One-Channel Wearable Mental Stress State Monitoring System
Abstract
:1. Introduction
- The development of a wearable system that integrates the information of EEGs and GSR, which can be used for the detection of elevations in stress. Usually, GSR systems are independent of EEGs and are used in research lab environments. Integrating both in a wearable system reduces the size of the device and the level of complexity while still maintaining a high accuracy.
- An EEG system comprising a 10–20 electrode system that uses the standard placement of electrodes on the scalp. They are high-density for use in research labs and clinical settings. Hence, it is difficult to use one-channel systems to identify and localize the source of stress. This study investigates and proposes a practical location for one-channel electrodes suitable for the detection of stress elevations using EEGs and GSR.
- This paper also proposes a frequency to induce sweat on the scalp and aid in the detection of stress using GSR.
2. Methodology
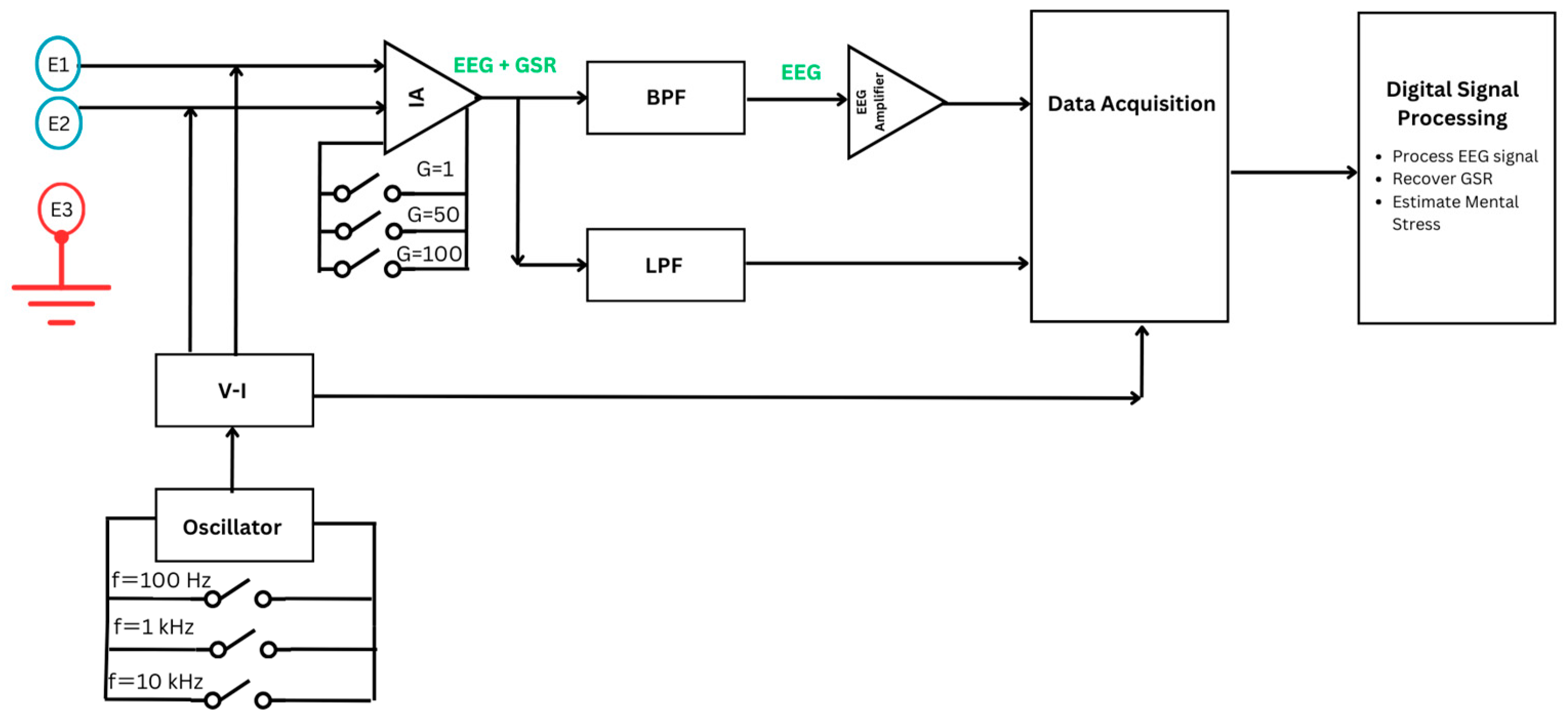
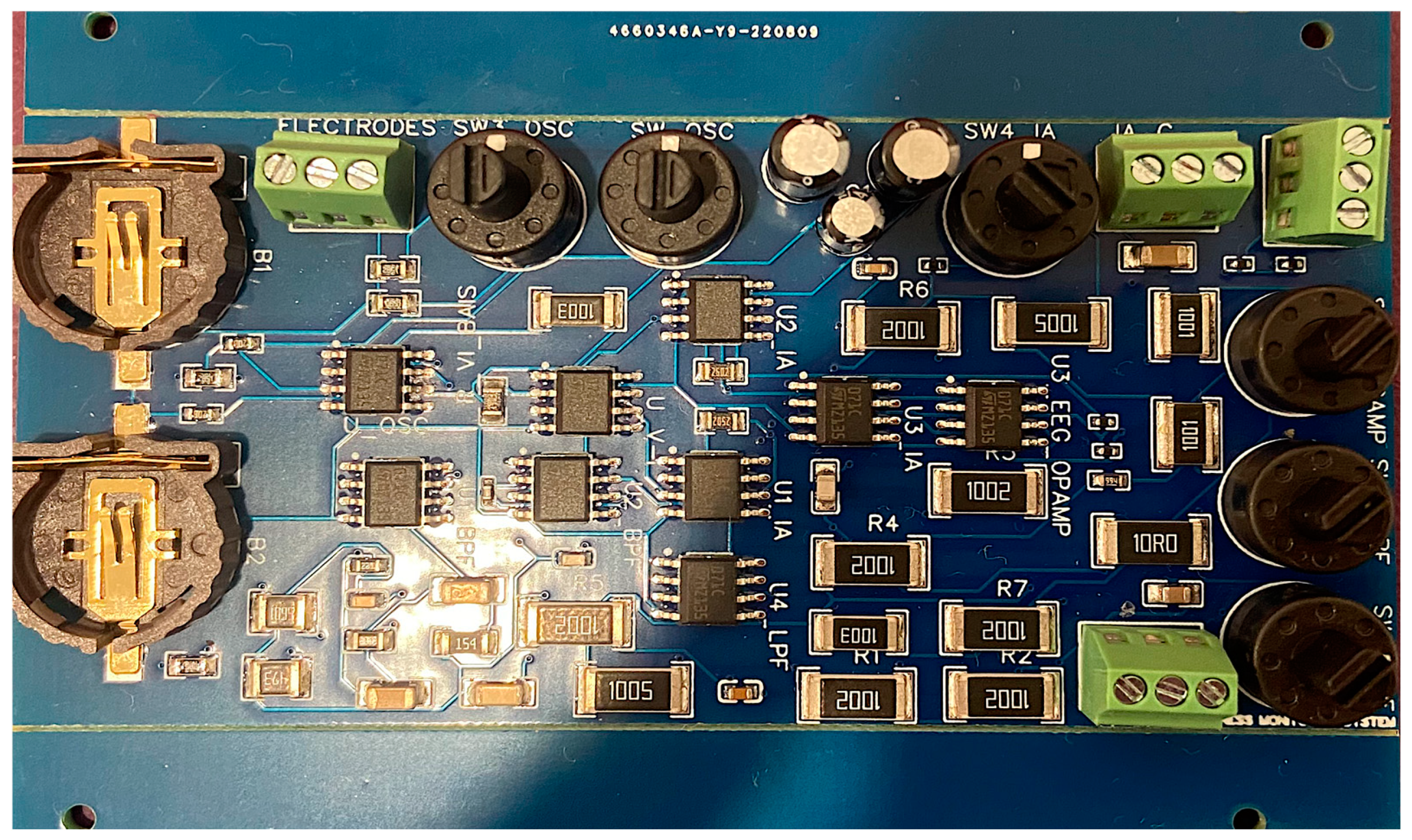
2.1. Data Acquisiton System
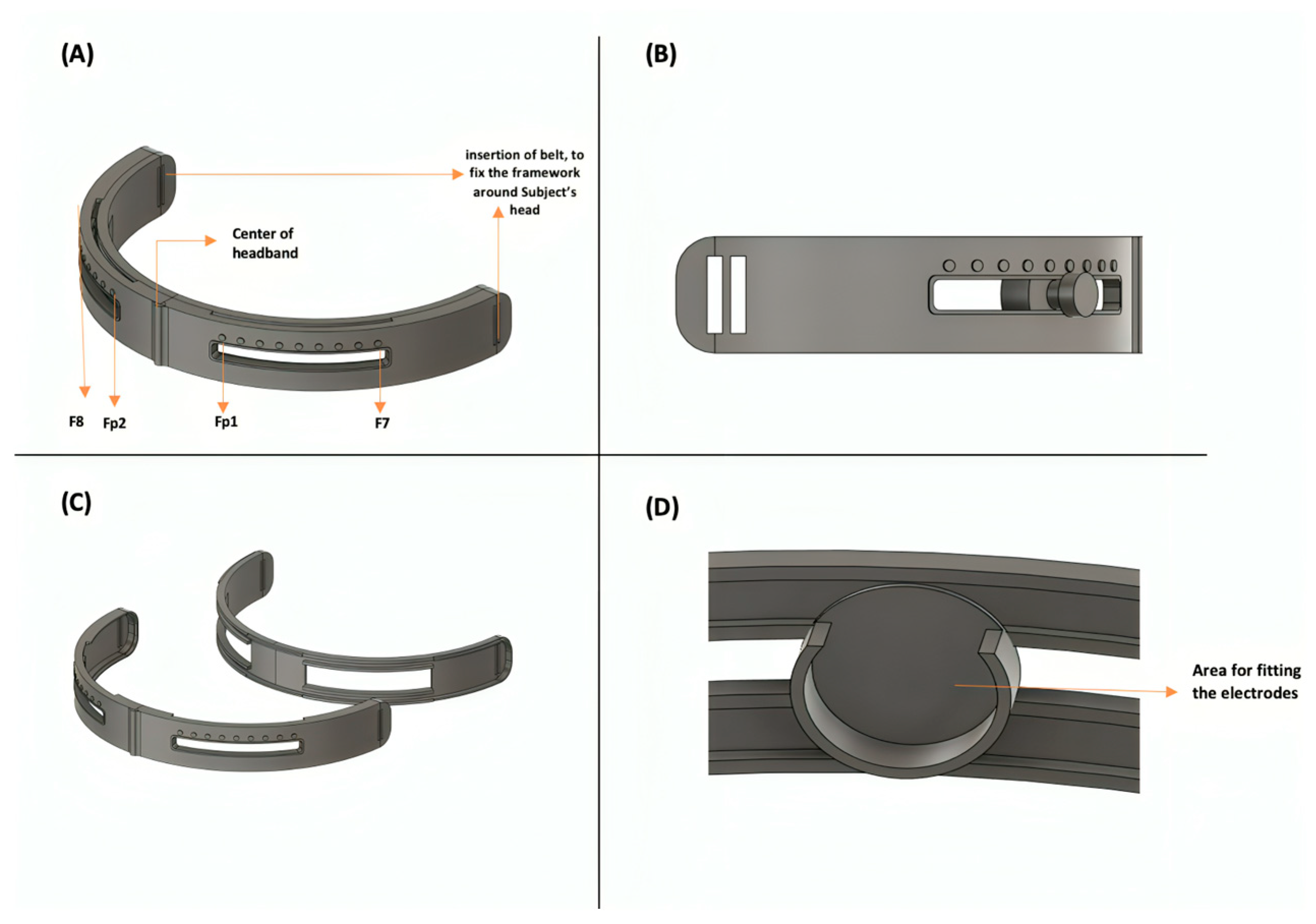
2.2. Mechanical Framework for Electrode
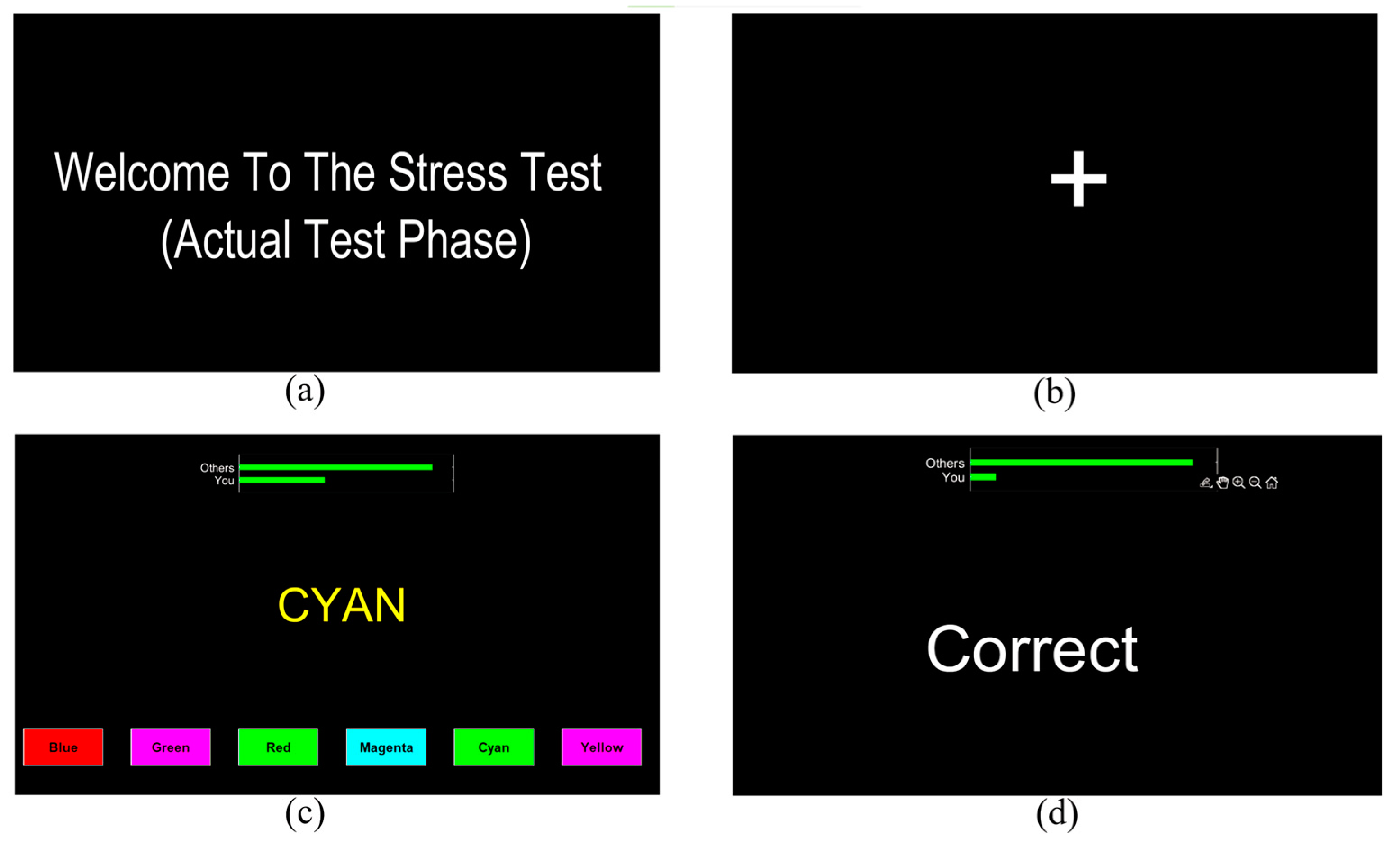
2.3. Mental Stress Task
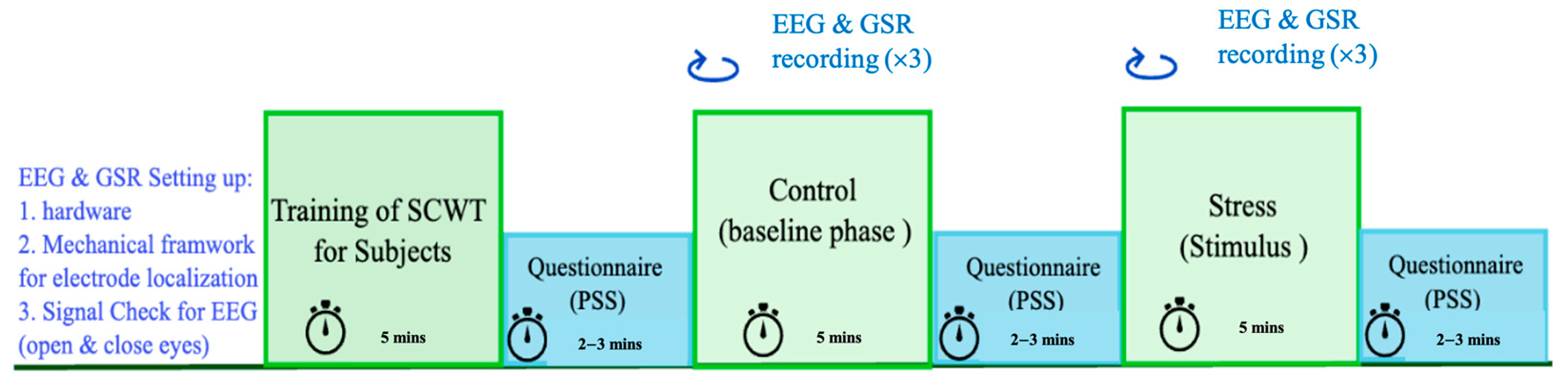
2.4. Experimental Protocol
2.5. Subjects
2.6. Data Analysis
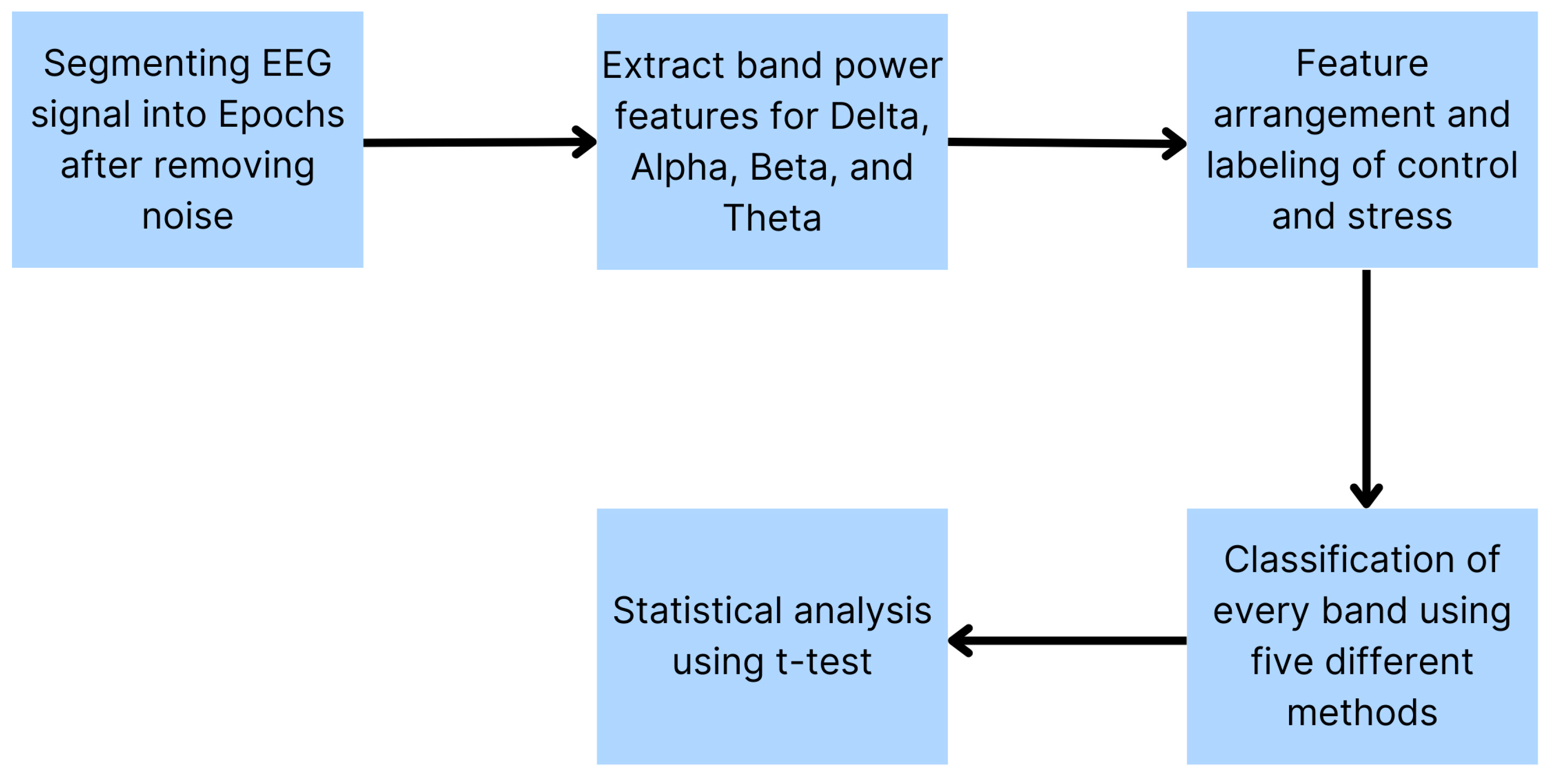
2.7. Signal Processing
2.8. Feature Extraction
3. Results
3.1. Hardware Testing of the Monitoring System to Detect Stress
3.2. Removal of Noise and Artifacts from EEG
3.3. Mental Stress Detection Using EEG
3.4. Classification of Stress Using EEG Only
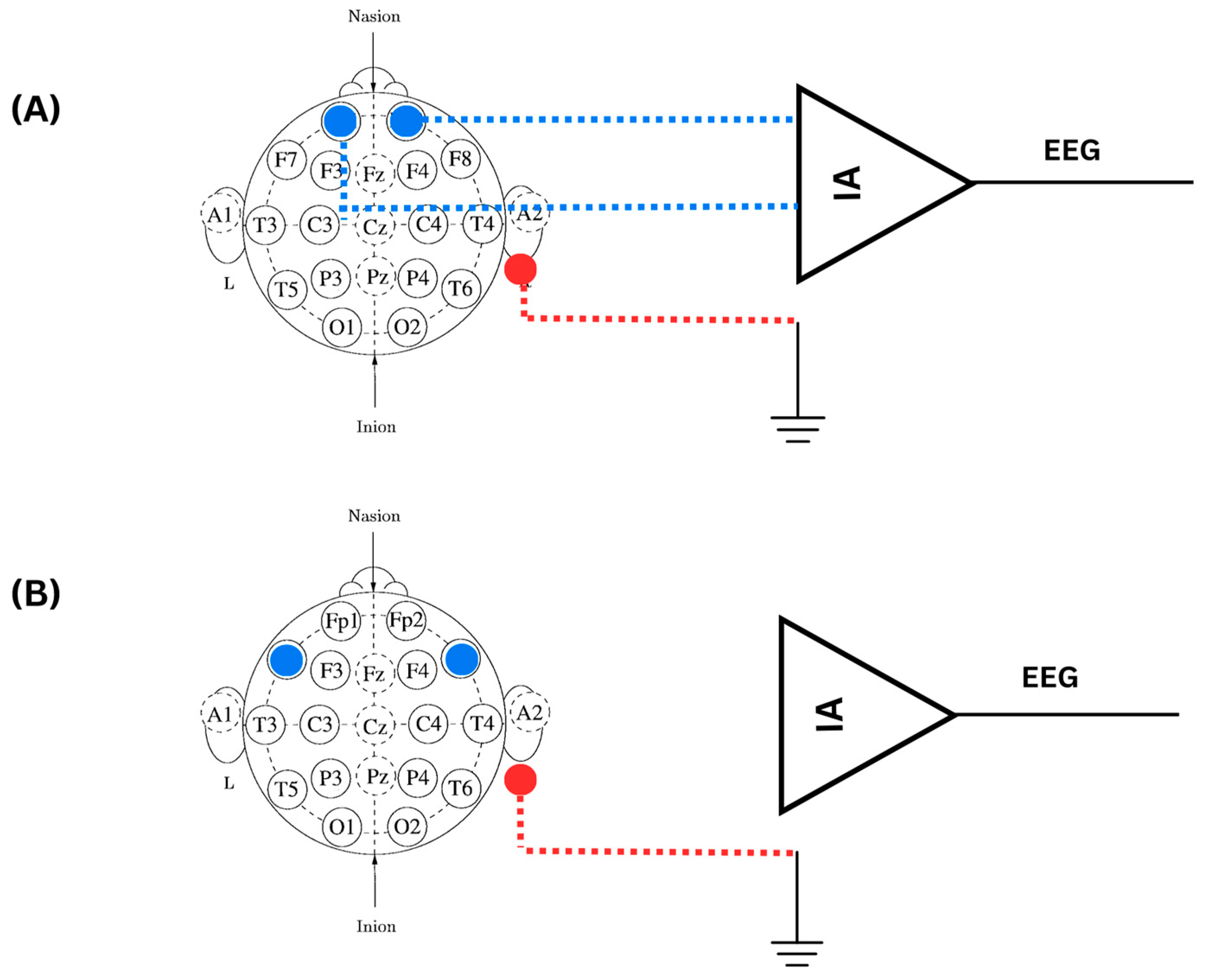
3.5. Optimum Electrode Location to Detect Stress Response in EEG
3.6. Subjective and Behavioural Data for Control and Stress Phase
3.7. Stress Detection through the Integration of EEGs and GSR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Acronym | Full Name | Description |
|---|---|---|
| EEG | Electroencephalogram | A method used to record the electrical activity of the brain, primarily used in this context for monitoring mental stress. |
| GSR | Galvanic Skin Response | A method of measuring the electrical conductance of the skin, which varies with its moisture level due to sweat, used as an indicator of psychological or physiological arousal. |
| SCWT | Stroop Color and Word Test | A psychological test involving the naming of the color of a word which is printed in a different color and that is used to induce stress and measure cognitive function. |
| SNS | Sympathetic Nervous System | Part of the autonomic nervous system that activates the fight-or-flight response, releasing stress hormones. |
| ANS | Autonomic Nervous System | Controls involuntary bodily functions such as heart rate, digestion, and respiratory rate and is involved in stress responses. |
| CNS | Central Nervous System | Comprises the brain and spinal cord and controls most functions of the body and mind. |
| RESPR | Respiration Rate | The number of breaths taken per minute, used in monitoring physiological responses to stress. |
| ECG | Electrocardiogram | A recording of the electrical activity of the heart, sometimes used in stress monitoring. |
| SC | Skin Conductance | Another term for galvanic skin response, measuring the conductance of the skin which varies with moisture level. |
| EDA | Electrodermal Activity | A measure of the skin’s ability to conduct electricity, which varies with its moisture level and is used to gauge emotional and physiological arousal. |
