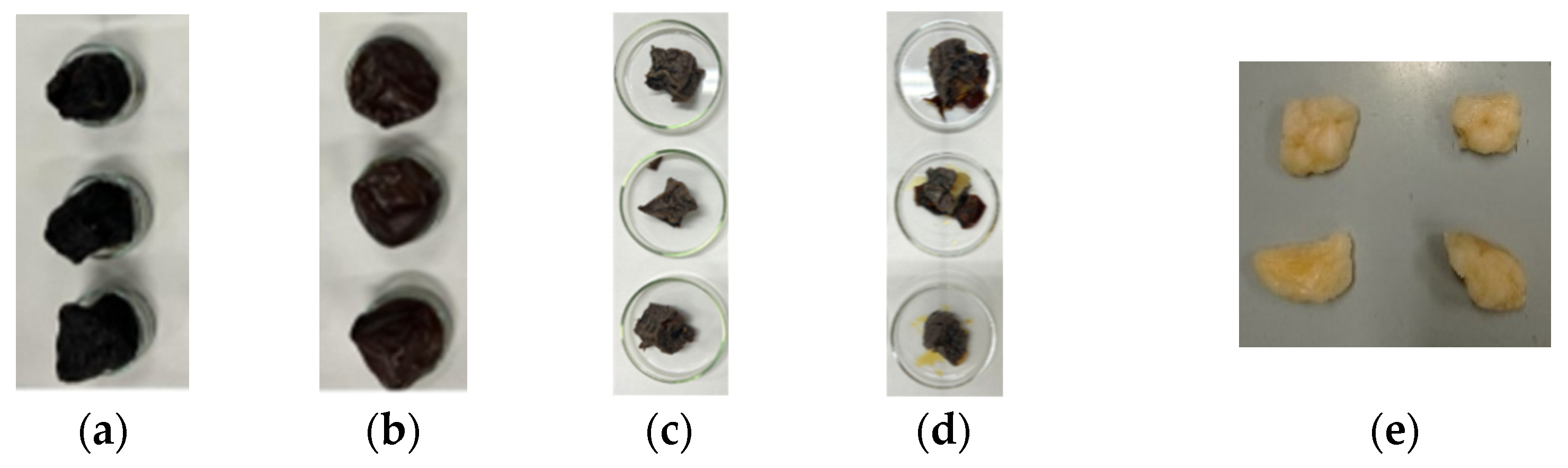
2.2. Sample Preparation and Water Content Evaluation
2.3. Calculation of Variability of Results
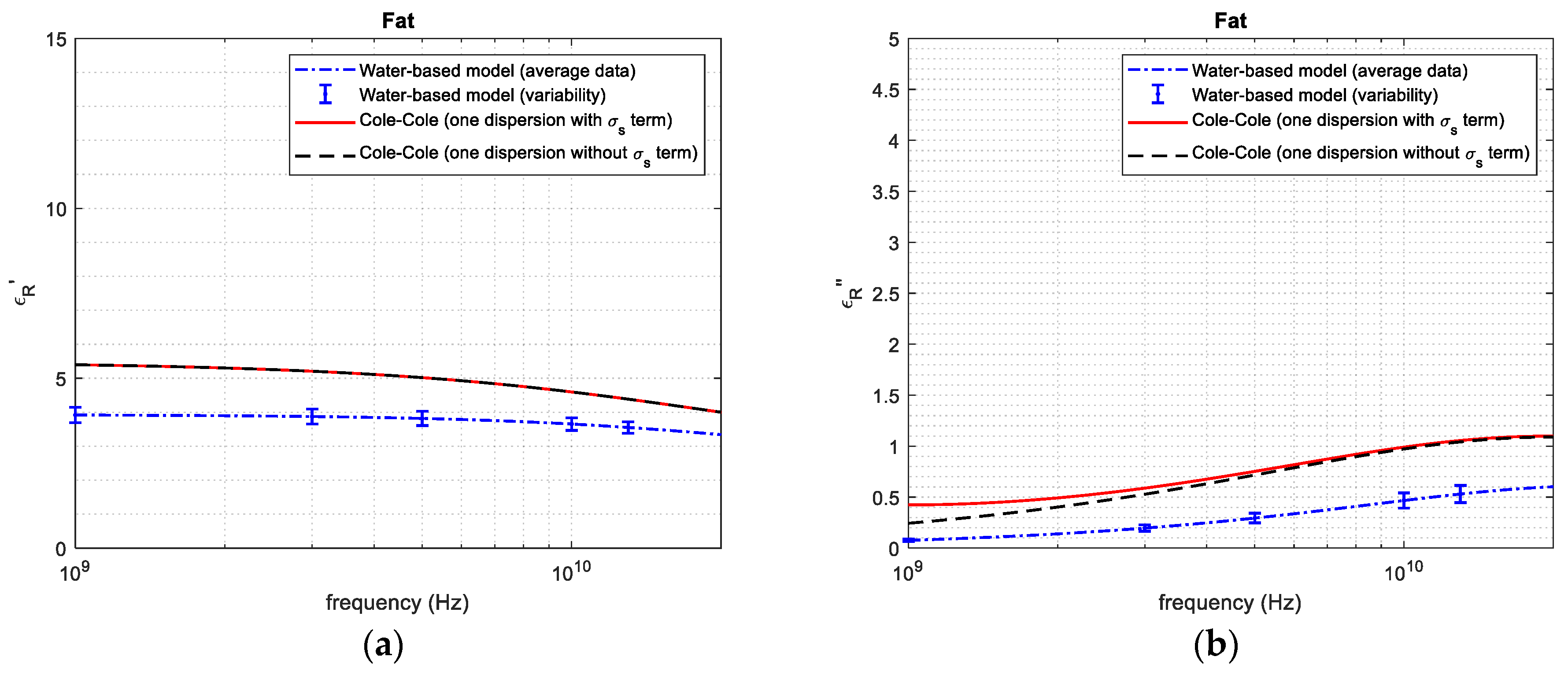
3. Results
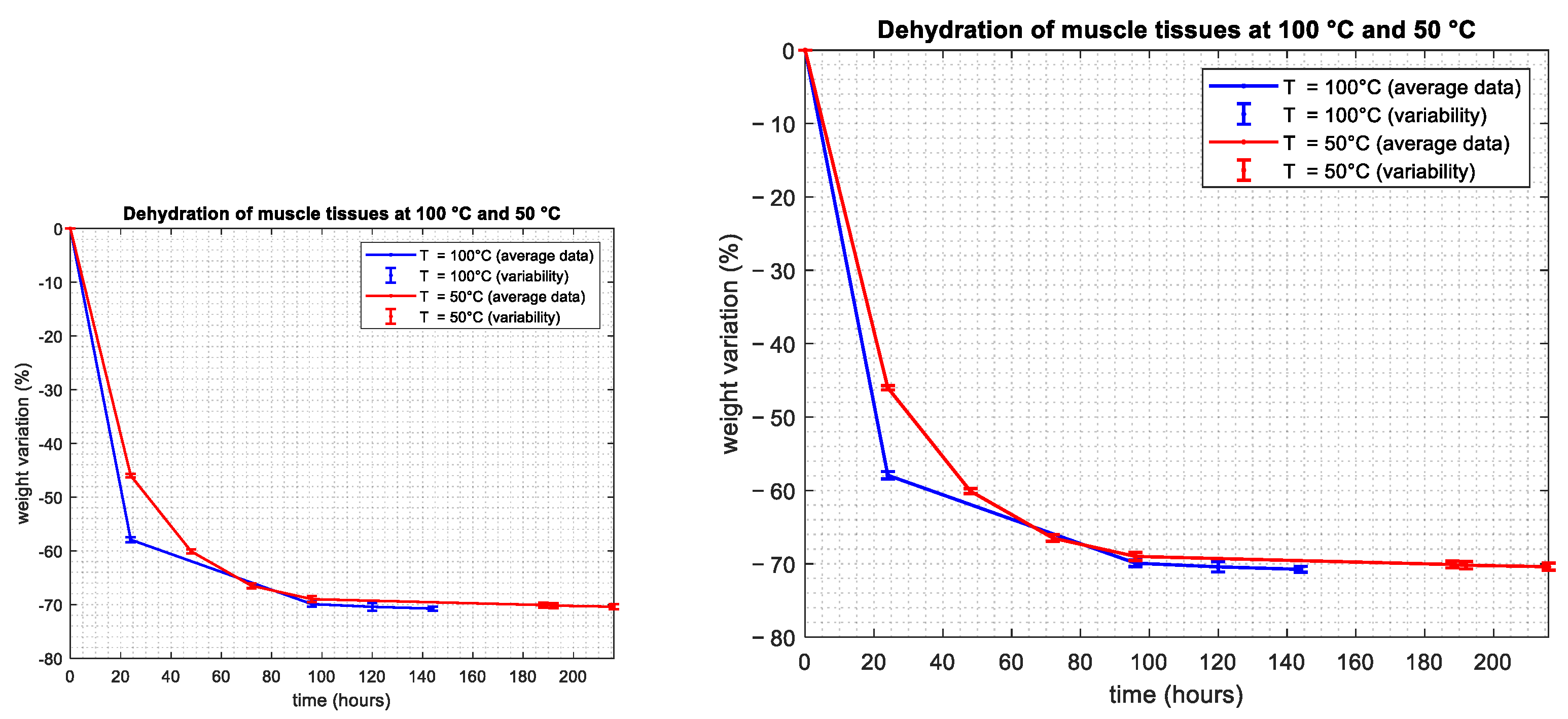
3.1. Water Content Evaluation: Dehydration Procedure
3.2. Dielectric Property Reconstruction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
References
- Mattsson, M.O.; Simkò, M. Emerging medical applications based on non-ionizing electromagnetic fields from 0 Hz to 10 THz. Med. Devices 2019, 12, 347–368. [Google Scholar] [CrossRef]
- Wang, Z.; Lim, E.G.; Tang, Y.; Leach, M. Medical Applications of Microwave Imaging. Sci. World J. 2014, 1, 147016. [Google Scholar] [CrossRef]
- Ahmed, M.; Brace, C.L.; Lee, F.T., Jr.; Goldberg, S.N. Principles of and Advances in Percutaneous Ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef]
- Malik, N.A.; Sant, P.; Ajmal, T.; Ur-Rehman, M. Implantable Antennas for BioMedical Applications. IEEE J. Electromagn. RF Microw. Med. Biol. 2021, 5, 84–96. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wang, Z.; Baumgartner, R.N.; Ross, R. Human Body Composition: Advances in Models and Methods. Annu. Rev. Nutr. 1997, 17, 527–558. [Google Scholar] [CrossRef] [PubMed]
- Paulides, M.M.; Stauffer, P.R.; Neufeld, E.; Maccarini, P.F.; Kyriakou, A.; Canters, R.A.; Diederich, C.J.; Bakker, J.F.; Van Rhoon, G.C. Simulation techniques in hyperthermia treatment planning. Int. J. Hyperthermia 2013, 29, 346–357. [Google Scholar] [CrossRef]
