ZIF-8-Derived Multifunctional Triethylamine Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZIF-8-In and ZnO/In2O3
2.3. Characterization
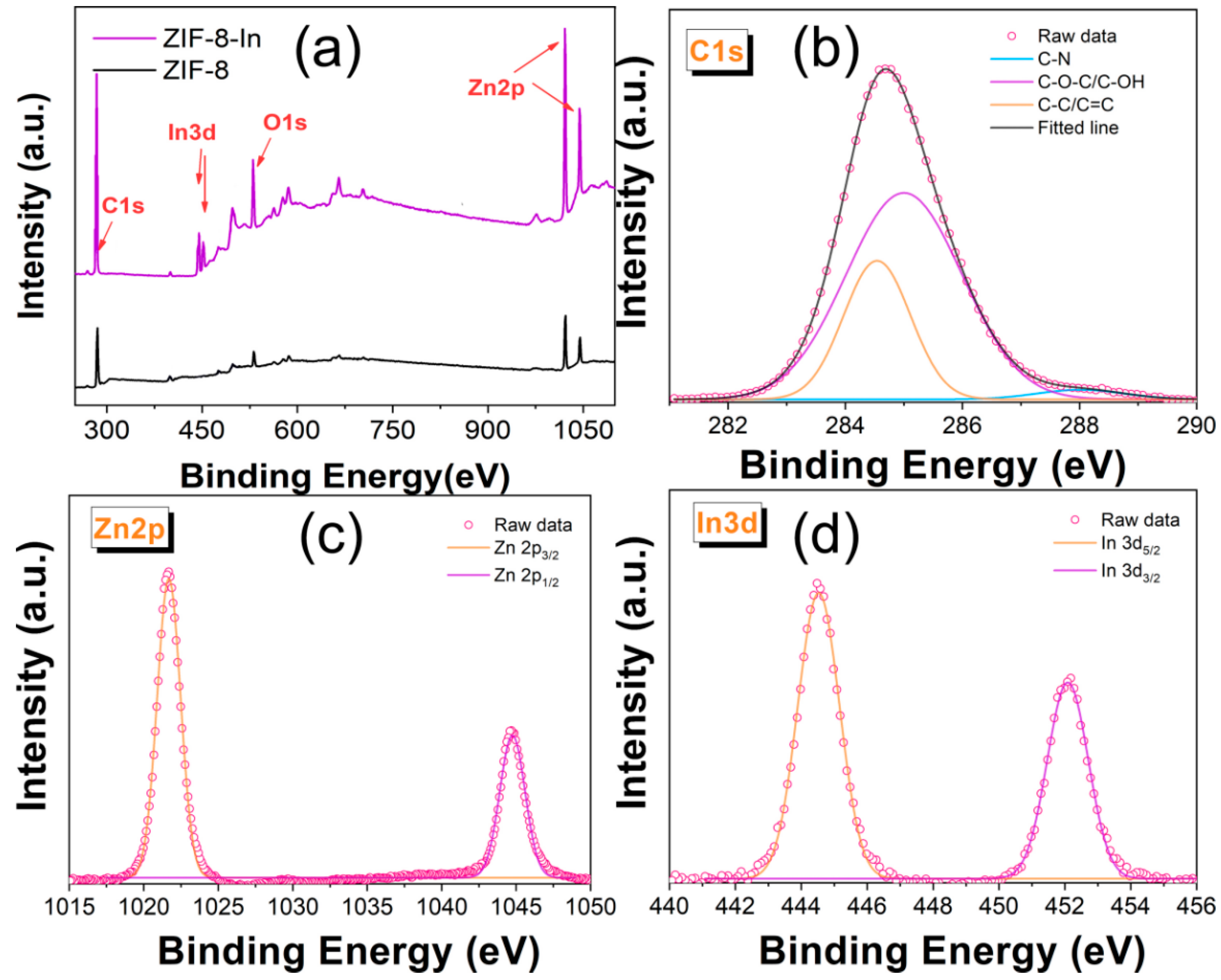
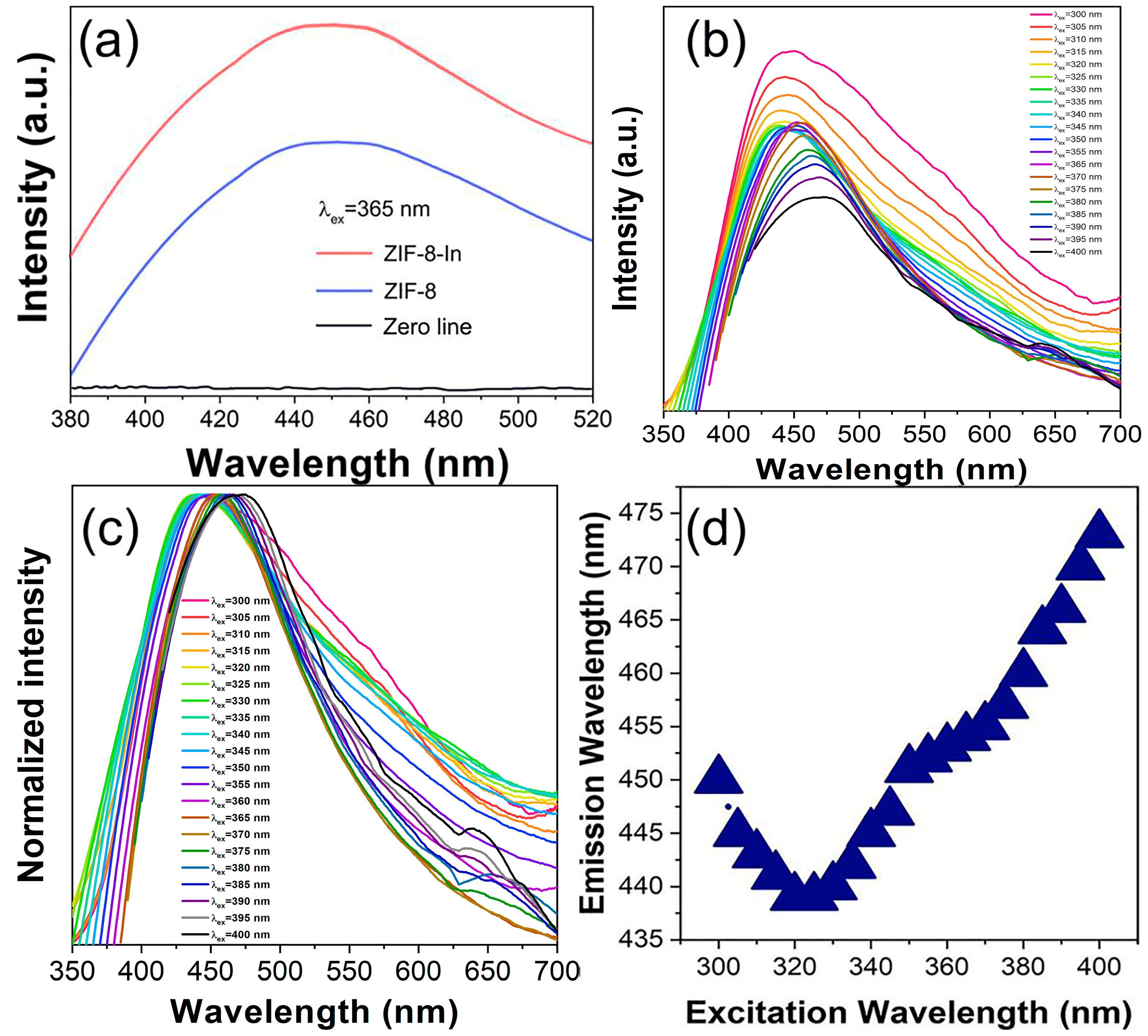
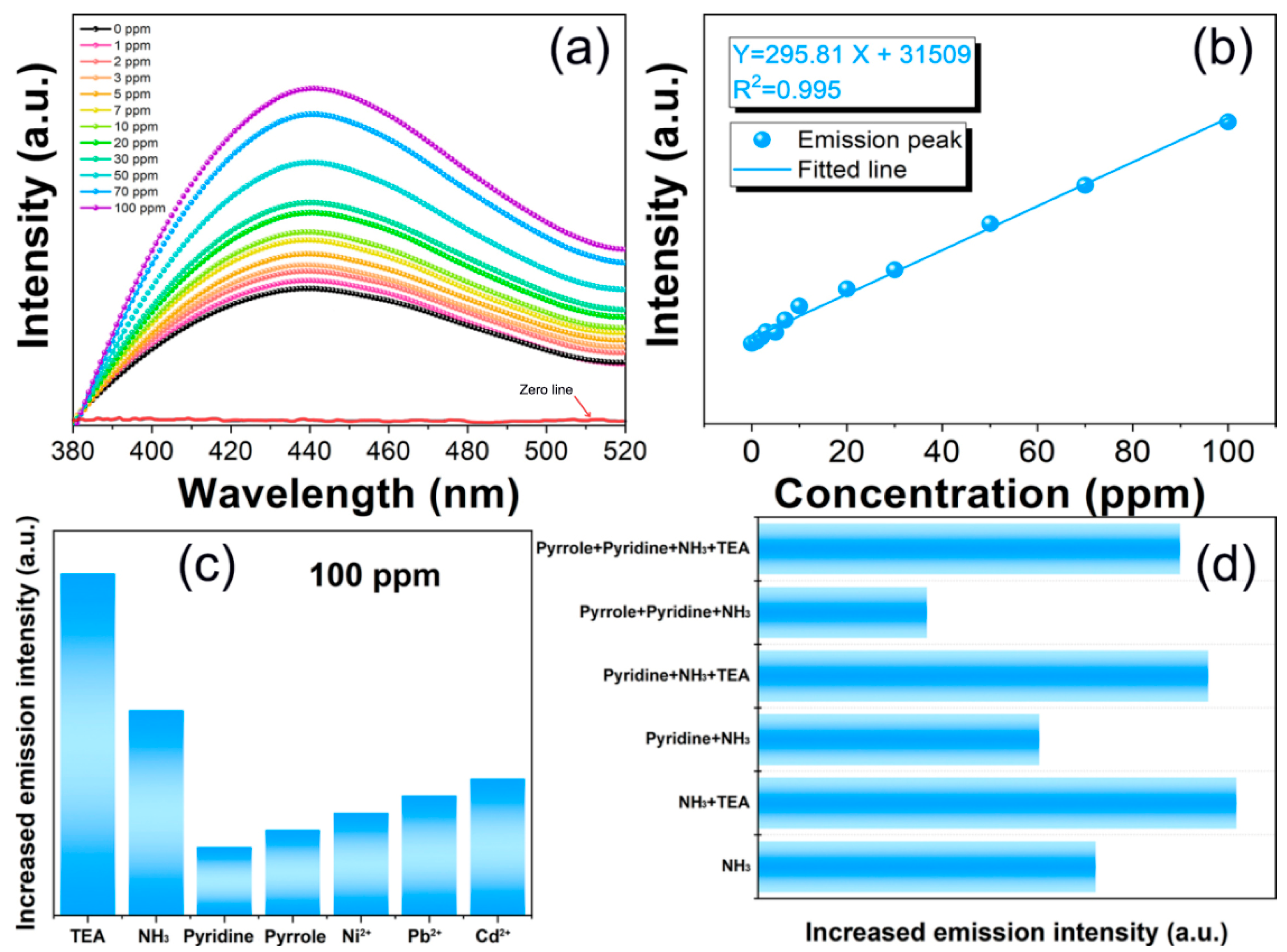
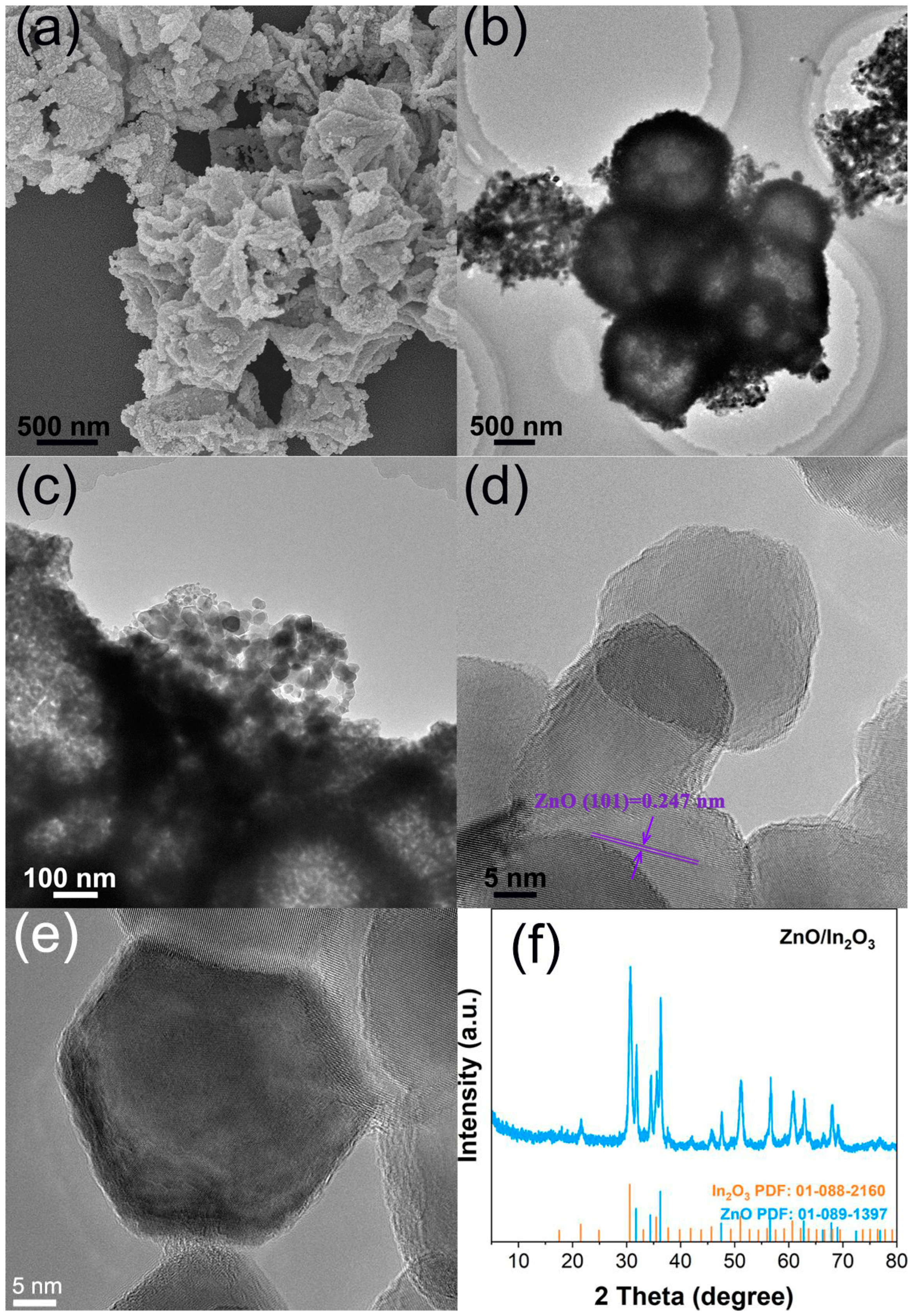
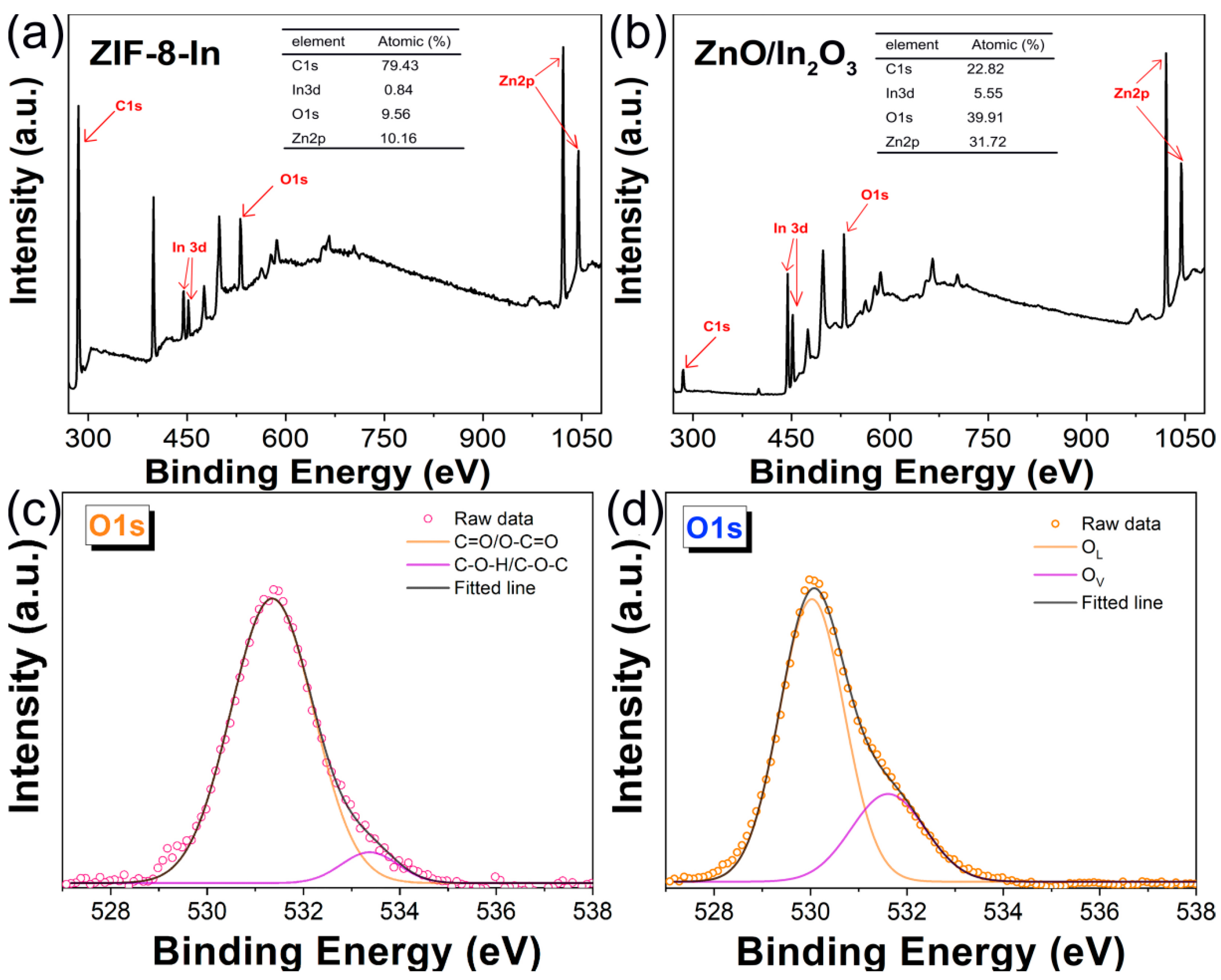
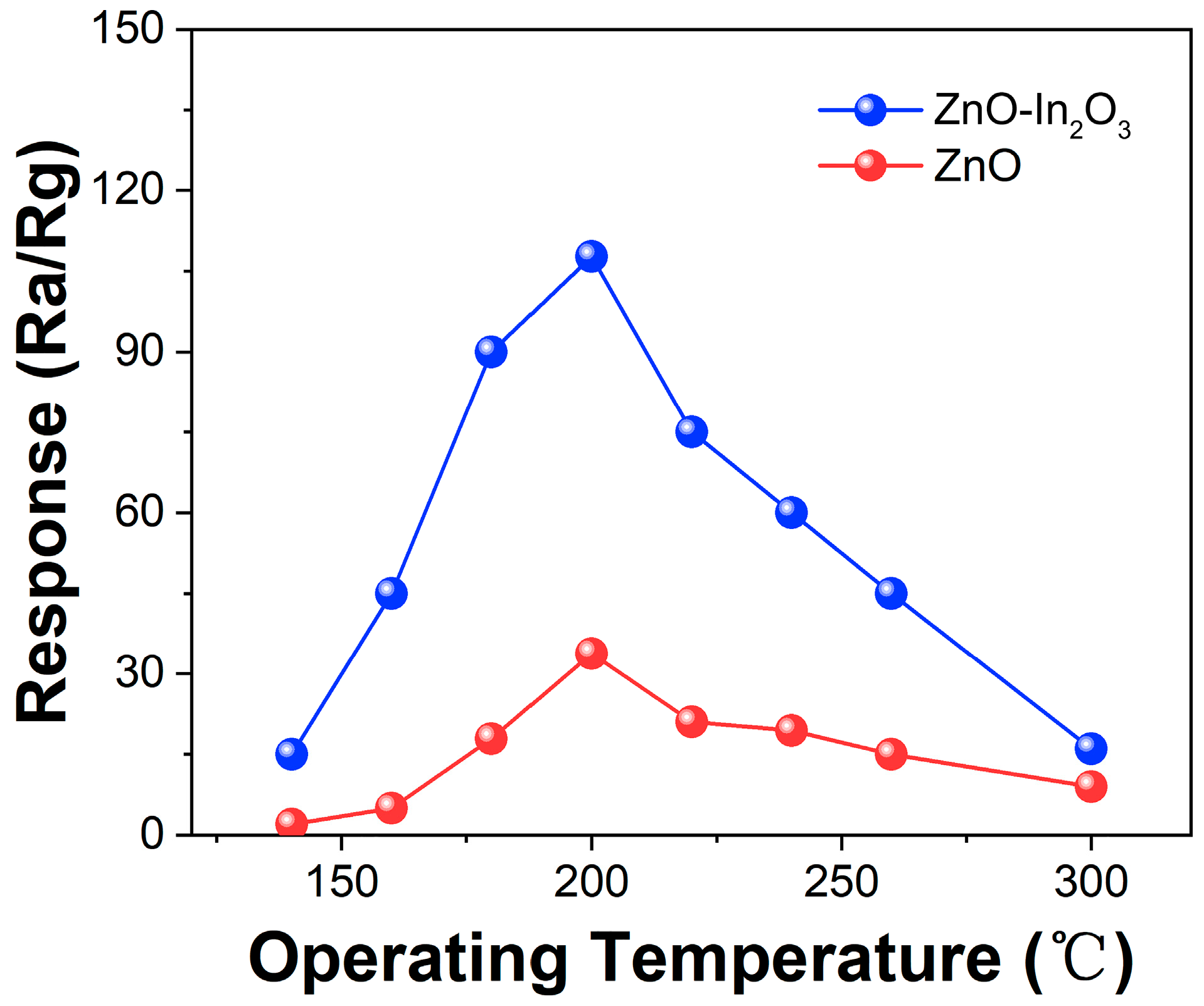
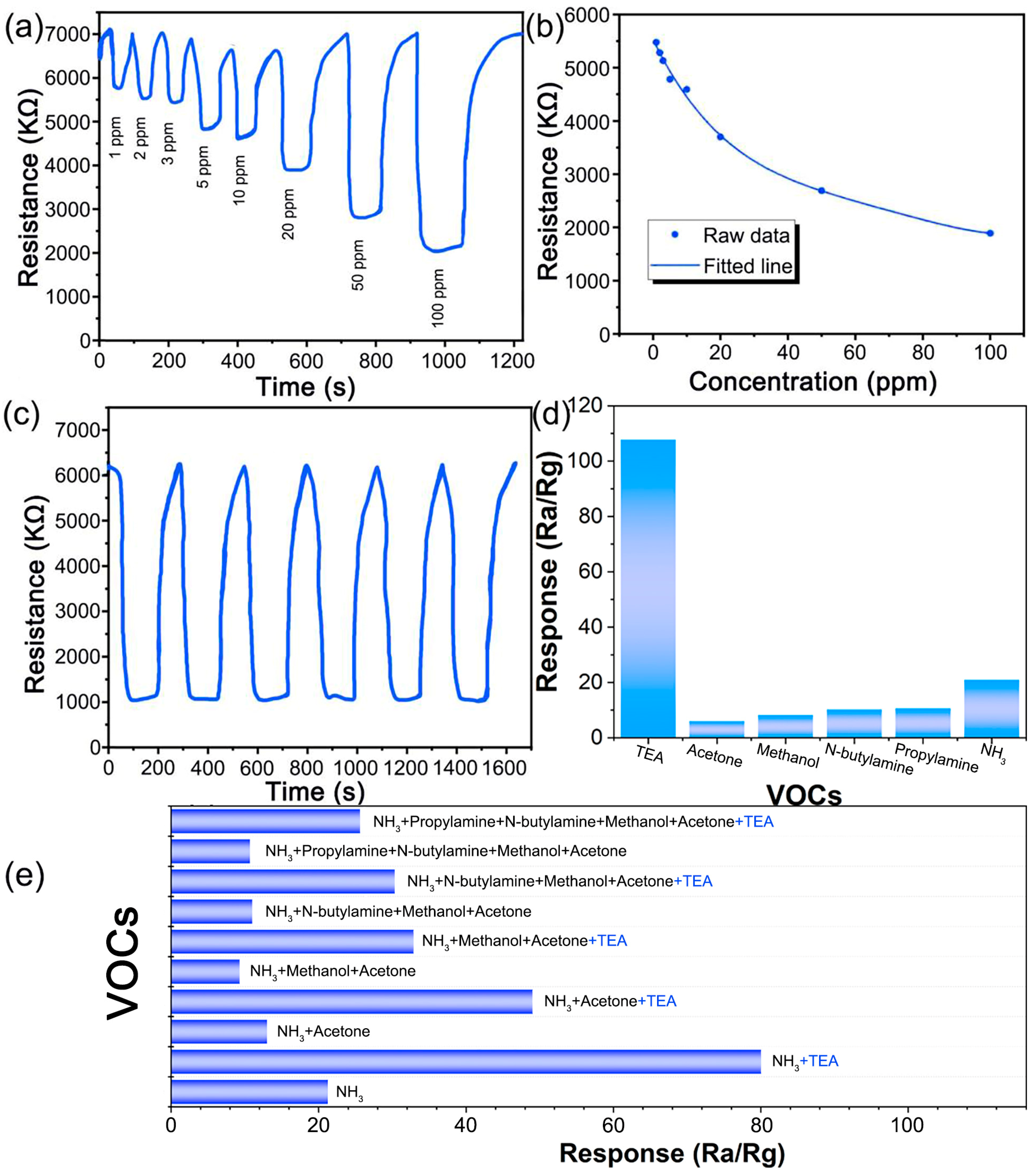
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Shen, Z.; Zhao, X.; Duanmu, F.; Yu, H.; Ji, H. Rational shape control of porous Co3O4 assemblies derived from MOF and their structural effects on n-butanol sensing. J. Hazard. Mater. 2019, 371, 352–361. [Google Scholar] [CrossRef]
- Zeng, Q.; Feng, J.; Lin, X.; Zhao, Y.; Liu, H.; Wang, S.; Dong, Z.; Feng, W. One-step facile synthesis of a NiO/ZnO biomorphic nanocomposite using a poplar tree leaf template to generate an enhanced gas sensing platform to detect n-butanol. J. Alloys Compd. 2020, 815, 150550. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y.; Hu, Y.; Pan, S.; Jiao, Z. Conductometric n-butanol gas sensor based on Tourmaline@ZnO hierarchical micro-nanostructures. Sens. Actuators B 2021, 337, 129793. [Google Scholar] [CrossRef]