| TSST | Trier Social Stress Test | A commonly used protocol to induce stress in research participants through public speaking and mental arithmetic tasks. |
| FP1 | Frontopolar 1 | An electrode position in the EEG system, located at the front of the scalp. |
| FP2 | Frontopolar 2 | An electrode position in the EEG system, located at the front of the scalp. |
| F7 | Frontal 7 | An electrode position in the EEG system, located at the front of the scalp, on the left side. |
| F8 | Frontal 8 | An electrode position in the EEG system, located at the front of the scalp, on the right side. |
| SLS | Selective Laser Sintering | A three-dimensional printing technology that uses a laser to sinter powdered material, binding it together to create a solid structure, often used for creating prototypes and end-use parts. |
| PA12 | Polyamide 12 | A type of thermoplastic polymer used in three-dimensional printing and other applications. |
| PSS | Perceived Stress Scale | A psychological instrument used to measure the perception of stress. |
| PSD | Power Spectral Density | A measure of the power present in a signal as a function of frequency, used in signal processing. |
| IRB | Institutional Review Board | A committee that reviews and approves research involving human subjects to ensure ethical standards are met. |
| ADC | Analog-to-Digital Converter | A device that converts analog signals to digital data for processing. |
| KNN | K-Nearest Neighbors | A machine learning algorithm used for classification and regression tasks. |
| SVM | Support Vector Machine | A supervised learning algorithm used for classification and regression analysis. |
| DT | Decision Tree | A decision support tool that uses a tree-like model of decisions and their possible consequences. |
| DA | Discriminant Analysis | A statistical technique used to classify a set of observations into predefined classes. |
| NB | Naive Bayes | A classification technique based on Bayes’s theorem with an assumption of independence between predictors. |
| LDA | Linear Discriminant Analysis | A classification and dimensionality reduction technique in machine learning. |
| V-I | Voltage–Current | Refers to the relationship between voltage and current in electrical circuits. |
| EMG | Electromyography | A technique for evaluating and recording the electrical activity produced by skeletal muscles. |
| EOG | Electrooculography | A technique for measuring the corneo-retinal standing potential that exists between the front and the back of the human eye. |
| LS | Low Stress | A category indicating a low level of stress, determined by the perceived stress scale. |
| MS | Medium Stress | A category indicating a medium level of stress, determined by the perceived stress scale. |
| HS | High Stress | A category indicating a high level of stress, determined by the perceived stress scale. |
| HRV | Heart Rate Variability | The variation in the time interval between heartbeats, used as an indicator of stress and autonomic nervous system activity. |
| Sens. | Sensitivity | Measures how well a machine learning model can detect positive instances. |
References
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Masri, G.; Al-Shargie, F.; Tariq, U.; Almughairbi, F.; Babiloni, F.; Al-Nashash, H. Mental Stress Assessment in the Workplace: A Review. IEEE Trans. Affect. Comput. 2023, 1–20. [Google Scholar] [CrossRef]
- Selye, H. Stress and the general adaptation syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Giannakakis, G.; Grigoriadis, D.; Giannakaki, K.; Simantiraki, O.; Roniotis, A.; Tsiknakis, M. Review on psychological stress detection using biosignals. IEEE Trans. Affect. Comput. 2019, 13, 440–460. [Google Scholar] [CrossRef]
