An Introduction to Ventra: A Programmable Abdominal Phantom for Training, Educational, Research, and Development Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Abdominal Compartment
2.2. Control Unit
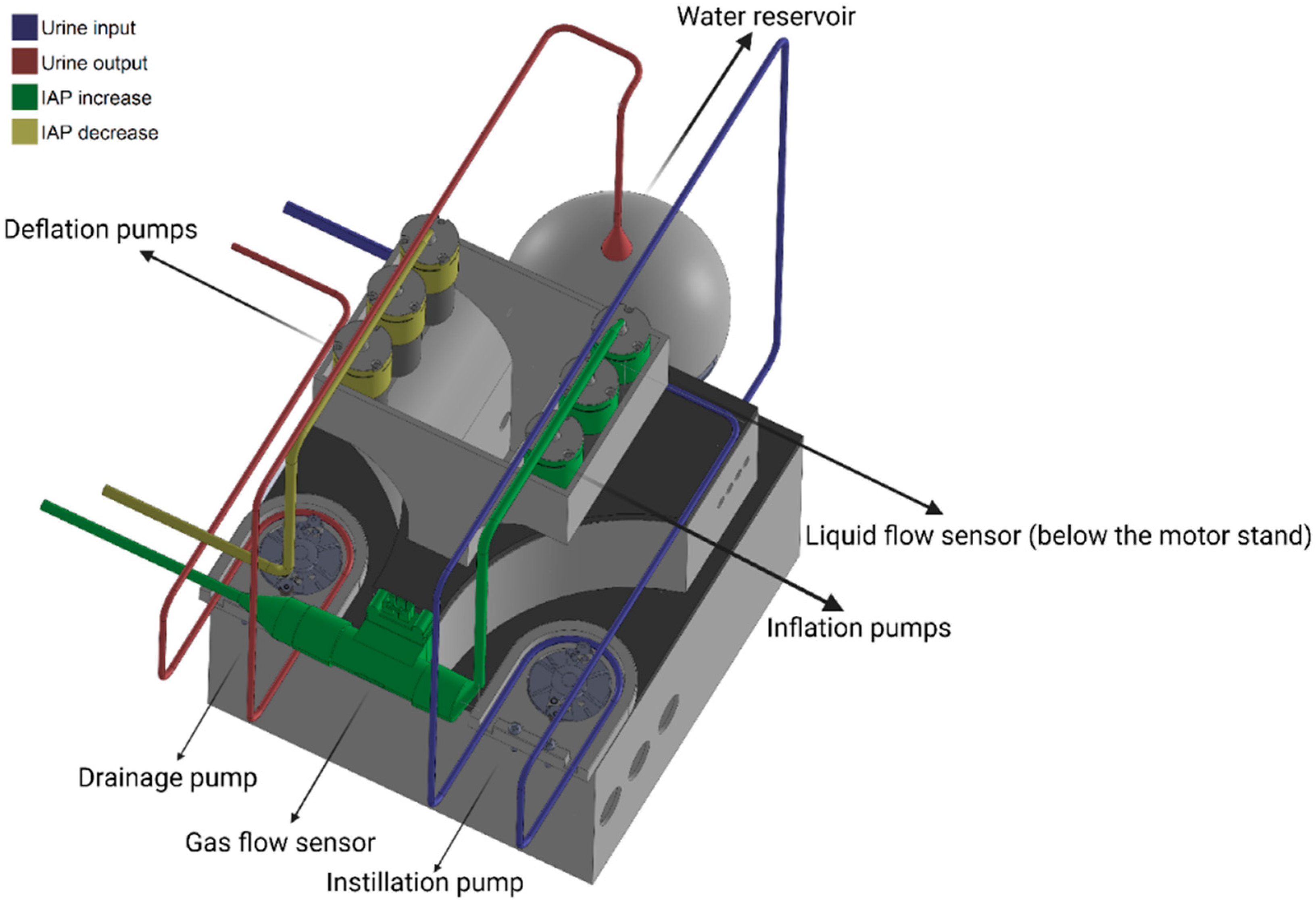
2.2.1. Electronic Circuit Design and Components