- Meng, L.; Bu, W.; Li, Y.; Qin, Q.; Zhou, Z.; Hu, C.; Chuai, X.; Wang, C.; Sun, P.; Lu, G. Highly selective triethylamine sensing based on SnO/SnO2 nanocomposite synthesized by one-step solvothermal process and sintering. Sens. Actuators B 2021, 342, 130018. [Google Scholar] [CrossRef]
- Guo, X.; Yin, D.; Khaing, K.K.; Wang, J.; Luo, Z.; Zhang, Y. Construction of MOF/COF hybrids for boosting sunlight-induced fenton-like photocatalytic removal of organic pollutants. Inorg. Chem. 2021, 60, 15557–15568. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, L.; Wang, X.; He, D.; Suo, H.; Zhao, C. Fabrication of ZnO/carbon quantum dots composite sensor for detecting NO gas. Sensors 2020, 20, 4961. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Sun, J.H.; Liu, J.M.; Zhang, H.W.; He, H.G.; Liu, X.M.; Liao, D.K.; Tong, Z.F.; Sun, L.X. UV-triggered carrier transport regulation of fibrous NiO/SnO2 heterostructures for triethylamine detection. Chem. Eng. J. 2023, 476, 146687. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Z.; Zhao, J.; Zhou, T.; Santiago, A.R.P.; He, T.; Zhang, J.; Liu, Q.; Hu, G. Unique Pd/PdO–In2O3 heterostructures for the highly efficient detection of triethylamine. J. Mater. Chem. A 2023, 11, 17056–17065. [Google Scholar] [CrossRef]