- Carneiro, D.; Novais, P.; Augusto, J.C.; Payne, N. New methods for stress assessment and monitoring at the workplace. IEEE Trans. Affect. Comput. 2017, 10, 237–254. [Google Scholar] [CrossRef]
- Ossewaarde, L.; Qin, S.; Van Marle, H.J.F.; van Wingen, G.A.; Fernández, G.; Hermans, E.J. Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage 2011, 55, 345–352. [Google Scholar] [CrossRef]
- Vanhollebeke, G.; Kappen, M.; De Raedt, R.; Baeken, C.; van Mierlo, P.; Vanderhasselt, M.-A. Effects of acute psychosocial stress on source level EEG power and functional connectivity measures. Sci. Rep. 2023, 13, 8807. [Google Scholar] [CrossRef]
- Vanhollebeke, G.; De Smet, S.; De Raedt, R.; Baeken, C.; van Mierlo, P.; Vanderhasselt, M.-A. The neural correlates of psychosocial stress: A systematic review and meta-analysis of spectral analysis EEG studies. Neurobiol. Stress 2022, 18, 100452. [Google Scholar] [CrossRef]
- Katmah, R.; Al-Shargie, F.; Tariq, U.; Babiloni, F.; Al-Mughairbi, F.; Al-Nashash, H. A review on mental stress assessment methods using EEG signals. Sensors 2021, 21, 5043. [Google Scholar] [CrossRef]
- Al-Shargie, F.M.; Tang, T.B.; Badruddin, N.; Kiguchi, M. Mental stress quantification using EEG signals. In Proceedings of the International Conference for Innovation in Biomedical Engineering and Life Sciences: ICIBEL2015, Putrajaya, Malaysia, 6–8 December 2015; Springer: Singapore, 2016; pp. 15–19. [Google Scholar]
- Attallah, O. An Effective Mental Stress State Detection and Evaluation System Using Minimum Number of Frontal Brain Electrodes. Diagnostics 2020, 10, 292. [Google Scholar] [CrossRef]
- Hou, X.; Liu, Y.; Sourina, O.; Tan, Y.R.E.; Wang, L.; Müller-Wittig, W. EEG Based Stress Monitoring. In Proceedings of the 2015 IEEE International Conference on Systems, Man, and Cybernetics, Kowloon Tong, Hong Kong, 9–12 October 2015; pp. 3110–3115. [Google Scholar]
- Karthikeyan, P.; Murugappan, M.; Yaacob, S. A review on stress inducement stimuli for assessing human stress using physiological signals. In Proceedings of the 2011 IEEE 7th International Colloquium on Signal Processing and Its Applications, Penang, Malaysia, 4–6 March 2011; pp. 420–425. [Google Scholar] [CrossRef]
- Jun, G.; Smitha, K.G. EEG based stress level identification. In Proceedings of the 2016 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Budapest, Hungary, 9–12 October 2016; pp. 003270–003274. [Google Scholar]
- Shanmugasundaram, G.; Yazhini, S.; Hemapratha, E.; Nithya, S. A comprehensive review on stress detection techniques. In Proceedings of the 2019 IEEE International Conference on System, Computation, Automation and Networking (ICSCAN), Pondicherry, India, 29–30 March 2019; pp. 1–6. [Google Scholar]
- Cantara, A.; Ceniza, A. Stress Sensor Prototype: Determining the Stress Level in using a Computer through Validated Self-Made Heart Rate (HR) and Galvanic Skin Response (GSR) Sensors and Fuzzy Logic Algorithm. Int. J. Eng. Res. Technol. 2016, 5, 28–37. [Google Scholar]
- Grimnes, S.; Jabbari, A.; Martinsen, Ø.G.; Tronstad, C. Electrodermal activity by DC potential and AC conductance measured simultaneously at the same skin site. Skin Res. Technol. 2011, 17, 26–34. [Google Scholar] [CrossRef]
- Nordbotten, B.J.; Tronstad, C.; Martinsen, Ø.G.; Grimnes, S. Estimation of skin conductance at low frequencies using measurements at higher frequencies for EDA applications. Physiol. Meas. 2014, 35, 1011. [Google Scholar] [CrossRef]