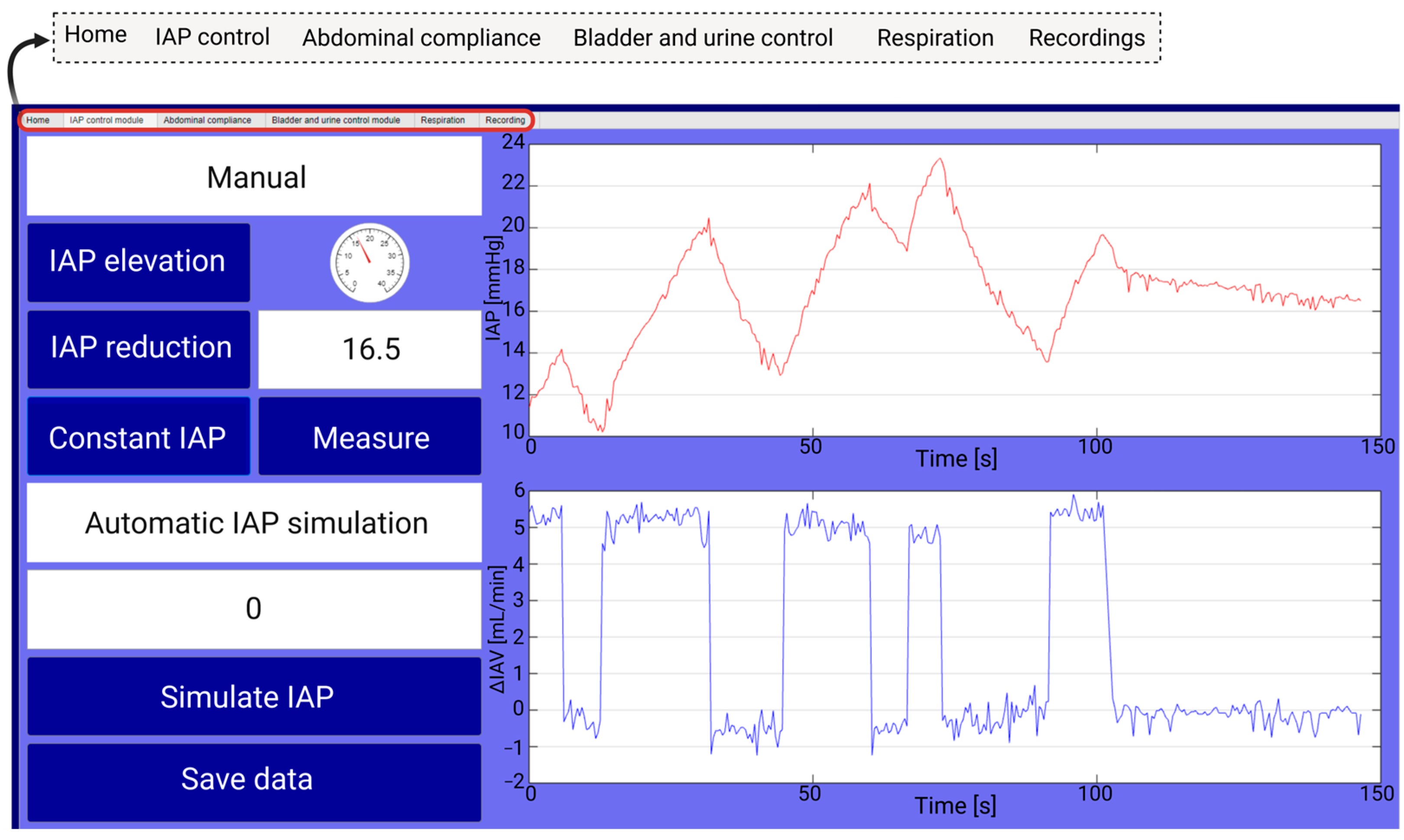
2.2.2. Graphical User Interface
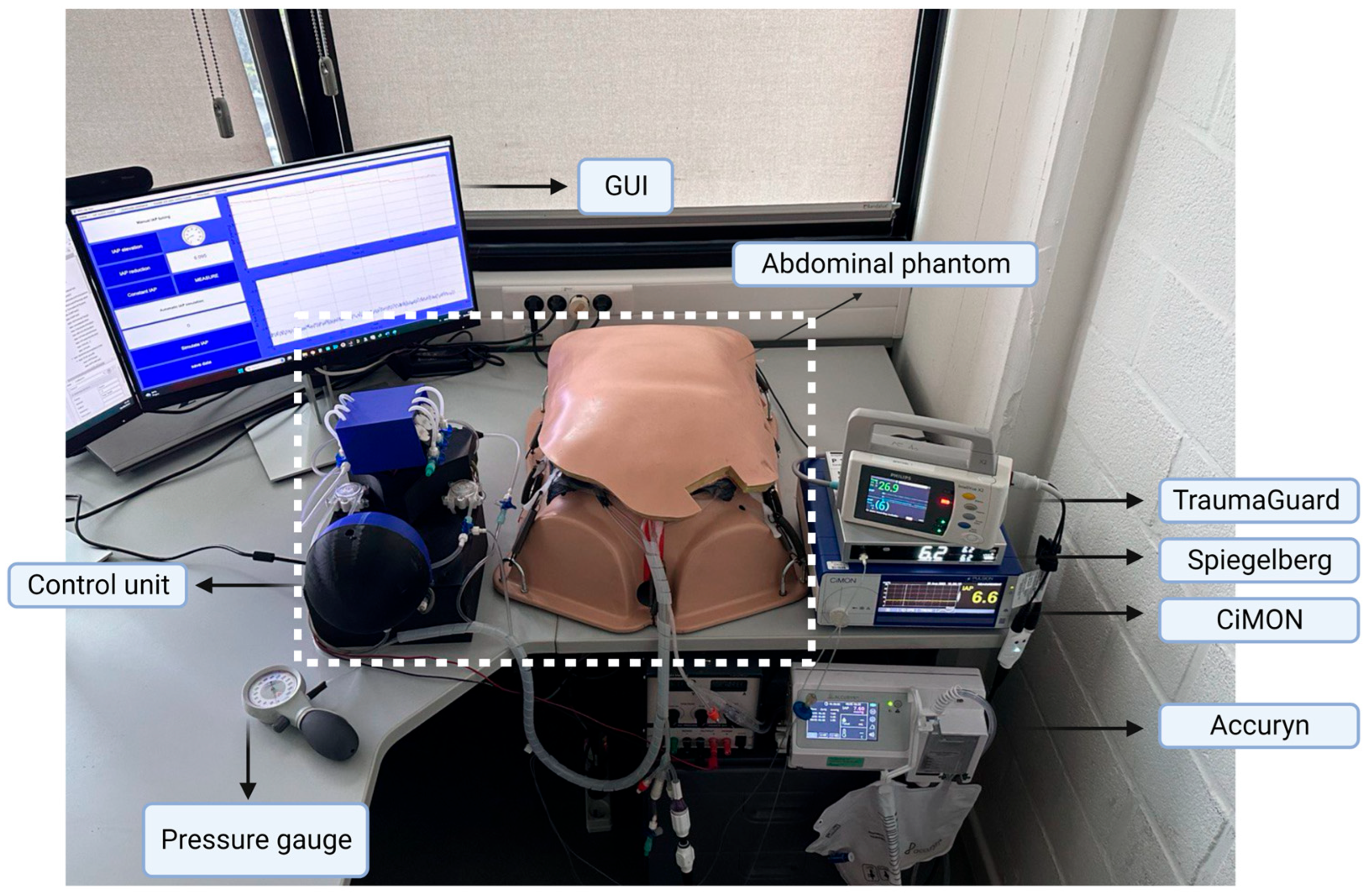
2.3. Validation Tests
2.4. Data Processing and Assessment
3. Results
3.1. Phantom Setup
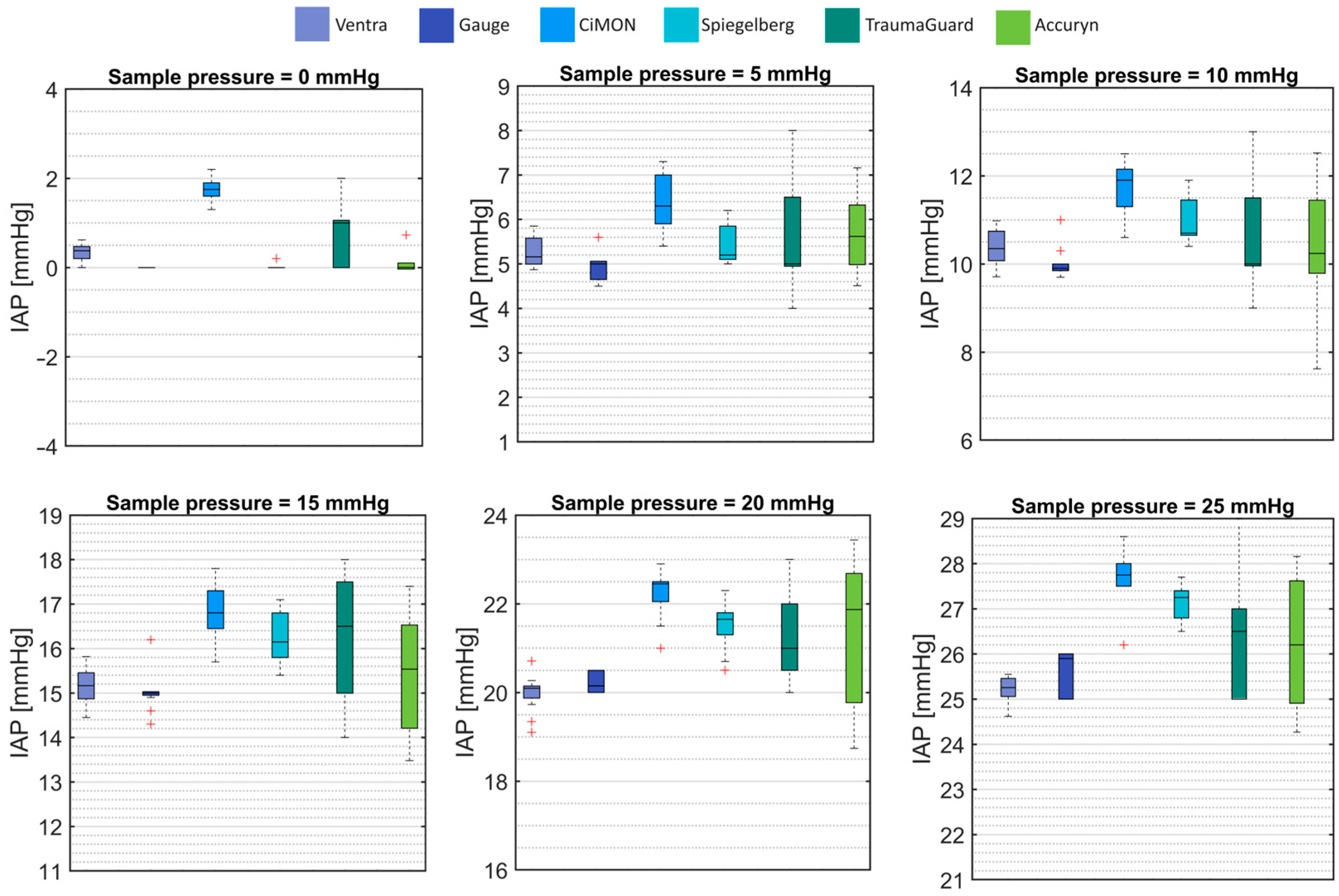
3.2. Intra-Abdominal Pressure Simulation