- Hu, T.; Li, Y.; Wang, Y.; Chen, Y.; Zhang, J.; Luo, E.; Lv, B.; Jia, J. Controlled evolution of surface microstructure and phase boundary of ZnO nanoparticles for the multiple sensitization effects on triethylamine detection. Nanoscale 2024, 16, 11774–11785. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Wang, G.-D.; Shi, W.-J.; Hou, L.; Wang, Y.-Y.; Zhu, Z. Efficient C2Hn hydrocarbons and VOC adsorption and separation in an MOF with lewis basic and acidic decorated active sites. ACS Appl. Mater. Interfaces 2020, 12, 41785–41793. [Google Scholar] [CrossRef]
- Dong, J.J.; Yang, L.H.; Xie, G.L.; Cui, W.H.; Fang, J.R.; Li, C. Catalytic ozone decomposition and adsorptive VOCs removal in bimetallic metal-organic frameworks. Nat. Commun. 2022, 13, 4991. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Okur, S.; Li, C.; Chandresh, A.; Mutruc, D.; Hecht, S.; Heinke, L. A photoprogrammable electronic nose with switchable selectivity for VOCs using MOF films. Chem. Sci. 2021, 12, 15700. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mao, J.; Huang, G.; Zhang, Y.; Huang, J.; She, H.; Liu, C.; Liu, H.; Wang, Q. Configuration of hetero-framework via integrating MOF and triazine-containing COF for charge-transfer promotion in photocatalytic CO2 reduction. Chem. Eng. J. 2022, 446, 137011. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, S.; Hu, C.; Li, Z.; Wang, J.; Feng, X. PorousZnO/Co3O4/CoO/Co composite derived from Zn-Co-ZIF asimproved performance anodes for lithium-ion batteries. Mater. Lett. 2019, 250, 75–78. [Google Scholar] [CrossRef]
- Ye, J.W.; Wang, L.X.; Zhu, B.C.; Cheng, B.; He, R.G. Light-enhanced metal-support interaction for synergetic photo/thermal catalytic formaldehyde oxidation. J. Mater. Sci. Technol. 2023, 167, 74–81. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Yang, X.; Copner, N.; Jiang, Y.; Li, K. Ethanol sensor based on cascaded tapered optical fiber with surface modification of ZIF-8. Sens. Actuators B 2024, 402, 135084. [Google Scholar] [CrossRef]
- Moscoso, F.G.; Rodriguez-Albelo, L.M.; Ruiz-Salvador, A.R.; Lopes-Costa, T.; Pedrosa, J.M. Enhancement of the intrinsic fluorescence of ZIF-8 via post-synthetic cation exchange with Cd2+ and its incorporation into PDMS films for selective sulfide optical sensing. Mater. Today Chem. 2023, 28, 101366. [Google Scholar] [CrossRef]