- Das, P.; Das, A.; Tibarewala, D.; Khasnobish, A. Design and development of portable galvanic skin response acquisition and analysis system. In Proceedings of the 2016 International Conference on Intelligent Control Power and Instrumentation (ICICPI), Kolkata, India, 21–23 October 2016; pp. 127–131. [Google Scholar]
- Posada-Quintero, H.F.; Chon, K.H. Innovations in electrodermal activity data collection and signal processing: A systematic review. Sensors 2020, 20, 479. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, S.; Seo, S.; Park, K. Highly wearable galvanic skin response sensor using flexible and conductive polymer foam. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 6631–6634. [Google Scholar]
- Banganho, A.; Santos, M.; da Silva, H.P. Design and Evaluation of an Electrodermal Activity Sensor (EDA) With Adaptive Gain. IEEE Sens. J. 2021, 21, 8639–8649. [Google Scholar] [CrossRef]
- Sequeira, H.; Roy, J.-C. Cortical and hypothalamo-limbic control of electrodermal responses. In Progress in Electrodermal Research; Springer: Boston, MA, USA, 1993; pp. 93–114. [Google Scholar]
- Boucsein, W. Principles of electrodermal phenomena. In Electrodermal Activity; Springer: Boston, MA, USA, 2012; pp. 1–86. [Google Scholar]
- Secerbegovic, A.; Ibric, S.; Nisic, J.; Suljanovic, N.; Mujcic, A. Mental workload vs. stress differentiation using single-channel EEG. In CMBEBIH 2017; Springer: Boston, MA, USA, 2017; pp. 511–515. [Google Scholar]
- Healey, J.A.; Picard, R.W. Detecting stress during real-world driving tasks using physiological sensors. IEEE Trans. Intell. Transp. Syst. 2005, 6, 156–166. [Google Scholar] [CrossRef]
- Panicker, S.S.; Gayathri, P. A survey of machine learning techniques in physiology based mental stress detection systems. Biocybern. Biomed. Eng. 2019, 39, 444–469. [Google Scholar] [CrossRef]
- Fowles, D.C. Electrodermal activity and antisocial behavior: Empirical findings and theoretical issues. In Progress in Electrodermal Research; Springer: Boston, MA, USA, 1993; pp. 223–237. [Google Scholar]
- Anusha, A.; Jose, J.; Preejith, S.; Jayaraj, J.; Mohanasankar, S. Physiological signal based work stress detection using unobtrusive sensors. Biomed. Phys. Eng. Express 2018, 4, 065001. [Google Scholar] [CrossRef]
- Tyagi, A.; Semwal, S.; Shah, G. A review of EEG sensors used for data acquisition. J. Comput. Appl. (IJCA) 2012, 12, 13–17. [Google Scholar]
- Sano, A.; Taylor, S.; McHill, A.W.; Phillips, A.J.; Barger, L.K.; Klerman, E.; Picard, R. Identifying Objective Physiological Markers and Modifiable Behaviors for Self-Reported Stress and Mental Health Status Using Wearable Sensors and Mobile Phones: Observational Study. J. Med. Internet Res. 2018, 20, e210. [Google Scholar] [CrossRef]
- Sani, M.; Norhazman, H.; Omar, H.; Zaini, N.; Ghani, S. Support vector machine for classification of stress subjects using EEG signals. In Proceedings of the 2014 IEEE Conference on Systems, Process and Control (ICSPC 2014), Kuala Lumpur, Malaysia, 12–14 December 2014; pp. 127–131. [Google Scholar]
- Zanetti, M.; Mizumoto, T.; Faes, L.; Fornaser, A.; De Cecco, M.; Maule, L.; Valente, M.; Nollo, G. Multilevel assessment of mental stress via network physiology paradigm using consumer wearable devices. J. Ambient. Intell. Humaniz. Comput. 2019, 12, 4409–4418. [Google Scholar] [CrossRef]
- Ahn, J.W.; Ku, Y.; Kim, H.C. A Novel Wearable EEG and ECG Recording System for Stress Assessment. Sensors 2019, 19, 1991. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, A.; Aztiria, A.; Basarab, A. Towards an automatic early stress recognition system for office environments based on multimodal measurements: A review. J. Biomed. Inform. 2016, 59, 49–75. [Google Scholar] [CrossRef]