- Peyman, A.; Kos, B.; Djokić, M.; Trotovšek, B.; Limbaeck-Stokin, C.; Serša, G.; Miklavčič, D. Variation in dielectric properties due to pathological changes in human liver. Bioelectromagnetics 2015, 36, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.P.; Clarke, R.N. Dielectric metrology with coaxial sensors. Meas. Sci. Technol. 2007, 18, 1372. [Google Scholar] [CrossRef]
- Stuchly, M.A.; Athey, T.W.; Samaras, G.M.; Taylor, G.E. Measurement of Radio Frequency Permittivity of Biological Tissues with an Open-Ended Coaxial Line: Part II—Experimental Results. IEEE Trans. Microw. Theory Techn. 1982, 30, 87–92. [Google Scholar] [CrossRef]
- Liporace, F.; Cavagnaro, M. Development of MR-based procedures for the implementation of patient-specific dielectric models for clinical use. J. Mech. Med. Biol. 2022, 23, 2340031. [Google Scholar] [CrossRef]
- Liporace, F.; Cavagnaro, M. wideband model to evaluate the dielectric properties of biological tissues from magnetic resonance acquisitions. In Proceedings of the 2023 17th European Conference on Antennas and Propagation (EuCAP), Florence, Italy, 26–31 March 2023. [Google Scholar]
- Sebek, J.; Albin, N.; Bortel, R.; Natarajan, B.; Prakash, P. Sensitivity of microwave ablation models to tissue biophysical properties: A first step toward probabilistic modelling and treatment planning. Med. Phys. 2016, 43, 2649. [Google Scholar] [CrossRef]