3.3. Abdominal Compliance
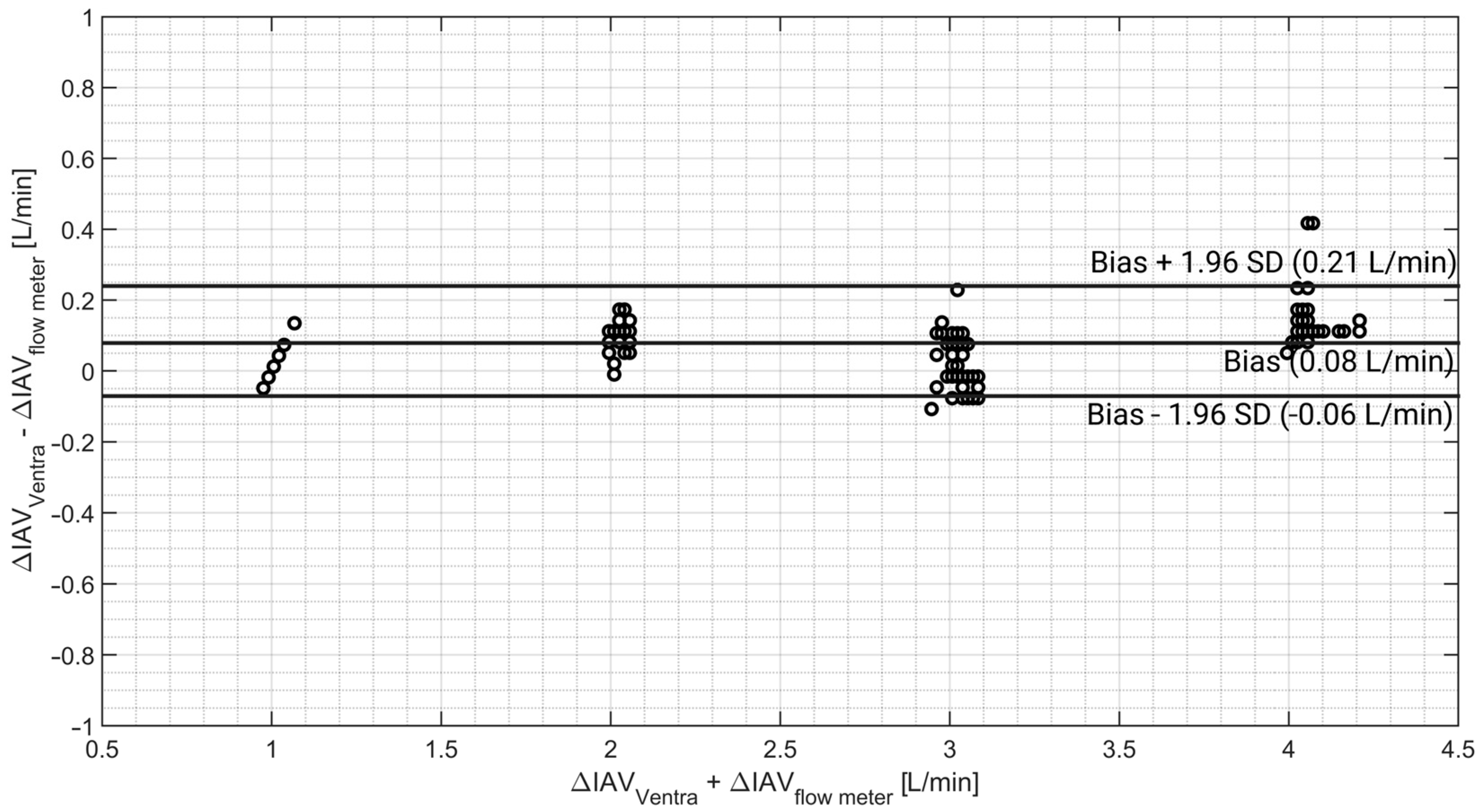
3.4. Abdominal Volume Monitoring
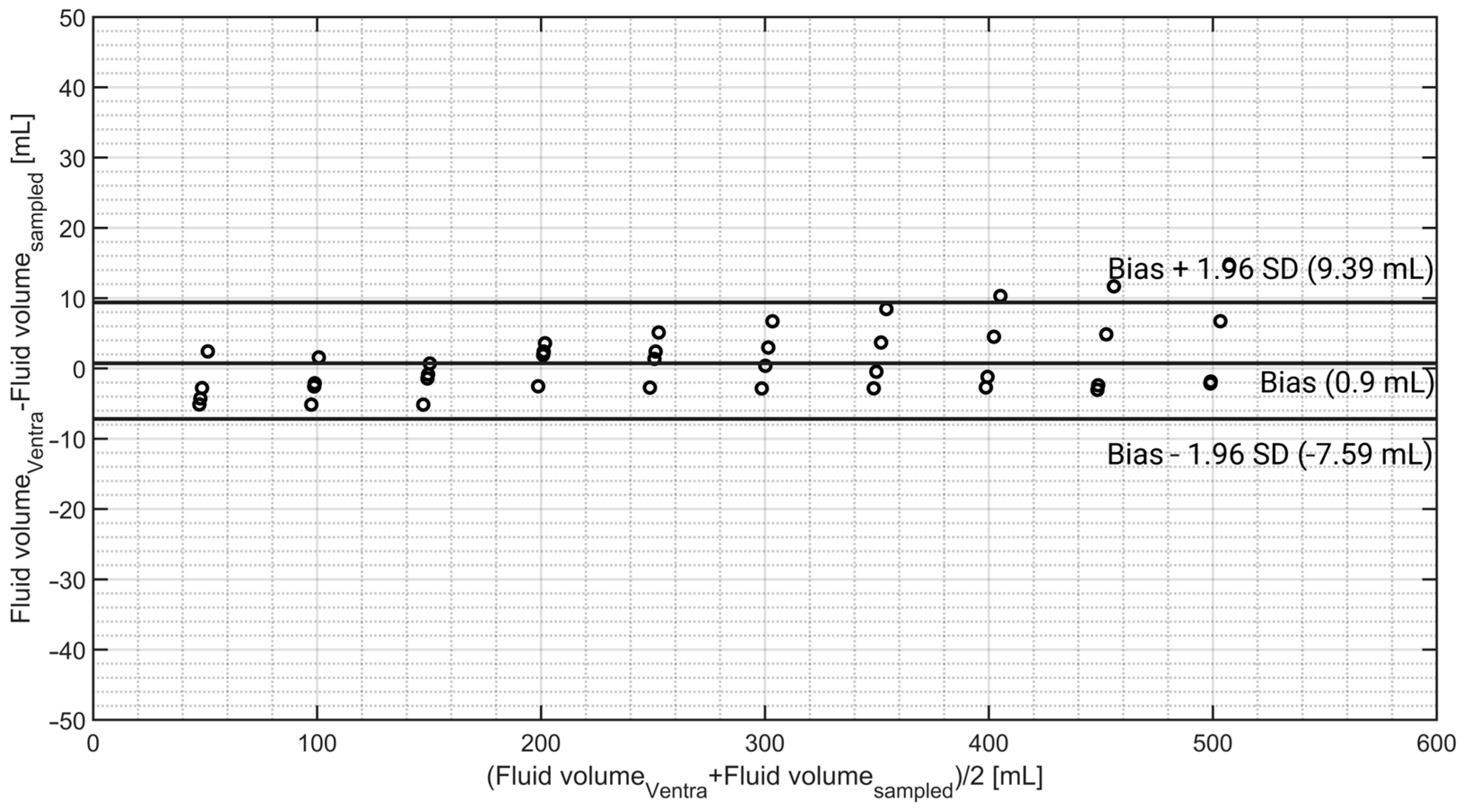
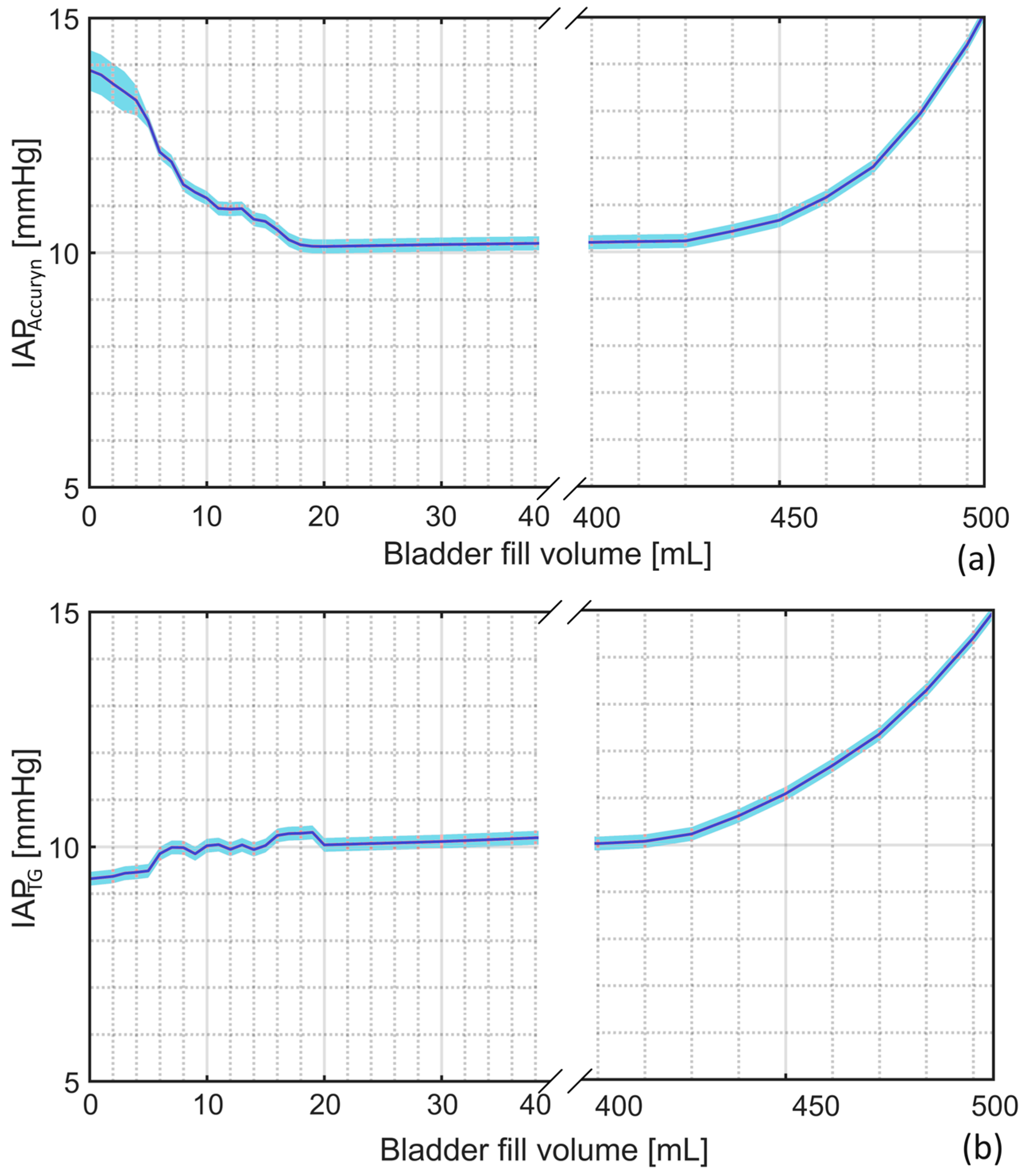
3.5. Bladder Fill Volume Monitoring
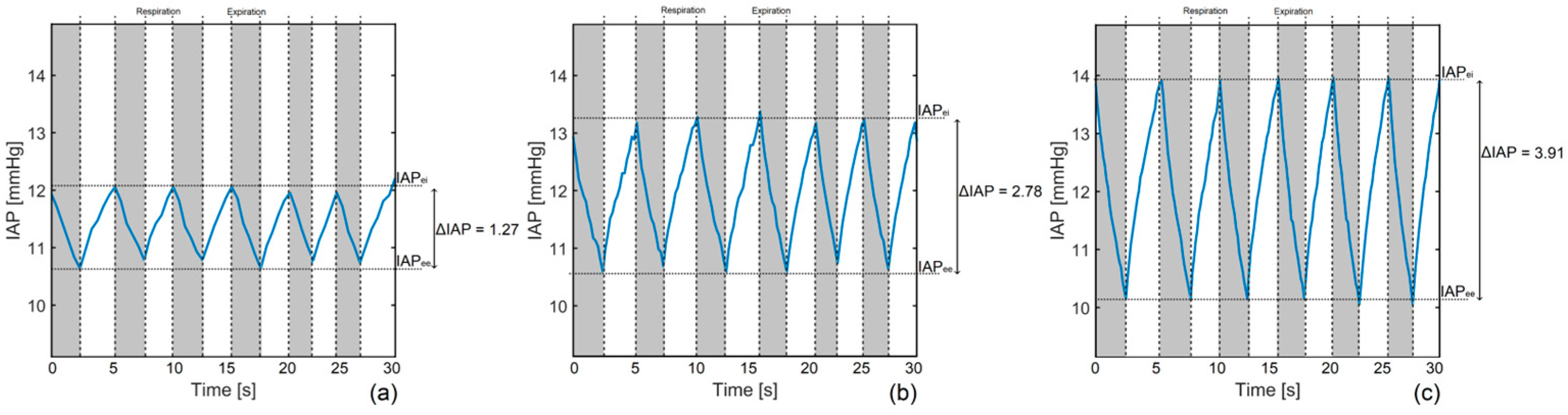
3.6. Respiration Simulation