- Seoane, F.; Mohino-Herranz, I.; Ferreira, J.; Alvarez, L.; Buendia, R.; Ayllón, D.; Llerena, C.; Gil-Pita, R. Wearable biomedical measurement systems for assessment of mental stress of combatants in real time. Sensors 2014, 14, 7120–7141. [Google Scholar] [CrossRef]
- Baumgartl, H.; Fezer, E.; Buettner, R. Two-Level Classification of Chronic Stress in EEG Recordings Two-Level Classification of Chronic Stress Using Machine Learning on Resting-State EEG Recordings. In Proceedings of the AMCIS 2020 Proceedings: 25th Americas Conference on Information Systems, Salt Lake City, UT, USA, 10–14 August 2020; pp. 1–10. [Google Scholar]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef]
- Bousefsaf, F.; Maaoui, C.; Pruski, A. Remote assessment of the Heart Rate Variability to detect mental stress. In Proceedings of the 2013 7th International Conference on Pervasive Computing Technologies for Healthcare and Workshops (PervasiveHealth), Venice, Italy, 5–8 May 2013; pp. 252–255. [Google Scholar] [CrossRef]
- Golden, Z.L.; Golden, C.J. Patterns of performance on the Stroop Color and Word Test in children with learning, attentional, and psychiatric disabilities. Psychol. Sch. 2002, 39, 489–495. [Google Scholar] [CrossRef]
- Derrfuss, J.; Brass, M.; Neumann, J.; Von Cramon, D.Y. Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and Stroop studies. Hum. Brain Mapp. 2005, 25, 22–34. [Google Scholar] [CrossRef]
- Williams, J.M.; Mathews, A.; MacLeod, C. The emotional Stroop task and psychopathology. Psychol. Bull. 1996, 120, 3. [Google Scholar] [CrossRef]
- Amatachaya, S.; Srisim, K.; Arrayawichanon, P.; Thaweewannakij, T.; Amatachaya, P. Dual-Task Obstacle Crossing Training Could Immediately Improve Ability to Control a Complex Motor Task and Cognitive Activity in Chronic Ambulatory Individuals With Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2019, 25, 260–270. [Google Scholar] [CrossRef]
- Katmah, R.; Al-Shargie, F.; Tariq, U.; Babiloni, F.; Al-Mughairbi, F.; Al-Nashash, H. Mental Stress Management Using fNIRS Directed Connectivity and Audio Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Al-Shargie, F.; Katmah, R.; Tariq, U.; Babiloni, F.; Al-Mughairbi, F.; Al-Nashash, H. Stress management using fNIRS and binaural beats stimulation. Biomed. Opt. Express 2022, 13, 3552–3575. [Google Scholar] [CrossRef]
- Yoo, J.; Park, J.W.; Kim, S.J. Development of User-friendly Bio-signal Acquisition System Based on LabVIEW. In Proceedings of the 2016 IEEE International Conference on Consumer Electronics (ICCE), Nantou County, Taiwan, 27–29 May 2016; pp. 1–2. [Google Scholar]
- Chi, Y.M.; Deiss, S.R.; Cauwenberghs, G. Non-contact low power EEG/ECG electrode for high density wearable biopotential sensor networks. In Proceedings of the 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 246–250. [Google Scholar]
- Sierra, A.D.S.; Ávila, C.S.; Casanova, J.G.; Bailador, G. A Stress-Detection System Based on Physiological Signals and Fuzzy Logic. IEEE Trans. Ind. Electron. 2011, 58, 4857–4865. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. Perceived stress scale. In Measuring Stress: A Guide for Health and Social Scientists; Oxford University Press: New York, NY, USA, 1994; pp. 235–283. [Google Scholar]
- Amin, A.H.U.; Malik, A.; Ahmad, R.F.; Badruddin, N.; Kamel, N.; Hussain, M.; Chooi, W.-T. Feature extraction and classification for EEG signals using wavelet transform and machine learning techniques. Australas. Phys. Eng. Sci. Med. 2015, 38, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liu, Y.; Sourina, O.; Mueller-Wittig, W. CogniMeter: EEG-based emotion, mental workload and stress visual monitoring. In Proceedings of the 2015 International Conference on Cyberworlds (CW), Visby, Sweden, 7–9 October 2015; pp. 153–160. [Google Scholar]