- Foster, K.R.; Scheppes, J.L. Dielectric Properties of Tumor and Normal Tissues at Radio through Microwave Frequencies. J. Microw. Power 1982, 16, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R.; Kell, D.B. The passive electrical properties of biological systems: Their significance in physiology, biophysics and biotechnology. Phys. Med. Biol. 1987, 32, 933–970. [Google Scholar] [CrossRef] [PubMed]
- Ciarleglio, G.; Russo, T.; Toto, E.; Santonicola, M.G. Fabrication of Alginate/Ozoile Gel Microspheres by Electrospray Process. Gels 2024, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Karabey, Y.; Kaynak, M.S.; Hincal, A.A. Potential use of freeze-drying technique for the estimation of tissue water content. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, P.P.; Marmarou, A. Use of magnetic resonance imaging for in vivo measurements of water content in human brain: Method and normal values. J. Neurosurg. 1999, 90, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Andreuccetti, D.; Fossi, R.; Petrucci, C. An Internet Resource for the Calculation of the Dielectric Properties of Body Tissues in the Frequency Range 10–100 GHz. IFAC-CNR, Florence (Italy), 1997. Based on Data Published by Gabriel, C.; et al. in 1996. Available online: http://niremf.ifac.cnr.it/tissprop/ (accessed on 30 May 2024).