- Xu, N.; Tang, Z.; Jiang, Y.P.; Fang, J.; Zhang, L.; Lai, X.; Sun, Q.J.; Fan, J.M.; Tang, X.G.; Liu, Q.X.; et al. Highly sensitive ratiometric fluorescent flexible sensor based on the RhB@ZIF-8@PVDF mixed-matrix membrane for broad-spectrum antibiotic detection. ACS Appl. Mater. Interfaces 2023, 15, 52993–53002. [Google Scholar] [CrossRef]
- Ren, X.; Xu, Z.; Liu, D.; Li, Y.; Zhang, Z.; Tang, Z. Conductometric NO2 gas sensors based on MOF-derived porous ZnO nanoparticles. Sens. Actuators B 2022, 357, 131384. [Google Scholar] [CrossRef]
- Tung, T.T.; Tran, M.T.; Feller, J.-F.; Castro, M.; Ngo, T.V.; Hassan, K.; Nine, M.J.; Losic, D. Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOC) biomarkers. Carbon 2019, 159, 333–344. [Google Scholar] [CrossRef]
- Hao, Z.; Song, S.; Li, B.; Jia, Q.-X.; Zheng, T.; Zhang, Z. A solvent driven dual responsive actuator based on MOF/polymer composite. Sens. Actuators B 2022, 358, 131448. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, F.-Y.; Wang, J.-S.; Yu, H.-Y.; Li, W.-T.; He, Y.-H.; Pang, M.; Li, Y.; Ruan, W.-J. Multi-dimensional ratiometric fluorescent sensing of organic disinfectants using a MOF composite with independent responsive components. Sens. Actuators B 2022, 367, 132041. [Google Scholar] [CrossRef]