- Richard, N.Y.; Benjamin, B.G.; Brian, L.S. An overview of power spectral density (PSD) calculations. Proc. SPIE 2005, 5869, 58690U. [Google Scholar] [CrossRef]
- Al-Shargie, F.M.; Hassanin, O.; Tariq, U.; Al-Nashash, H. EEG-based semantic vigilance level classification using directed connectivity patterns and graph theory analysis. IEEE Access 2020, 8, 115941–115956. [Google Scholar] [CrossRef]
- Bobade, P.; Vani, M. Stress Detection with Machine Learning and Deep Learning using Multimodal Physiological Data. In Proceedings of the 2020 Second International Conference on Inventive Research in Computing Applications (ICIRCA), Coimbatore, India, 15–17 July 2020; pp. 51–57. [Google Scholar] [CrossRef]
- Badr, Y.; Al-Shargie, F.; Tariq, U.; Babiloni, F.; Al-Mughairbi, F.; Al-Nashash, H. Mental Stress Detection and Mitigation using Machine Learning and Binaural Beat Stimulation. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–5. [Google Scholar]
- Blanco, J.A.; Vanleer, A.C.; Calibo, T.K.; Firebaugh, S.L. Single-Trial Cognitive Stress Classification Using Portable Wireless Electroencephalography. Sensors 2019, 19, 499. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Wu, S.-K.; Yu, S.-S.; Tsai, M.-H. Analyzing Brain Waves of Table Tennis Players with Machine Learning for Stress Classification. Appl. Sci. 2022, 12, 8052. [Google Scholar] [CrossRef]
- Calibo, T.K.; Blanco, J.A.; Firebaugh, S.L. Cognitive stress recognition. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Minneapolis, MN, USA, 6–9 May 2013; pp. 1471–1475. [Google Scholar] [CrossRef]

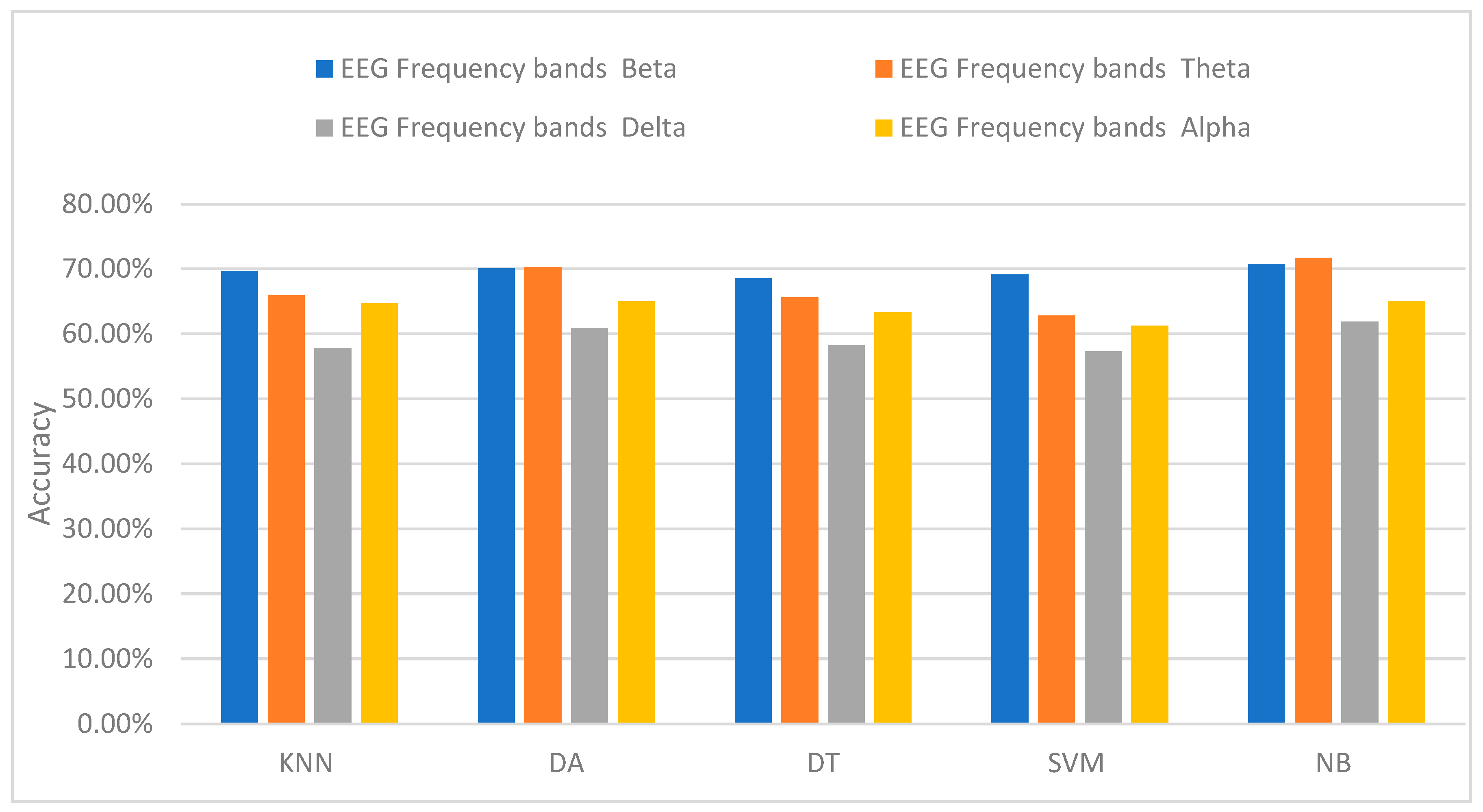


| Accuracy and Sensitivity (Sens.) for Each Classifier (%) | EEG Frequency Bands | |||
|---|---|---|---|---|
| Beta | Theta | Delta | Alpha | |
| KNN | 69.73% ± 0.125 Sens. 64.45% | 65.93% ± 0.079 Sens. 59.02% | 57.84% ± 0.093 Sens. 56.86% | 64.7% ± 0.11 Sens. 57.59% |
| LDA | 70.32% ± 0.134 Sens. 65.75% | 64.25% ± 0.082 Sens. 63.35% | 60.86% ± 0.091 Sens. 60.69% | 65.04% ± 0.11 Sens. 63.95% |
| DT | 68.57% ± 0.121 Sens. 63.38% | 65.63% ± 0.074 Sens. 61.15% | 58.25% ± 0.089 Sens. 54.78% | 63.33% ± 0.13 Sens. 63.1% |
| SVM | 69.16% ± 0.123 Sens. 63.99% | 62.85% ± 0.065 Sens. 60.93% | 57.29% ± 0.085 Sens. 53.48% | 61.23% ± 0.09 Sens. 60.465% |
| NB | 70.74% ± 0.105 Sens. 68.60% | 67.7% ± 0.078 Sens. 67.44% | 61.86% ± 0.091 Sens. 58.13% | 65.07% ± 0.01 Sens. 61.63% |
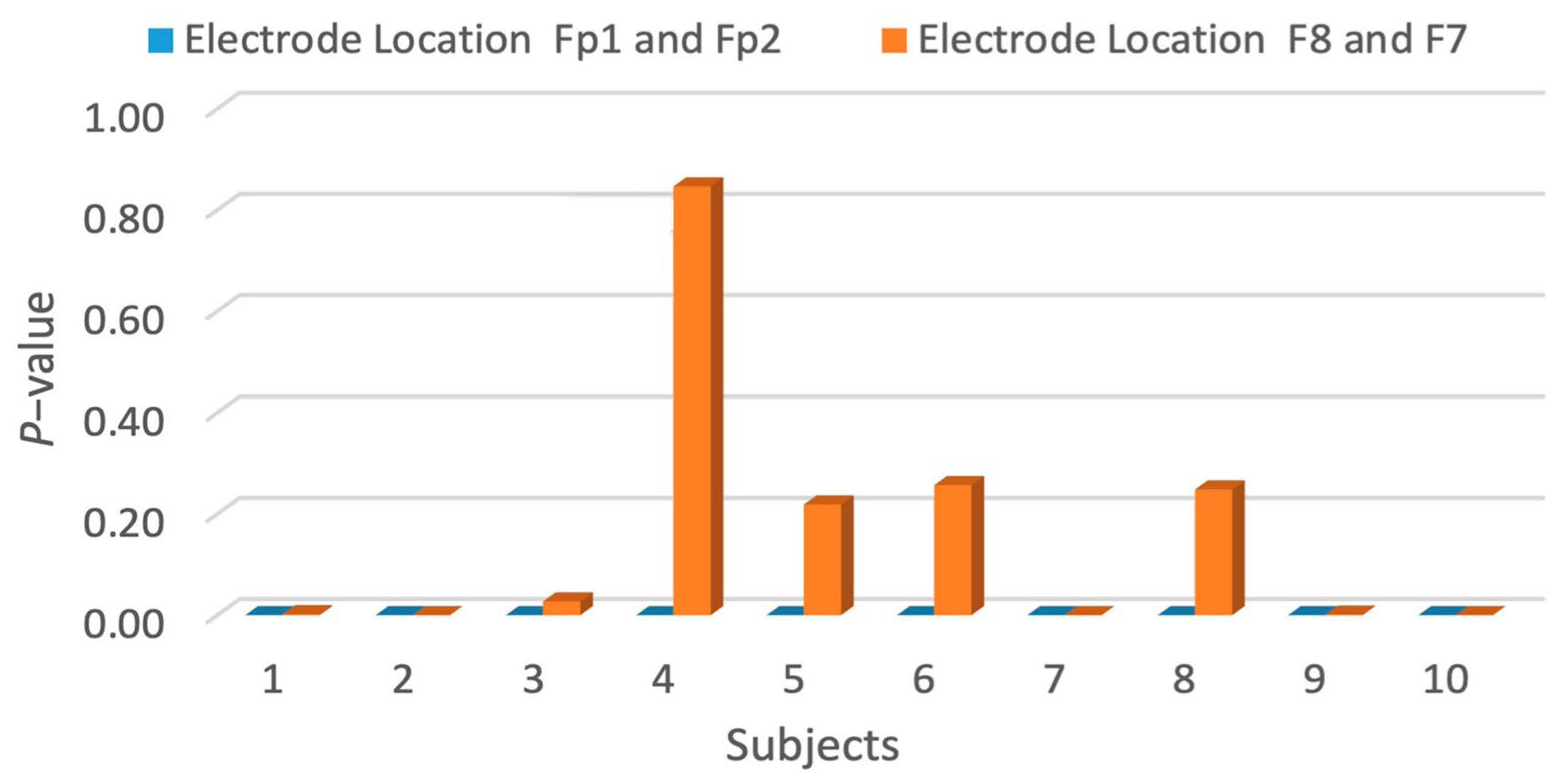
| p-Value for Each Electrode Location | |||
|---|---|---|---|
| Subjects | Subjects | ||
| # | Fp1 and Fp2 | # | F8 and F7 |
| 1 | 2.22 × 10−10 | 11 | 1.85 × 10−3 |
| 2 | 4.16 × 10−18 | 12 | 3.44 × 10−5 |
| 3 | 5.97 × 10−13 | 13 | 2.73 × 10−2 |
| 4 | 9.42 × 10−17 | 14 | 8.47 × 10−1 |
| 5 | 8.29 × 10−42 | 15 | 2.19 × 10−1 |
| 6 | 1.09 × 10−6 | 16 | 2.57 × 10−1 |
| 7 | 2.56 × 10−13 | 17 | 4.67 × 10−5 |
| 8 | 1.90 × 10−54 | 18 | 2.48 × 10−1 |
| 9 | 1.16 × 10−10 | 19 | 1.25 × 10−3 |
| 10 | 6.67 × 10−5 | 20 | 9.37 × 10−5 |
| Subjects | Control | Stress | Subjects | Control | Stress |
|---|---|---|---|---|---|
| 1 | 6 (LS) | 5(LS) | 11 | 27 (HS) | 24 (MS) |
| 2 | 12 (LS) | 17 (MS) | 12 | 13 (LS) | 20 (MS) |
| 3 | 18 (MS) | 19 (MS) | 13 | 17 (MS) | 20(MS) |
| 4 | 7 (LS) | 6 (LS) | 14 | 18 (MS) | 20 (MS) |
| 5 | 21 (MS) | 24 (MS) | 15 | 20 (MS) | 22 (MS) |
| 6 | 26 (MS) | 28 (HS) | 16 | 25 (MS) | 27 (MS) |
| 7 | 26 (MS) | 28 (HS) | 17 | 4 (LS) | 25 (MS) |
| 8 | 23 (MS) | 25 (MS) | 18 | 26 (MS) | 28 (HS) |
| 9 | 11 (LS) | 15 (MS) | 19 | 25 (MS) | 28 (HS) |