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef]
- Schepps, J.L.; Foster, K.R. The UHF and microwave dielectric properties of normal and tumor tissues: Variation in dielectric properties with tissue water content. Phys. Med. Biol. 1980, 25, 114–159. [Google Scholar] [CrossRef] [PubMed]
- Fricke, H. A mathematical treatment of the electric conductivity and capacity of disperse systems: I. The electric conductivity of a suspension of homogeneous spheroids. Phys. Rev. 1924, 24, 575–587. [Google Scholar] [CrossRef]
- Farace, P.; Pontalti, R.; Cristoforetti, L.; Antolini, R.; Scarpa, M. An automated method for mapping human tissue permittivities by MRI in hyperthermia treatment planning. Phys. Med. Biol. 1997, 42, 2159–2174. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Foster, K.R. Dielectric properties of low-water-content tissues. Phys. Med. Biol. 1985, 30, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Reinoso, R.; Telfe, B.A.; Rowland, M. Tissue water content in rats measured by desiccation. J. Pharmacol. Toxicol. Methods 1997, 38, 87–92. [Google Scholar] [CrossRef]
- Liporace, F.; Ciarleglio, G.; Santonicola, M.G.; Cavagnaro, M. Dielectric Characterization of Biological Tissues at Microwave Frequencies Based on Water Content. In Proceedings of the 2024 18th European Conference on Antennas and Propagation (EuCAP), Glasgow, UK, 17–22 March 2024; pp. 1–5. [Google Scholar]
- Entris II Advanced Line Operating Instructions; Sartorius Lab Instruments GmbH & Co.: Goettingen, Germany, 2020.
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0; National Institute of Standards and Technology, Standard Reference Data Program: Gaithersburg, MD, USA, 2018.
- Mohammed, B.M.; Mohamed, M.; Naqvi, S.A.R.; Bialkowski, K.S.; Mills, P.C.; Abbosh, A.M. Using Dielectric Properties of Organ Matter and Water Content to Characterize Tissues at Different Health and Age Conditions. IEEE J. Electromagn. RF Microw. Med. Biol. 2020, 4, 69–76. [Google Scholar] [CrossRef]
- Taylor, B.N.; Kuyatt, C.E. Guidelines for Evaluating and Expressing the Uncertainty of NIST Measurement Results; US Department of Commerce, Technology Administration, National Institute of Standards and Technology: Gaithersburg, MD, USA, 1994.
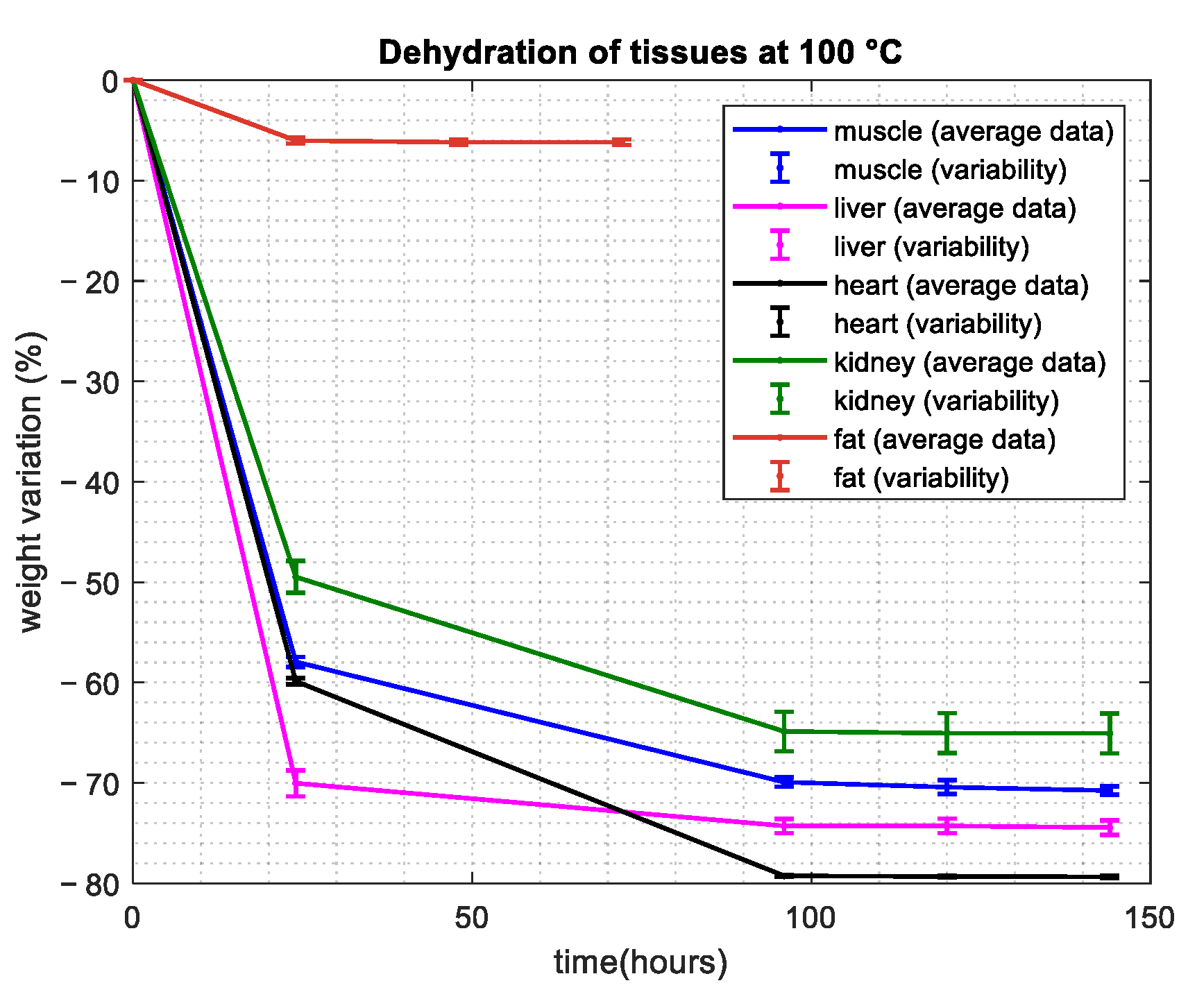
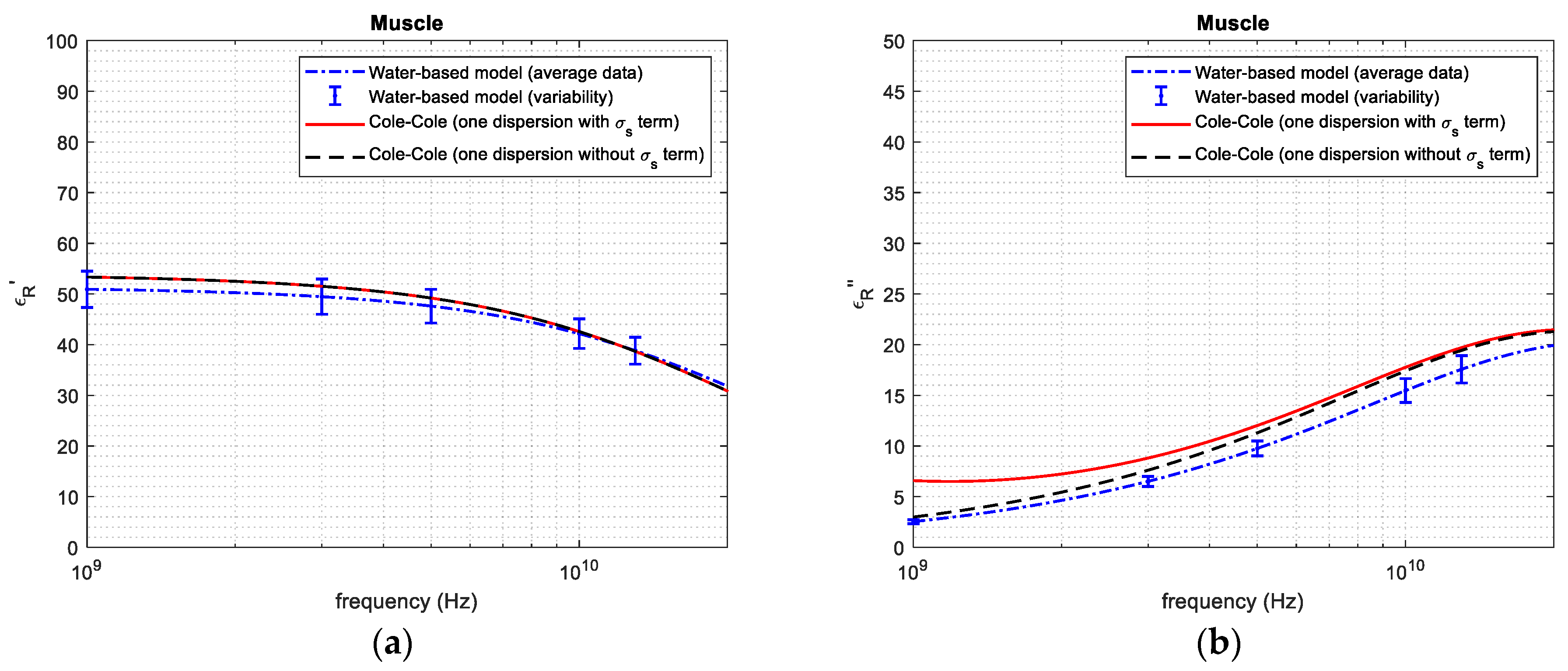
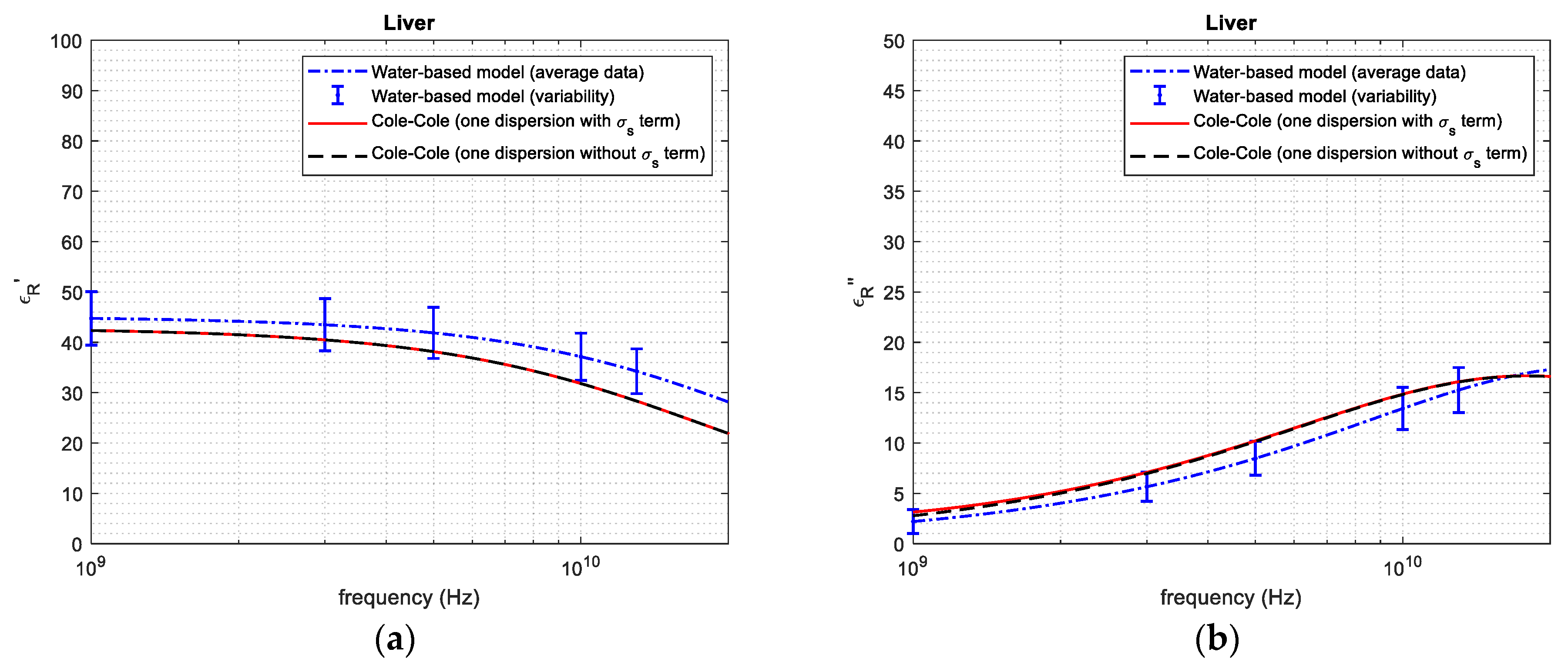
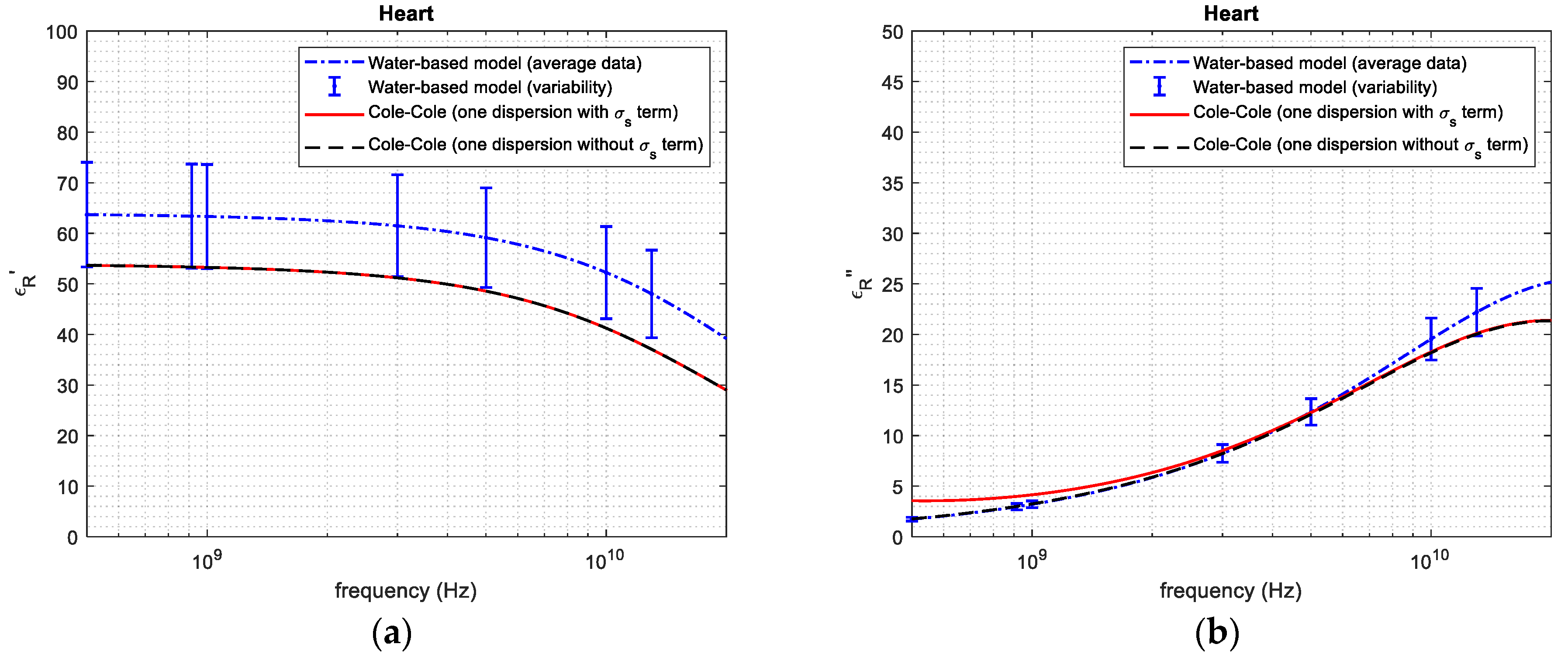