- Yuan, H.; Li, N.; Fan, W.; Cai, H.; Zhao, D. Metal-organic framework based gas sensors. Adv. Sci. 2022, 9, 2104374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, Y.; Feng, X.; Yu, S.H.; Seok, J.; Muller, D.A.; Abrua, H.D. Sulfur encapsulation by MOF-derived CoS2 embeddedin carbon hosts for high-performance Li-S batteries. J. Mater. Chem. A 2019, 7, 21128–21139. [Google Scholar] [CrossRef]
- Shaikh, S.K.; Ganbavle, V.V.; Inamdar, S.I.; Rajpure, K.Y. Multifunctional zinc oxide thin films for high-performance UV photodetectors and nitrogen dioxide gas sensors. RSC Adv. 2016, 6, 25641. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; White, J.F.; Gengenbach, T.R.; Caruso, R.A.; Doherty, C.M. Fabrication of a reusable carbon dot/gold nanoparticle/metal-organic framework film for fluorescence detection of lead ions in water. ACS Appl. Mater. Interfaces 2022, 14, 35755–35768. [Google Scholar] [CrossRef]
- Kong, D.; Han, J.; Gao, Y.; Gao, Y.; Zhou, W.; Liu, G.; Lu, G. Lower coordination Co3O4 mesoporous hierarchical microspheres for comprehensive sensitization of triethylamine vapor sensor. J. Hazard. Mater. 2022, 430, 128469. [Google Scholar] [CrossRef]