| 10 | 26 (MS) | 29 (HS) | 20 | 21 (MS) | 22 (MS) |
| Accuracy/Sensitivity of EEG + GSR | ||
|---|---|---|
| Accuracy/Sensitivity at Beta band of EEG | ||
| Classification type | EEG + GSR (100 Hz) | EEG |
| KNN | Accuracy: 83.77%, Sensitivity: 81.16% | Accuracy: 69.73% |
| DA | Accuracy: 43.39%, Sensitivity: 41.57% | Accuracy: 70.32% |
| DT | Accuracy: 67.38%, Sensitivity: 62.85% | Accuracy: 68.57% |
| SVM | Accuracy: 80.04%, Sensitivity: 79.05% | Accuracy: 69.16% |
| NB | Accuracy: 72.58%, Sensitivity: 73.52% | Accuracy: 70.74% |
| Accuracy at Theta band of EEG | ||
| Classification type | EEG + GSR (100 Hz) | EEG |
| KNN | Accuracy: 79.58%, Sensitivity:78.97% | Accuracy: 69.73% |
| DA | Accuracy: 52.06%, Sensitivity: 54.27% | Accuracy: 70.32% |
| DT | Accuracy: 58.85%, Sensitivity: 57.31% | Accuracy: 68.57% |
| SVM | Accuracy: 73.57%, Sensitivity:73.37% | Accuracy: 69.16% |
| NB | Accuracy: 76.04%, Sensitivity: 77.2% | Accuracy: 70.74% |
| Accuracy at Beta band of EEG | ||
| Classification type | EEG + GSR (1 kHz) | EEG |
| KNN | Accuracy: 75.44%, Sensitivity: 73.89% | Accuracy: 69.73% |
| DA | Accuracy: 42.77%, Sensitivity: 48.82% | Accuracy: 70.32% |
| DT | Accuracy: 58.70%, Sensitivity: 60.92% | Accuracy: 68.57% |
| SVM | Accuracy: 74.46%, Sensitivity: 72.19% | Accuracy: 69.16% |
| NB | Accuracy: 72.56%, Sensitivity: 71.63% | Accuracy: 70.74% |
| Accuracy at Theta band of EEG | ||
| Classification type | EEG + GSR (1 kHz) | EEG |
| KNN | Accuracy: 73.33%, Sensitivity: 68.64% | Accuracy: 65.93% |
| DA | Accuracy: 56.23%, Sensitivity: 58.79% | Accuracy: 64.25% |
| DT | Accuracy: 55.91%, Sensitivity: 56.72% | Accuracy: 65.62% |
| SVM | Accuracy: 69.63%, Sensitivity: 67.52% | Accuracy: 62.85% |
| NB | Accuracy: 73.71%, Sensitivity: 77.56% | Accuracy: 67.70% |
| Accuracy at Beta band for EEG | ||
| Classification type | EEG + GSR (10 kHz) | EEG |
| KNN | Accuracy: 83.44%, Sensitivity: 84.22% | Accuracy: 69.73% |
| DA | Accuracy: 42.89%, Sensitivity: 41.90% | Accuracy: 70.32% |
| DT | Accuracy: 43.06%, Sensitivity: 42.36% | Accuracy: 68.57% |
| SVM | Accuracy: 79.25%, Sensitivity: 74.44% | Accuracy: 69.16% |
| NB | Accuracy: 64.52%, Sensitivity: 62.78% | Accuracy: 70.74% |
| Accuracy at Theta band for EEG | ||
| Classification type | EEG + GSR (10 kHz) | EEG |
| KNN | Accuracy: 83.83%, Sensitivity: 82.90% | Accuracy: 69.73% |
| DA | Accuracy: 45.55%, Sensitivity: 43.73% | Accuracy: 70.32% |
| DT | Accuracy: 58.74%, Sensitivity: 54.27% | Accuracy: 68.57% |
| SVM | Accuracy: 77.98%, Sensitivity: 73.53% | Accuracy: 69.16% |
| NB | Accuracy: 64.55%, Sensitivity: 62.39% | Accuracy: 70.74% |
| Accuracy/Sensitivity at Beta band of EEG | ||
| Classification type | EEG + GSR (100 Hz) | EEG |
| KNN | Accuracy: 83.77%, Sensitivity: 81.16% | Accuracy: 69.73% |
| DA | Accuracy: 43.39%, Sensitivity: 41.57% | Accuracy: 70.32% |
| DT | Accuracy: 67.38%, Sensitivity: 62.85% | Accuracy: 68.57% |
| SVM | Accuracy: 80.04%, Sensitivity: 79.05% | Accuracy: 69.16% |
| NB | Accuracy: 72.58%, Sensitivity: 73.52% | Accuracy: 70.74% |
| Accuracy at Theta band of EEG | ||
| Classification type | EEG + GSR (100 Hz) | EEG |
| KNN | Accuracy: 79.58%, Sensitivity:78.97% | Accuracy: 69.73% |
| DA | Accuracy: 52.06%, Sensitivity: 54.27% | Accuracy: 70.32% |
| DT | Accuracy: 58.85%, Sensitivity: 57.31% | Accuracy: 68.57% |
| SVM | Accuracy: 73.57%, Sensitivity:73.37% | Accuracy: 69.16% |
| NB | Accuracy: 76.04%, Sensitivity: 77.2% | Accuracy: 70.74% |
| Accuracy at Beta band of EEG | ||
| Classification type | EEG + GSR (1 kHz) | EEG |
| KNN | Accuracy: 75.44%, Sensitivity: 73.89% | Accuracy: 69.73% |