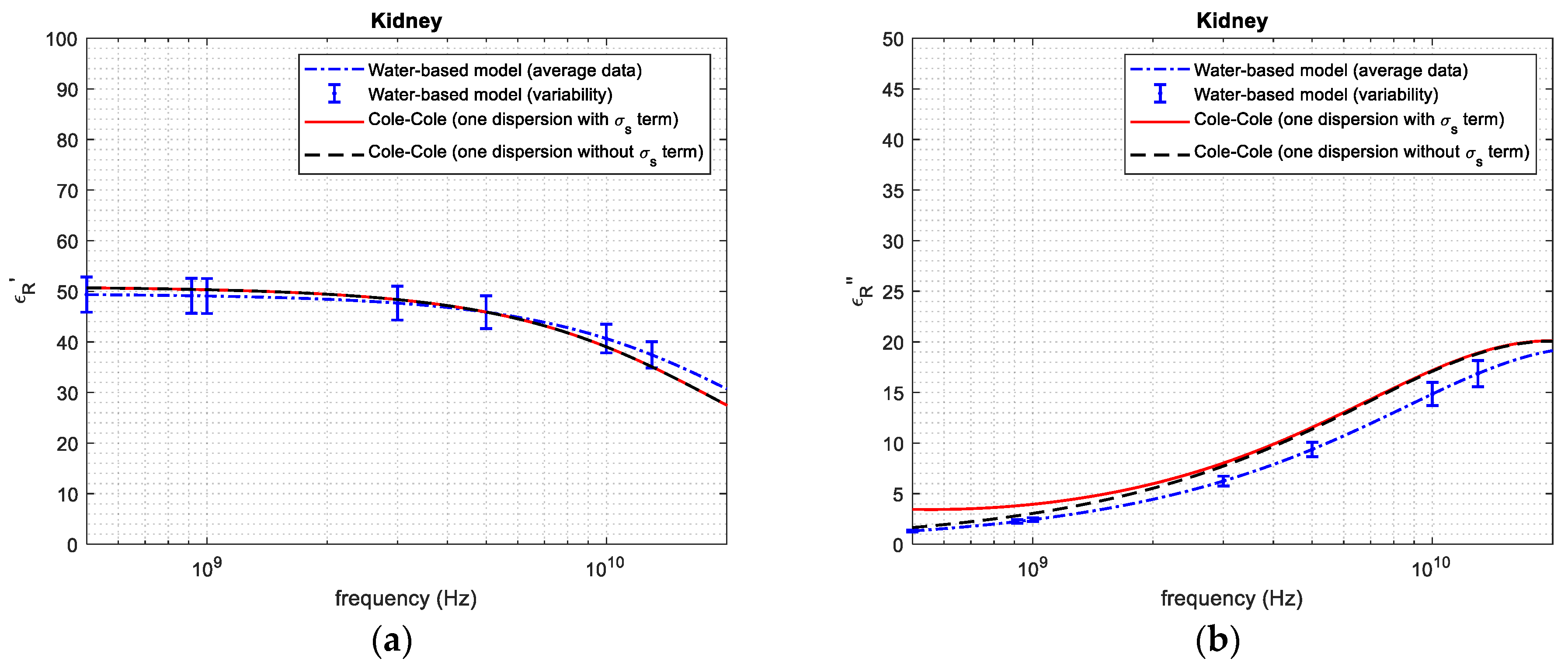
| Tissue | Average and Standard Deviation—Mass Basis (%) | Average and Standard Deviation—Volume Basis (%) | Reference from the Literature—Volume Basis (%) |
|---|---|---|---|
| Muscle | 70.8 ± 0.700 | 75.2 ± 5.22 | 73–78 [14] |
| Liver | 74.4 ± 1.60 | 70.1 ± 5.47 | 73–77 [14] |
| Heart | 79.3 ± 0.230 | 87.9 ± 6.52 | 86–87 [31] |
| Kidney | 65.1 ± 3.99 | 73.1 ± 5.31 | 78–79 [14] |
| Fat | 6.20 ± 0.620 | 5.20 ± 10.9 | 5–20 [14] |
| Tissue | (ps) | |||
|---|---|---|---|---|
| Muscle | 4 | 51.47 | 6.36 | 0.1 |
| Liver | 4 | 46.99 | 6.36 | 0.1 |
| Heart | 4 | 64.02 | 6.36 | 0.1 |
| Kidney | 4 | 49.59 | 6.36 | 0.1 |
| Fat | 2.5 | 3.930 | 6.36 | 0.1 |
| Frequency | (Water-Based Model) | (Reference from the Literature [18]) | (%) | (Water-Based Model) | (Reference from the Literature [18]) | (%) | |
|---|---|---|---|---|---|---|---|
| 1 GHz | 50.98 | 53.91 | 5.435 | 2.560 | 2.910 | 12.03 | |
| Muscle tissue | 3 GHz | 49.46 | 51.55 | 4.054 | 6.511 | 7.511 | 13.31 |
| 5 GHz | 47.60 | 49.23 | 3.311 | 9.791 | 11.30 | 13.35 | |
| 10 GHz | 42.16 | 42.61 | 1.056 | 15.46 | 17.60 | 12.16 | |
| 20 GHz | 31.86 | 30.86 | 3.240 | 19.78 | 21.41 | 7.613 | |