- Park, J.Y.; Kwak, Y.; Lim, H.-R.; Park, S.-W.; Lim, M.S.; Cho, H.-B.; Myung, N.V.; Choa, Y.-H. Tuning the sensing responses towards room-temperature hypersensitive methanol gas sensor using exfoliated graphene-enhanced ZnO quantum dot nanostructures. J. Hazard. Mater. 2022, 438, 129412. [Google Scholar] [CrossRef]
- Cheng, M.; Wu, Z.; Liu, G.; Zhao, L.; Gao, Y.; Li, S.; Zhang, B.; Yan, X.; Lu, G. Carbon dots decorated hierarchical litchi-like In2O3 nanospheres for highly sensitive and selective NO2 detection. Sens. Actuators B 2020, 304, 127272. [Google Scholar] [CrossRef]
- Jin, Q.; Wen, W.; Wang, Z.-X.; Wang, R.-H.; Zheng, S.; Ye, Z.; Wu, J.-M. Nanoarchitectonics of nest-like MnO2/TiO2 thin film for triethylamine sensing. Sens. Actuators B 2022, 353, 131137. [Google Scholar] [CrossRef]
- Wang, X.; Han, W.; Yang, J.; Jiang, B.; Cheng, P.; Wang, Y.; Sun, P.; Zhang, H.; Sun, Y.; Lu, G. Facet effect on diverse WO3 morphologies and ideal work function for ppb-level triethylamine detection. Sens. Actuators B 2022, 363, 131849. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, B.; Yang, X.; Zhang, S.; Wang, Y.; Wang, G.; Zhang, Z. Sensing platform of PdO-ZnO-In2O3 nanofibers using MOF templated catalysts for triethylamine detection. Sens. Actuators B 2021, 343, 130126. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Feng, Y.; Zeng, H.; Liu, X.; Xu, Y.; Yang, X.; Wang, G. Small hollow ternary composite oxide semiconductor nanomaterials for conductometric HCHO sensors. Sens. Actuators B 2023, 385, 133703. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Yu, S.; Jiao, X.; Chen, D. High performance hollow metal-organic framework nanoshell-based etalons for volatile organic compounds detection. Adv. Mater. Technol. 2016, 1, 1600127. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Jiao, Z.; Yang, X. ZIF-8-Derived Multifunctional Triethylamine Sensor. Sensors 2024, 24, 5425. https://doi.org/10.3390/s24165425
Xiao S, Jiao Z, Yang X. ZIF-8-Derived Multifunctional Triethylamine Sensor. Sensors. 2024; 24(16):5425. https://doi.org/10.3390/s24165425
Chicago/Turabian StyleXiao, Shuo, Zheng Jiao, and Xuechun Yang. 2024. "ZIF-8-Derived Multifunctional Triethylamine Sensor" Sensors 24, no. 16: 5425. https://doi.org/10.3390/s24165425