| DA | Accuracy: 42.77%, Sensitivity: 48.82% | Accuracy: 70.32% |
| DT | Accuracy: 58.70%, Sensitivity: 60.92% | Accuracy: 68.57% |
| SVM | Accuracy: 74.46%, Sensitivity: 72.19% | Accuracy: 69.16% |
| NB | Accuracy: 72.56%, Sensitivity: 71.63% | Accuracy: 70.74% |
| Accuracy at Theta band of EEG | ||
| Classification type | EEG + GSR (1 kHz) | EEG |
| KNN | Accuracy: 73.33%, Sensitivity: 68.64% | Accuracy: 65.93% |
| DA | Accuracy: 56.23%, Sensitivity: 58.79% | Accuracy: 64.25% |
| DT | Accuracy: 55.91%, Sensitivity: 56.72% | Accuracy: 65.62% |
| SVM | Accuracy: 69.63%, Sensitivity: 67.52% | Accuracy: 62.85% |
| NB | Accuracy: 73.71%, Sensitivity: 77.56% | Accuracy: 67.70% |
| Accuracy at Beta band for EEG | ||
| Classification type | EEG + GSR (10 kHz) | EEG |
| KNN | Accuracy: 83.44%, Sensitivity: 84.22% | Accuracy: 69.73% |
| DA | Accuracy: 42.89%, Sensitivity: 41.90% | Accuracy: 70.32% |
| DT | Accuracy: 43.06%, Sensitivity: 42.36% | Accuracy: 68.57% |
| SVM | Accuracy: 79.25%, Sensitivity: 74.44% | Accuracy: 69.16% |
| NB | Accuracy: 64.52%, Sensitivity: 62.78% | Accuracy: 70.74% |
| Accuracy at Theta band for EEG | ||
| Classification type | EEG + GSR (10 kHz) | EEG |
| KNN | Accuracy: 83.83%, Sensitivity: 82.90% | Accuracy: 69.73% |
| DA | Accuracy: 45.55%, Sensitivity: 43.73% | Accuracy: 70.32% |
| DT | Accuracy: 58.74%, Sensitivity: 54.27% | Accuracy: 68.57% |
| SVM | Accuracy: 77.98%, Sensitivity: 73.53% | Accuracy: 69.16% |
| NB | Accuracy: 64.55%, Sensitivity: 62.39% | Accuracy: 70.74% |
| Study | Number of Channels | Type of Stressor | Electrode Location on Brain Region | Methodology | Accuracy |
|---|---|---|---|---|---|
| [57] | 14 | SCWT | Frontal, Temporal, Occipital | SCWT was used in 18 patients to elicit stress. Each session consisted of 24 trials; each lasted for 1 s. Logistic regression, KNN, and QDA were developed to obtain the accuracy. | Accuracy: 70.71% Alpha waves are significant at the pre-frontal lobe |
| [58] | 10 | SCWT, mental arithmetic | Frontal, Central, Temporal, Parietal, Occipital | Twelve subjects took the SCWT. They rested for 60 s (control) and then took the SCWT twice for 30 s. They used PSD for feature extraction and logistic regression. XGBoost, SVM, DT, and random forest were used for classification of mental stress. | Accuracy is 86.49% |
| [59] | 14 | SCWT | Frontal, Temporal, occipital | The SCWT was designed to wait only one second to obtain the user’s response. This was performed for 10 sessions in order to induce mental stress. Logistic regression and KNN were used to further classify stress. | Accuracy: 73.96% |
| [45] | 30 | SCWT | Right prefrontal region | Highest sensitivity to stress was at the right pre-frontal lobe region. High accuracy due to the combination of EEG with FNIRs and stress mitigation was found. | Accuracy of EEG increased by 20.83% |
| This paper | 1 | SCWT | Fp1 and FP2 (prefrontal region) | Through sensor fusion, EEG and GSR were used to classify stress. This improved overall accuracy. | Accuracy: 83.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Kader, L.; Al-Shargie, F.; Tariq, U.; Al-Nashash, H. One-Channel Wearable Mental Stress State Monitoring System. Sensors 2024, 24, 5373. https://doi.org/10.3390/s24165373
Abdul Kader L, Al-Shargie F, Tariq U, Al-Nashash H. One-Channel Wearable Mental Stress State Monitoring System. Sensors. 2024; 24(16):5373. https://doi.org/10.3390/s24165373
Chicago/Turabian StyleAbdul Kader, Lamis, Fares Al-Shargie, Usman Tariq, and Hasan Al-Nashash. 2024. "One-Channel Wearable Mental Stress State Monitoring System" Sensors 24, no. 16: 5373. https://doi.org/10.3390/s24165373