| 1 GHz | 44.75 | 42.34 | 5.692 | 2.281 | 2.790 | 18.24 | |
| 3 GHz | 43.48 | 40.49 | 7.384 | 5.650 | 6.771 | 16.55 | |
| Liver tissue | 5 GHz | 41.87 | 38.27 | 9.407 | 8.470 | 10.21 | 17.04 |
| 10 GHz | 37.14 | 31.74 | 17.01 | 13.43 | 14.81 | 9.318 | |
| 20 GHz | 28.11 | 22.01 | 27.71 | 17.27 | 16.59 | 4.099 | |
| 1 GHz | 63.33 | 53.25 | 18.93 | 3.210 | 3.251 | 1.261 | |
| 3 GHz | 61.47 | 51.17 | 20.13 | 8.230 | 8.220 | 0.1216 | |
| Heart tissue | 5 GHz | 59.13 | 48.53 | 21.84 | 12.28 | 12.11 | 1.404 |
| 10 GHz | 52.24 | 41.02 | 27.35 | 19.54 | 18.21 | 7.304 | |
| 20 GHz | 39.77 | 29.65 | 34.13 | 25.15 | 21.35 | 17.79 | |
| 1 GHz | 49.06 | 50.30 | 2.465 | 2.431 | 3.050 | 20.29 | |
| 3 GHz | 47.66 | 48.35 | 1.427 | 6.250 | 7.700 | 18.83 | |
| Kidney tissue | 5 GHz | 45.87 | 45.89 | 0.0438 | 9.370 | 11.44 | 18.09 |
| 10 GHz | 40.64 | 39.06 | 4.045 | 14.84 | 17.05 | 12.96 | |
| 20 GHz | 31.01 | 27.70 | 11.94 | 19.04 | 20.06 | 5.085 | |
| 1 GHz | 3.91 | 5.39 | 27.5 | 0.0760 | 0.250 | 69.6 | |
| 3 GHz | 3.87 | 5.21 | 25.7 | 0.191 | 0.521 | 63.3 | |
| Fat tissue | 5 GHz | 3.82 | 5.00 | 23.6 | 0.290 | 0.721 | 59.8 |
| 10 GHz | 3.65 | 4.59 | 20.5 | 0.467 | 0.970 | 51.8 | |
| 20 GHz | 3.35 | 4.01 | 16.5 | 0.601 | 1.092 | 44.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liporace, F.; Ciarleglio, G.; Santonicola, M.G.; Cavagnaro, M. Reconstruction of the Permittivity of Ex Vivo Animal Tissues in the Frequency Range 1–20 GHz Using a Water-Based Dielectric Model. Sensors 2024, 24, 5338. https://doi.org/10.3390/s24165338
Liporace F, Ciarleglio G, Santonicola MG, Cavagnaro M. Reconstruction of the Permittivity of Ex Vivo Animal Tissues in the Frequency Range 1–20 GHz Using a Water-Based Dielectric Model. Sensors. 2024; 24(16):5338. https://doi.org/10.3390/s24165338
Chicago/Turabian StyleLiporace, Flavia, Gianluca Ciarleglio, Maria Gabriella Santonicola, and Marta Cavagnaro. 2024. "Reconstruction of the Permittivity of Ex Vivo Animal Tissues in the Frequency Range 1–20 GHz Using a Water-Based Dielectric Model" Sensors 24, no. 16: 5338. https://doi.org/10.3390/s24165338
APA StyleLiporace, F., Ciarleglio, G., Santonicola, M. G., & Cavagnaro, M. (2024). Reconstruction of the Permittivity of Ex Vivo Animal Tissues in the Frequency Range 1–20 GHz Using a Water-Based Dielectric Model. Sensors, 24(16), 5338. https://doi.org/10.3390/s24165338