4. Discussion
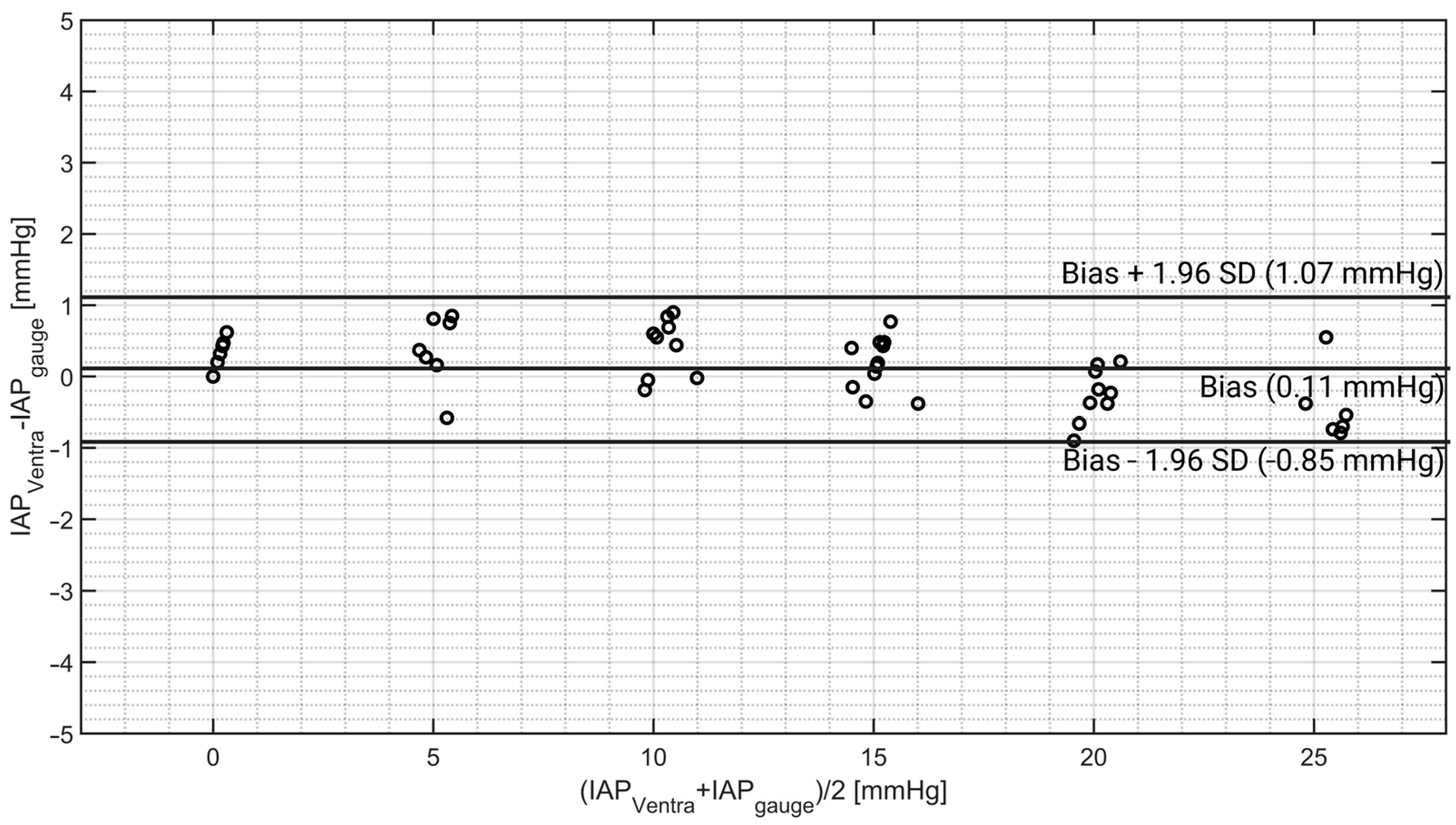
4.1. IAP Simulation
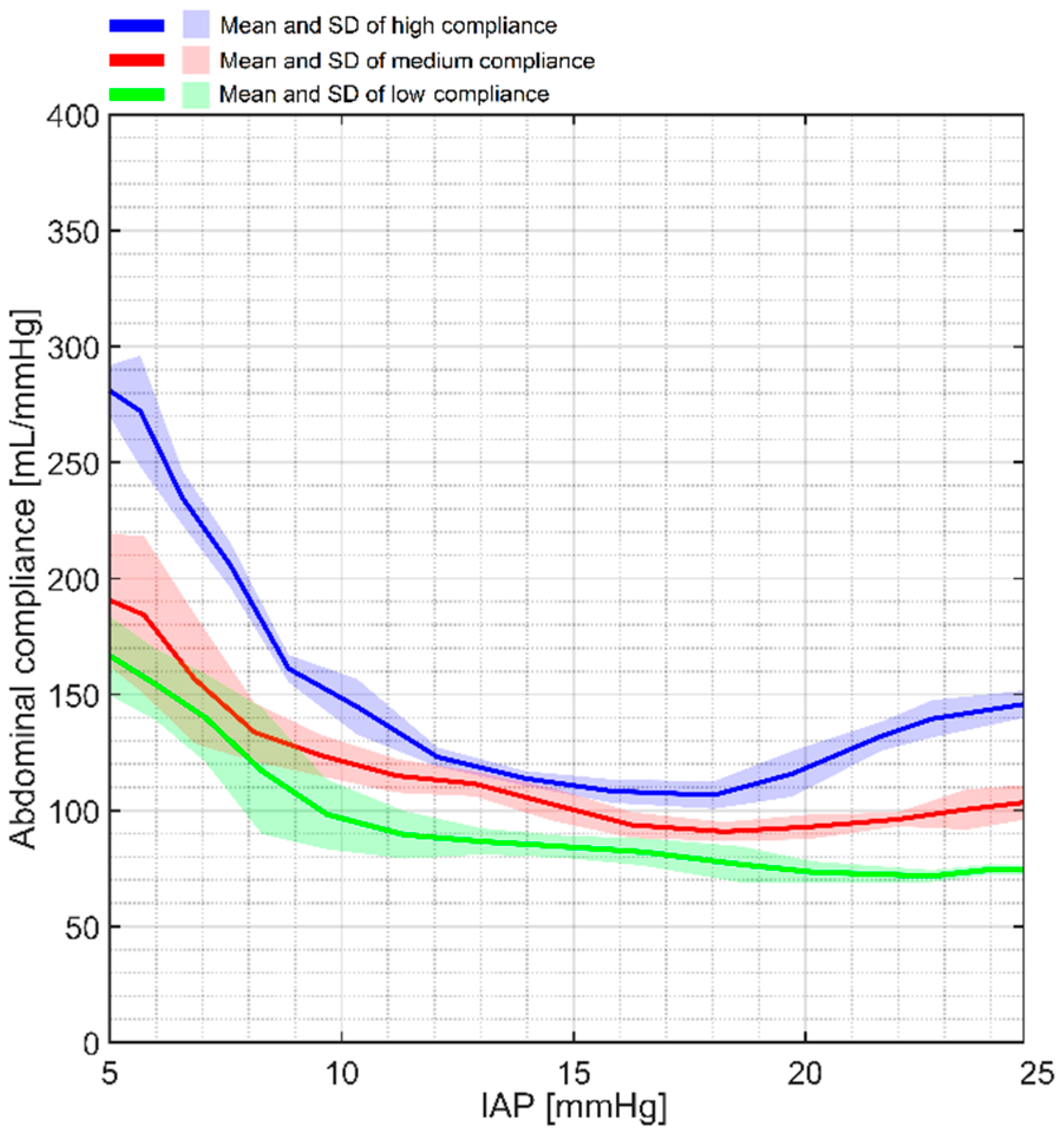
4.2. Abdominal Compliance
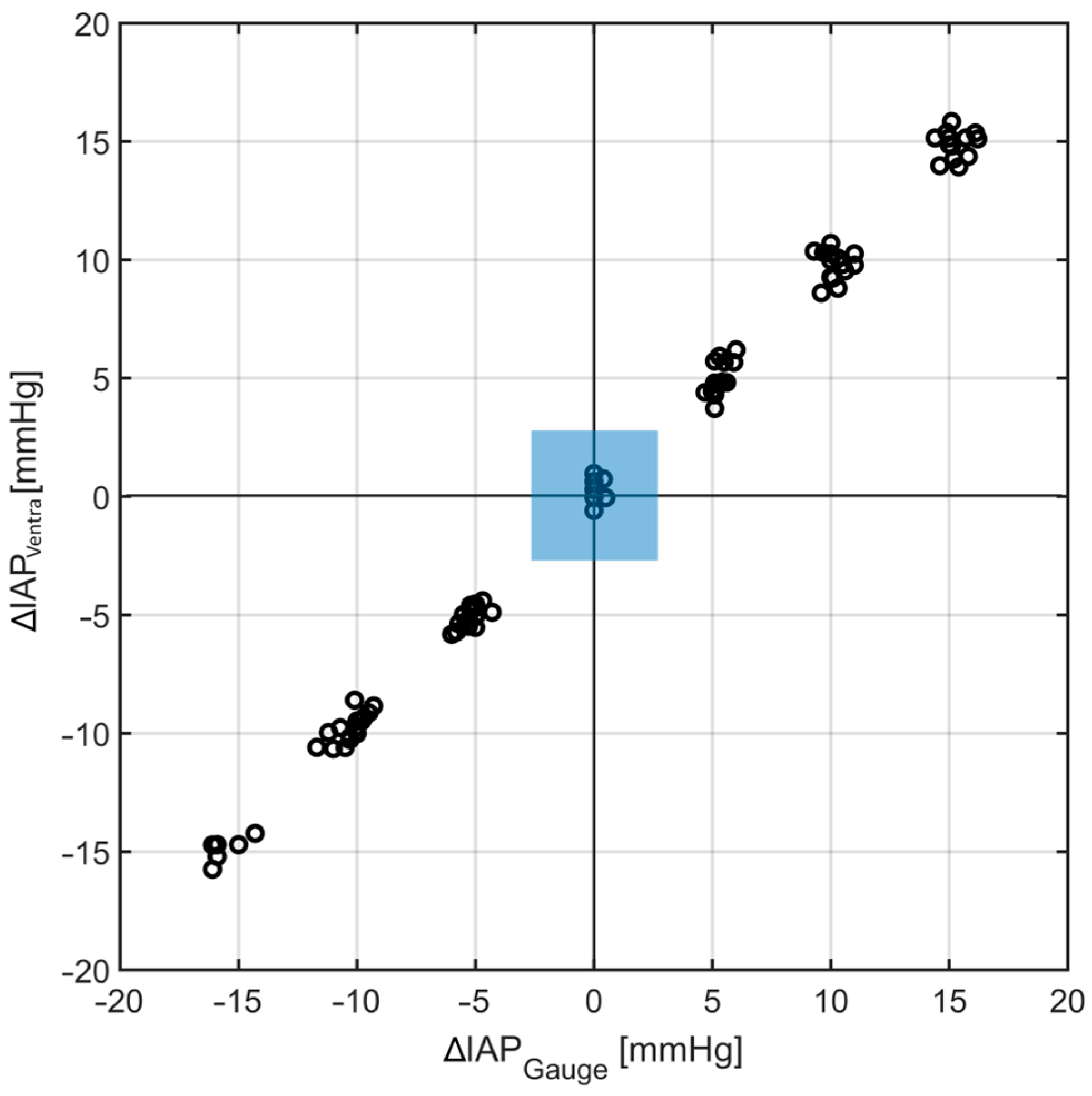
4.3. Intra-Abdominal Volume Changes
4.4. Bladder Fill Volume
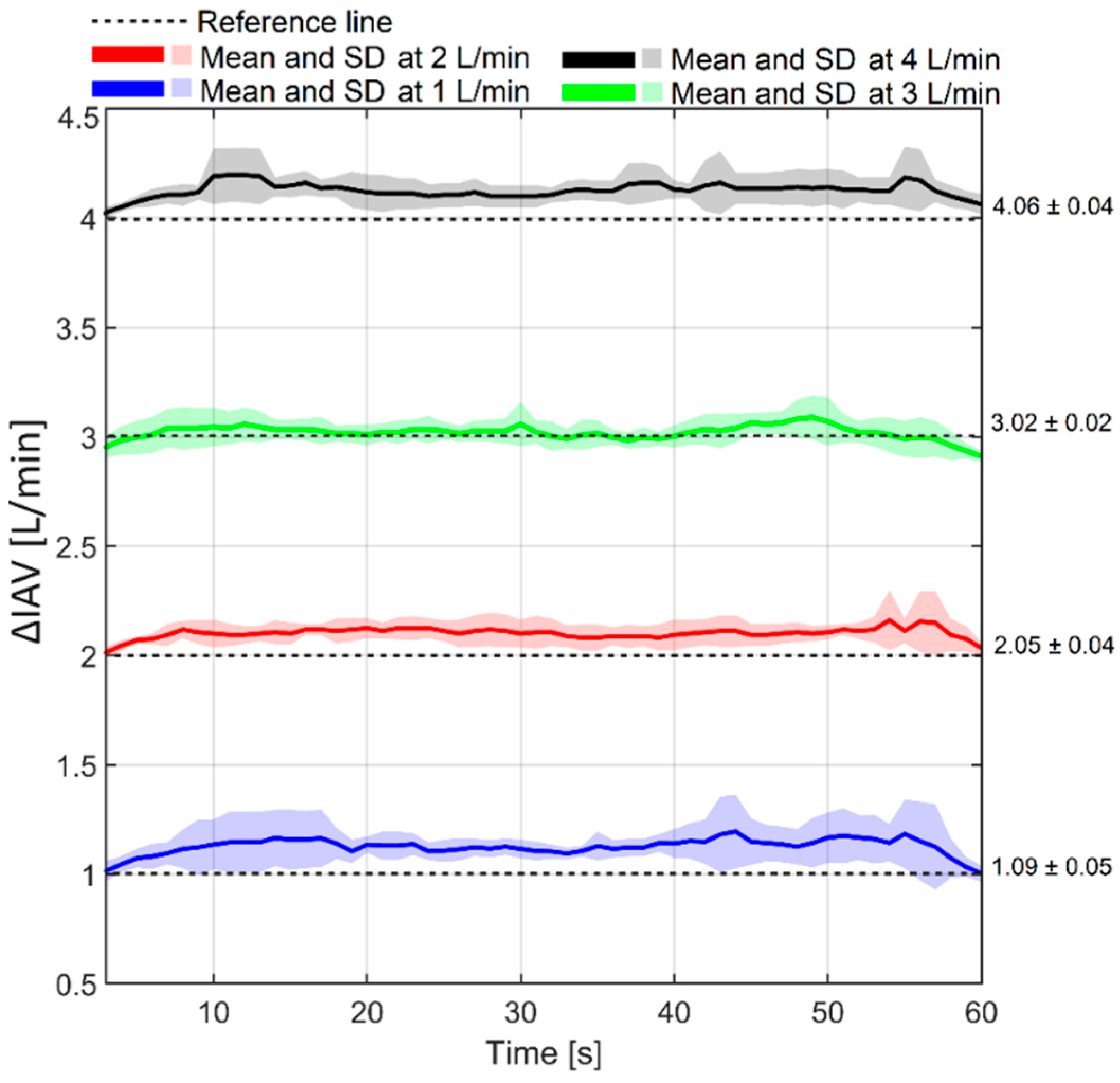
4.5. Respiration-Related IAP
4.6. Limitations
4.7. Future Developments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, B.M.; Howard, C. A 6th Vital Sign—Potential Use of Nasogastric Tube for Intra-abdominal Pressure Monitoring Method to Detect Feeding Intolerance in Very Low Birth-Weight Preterm Infants (<1500 g). Adv. Neonatal. Care 2015, 15, 176–181. [Google Scholar]
- Smit, M.; van Meurs, M.; Zijlstra, J.G. Intra-abdominal hypertension and abdominal compartment syndrome in critically ill patients: A narrative review of past, present, and future steps. Scand. J. Surg. 2022, 111, 14574969211030128. [Google Scholar] [CrossRef] [PubMed]
- Strang, S.G.; Breederveld, R.S.; Cleffken, B.I.; Verhofstad, M.H.J.; Van Waes, O.J.F.; Van Lieshout, E.M.M. Prevalence of intra-abdominal hypertension and markers for associated complications among severe burn patients: A multicenter prospective cohort study (BURNIAH study). Eur. J. Trauma Emerg. Surg. 2022, 48, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Buglevski, M.; Perdigoto, R.; Marcelino, P.; Saliba, F.; Blot, S.; Starkopf, J. Intra-abdominal hypertension and abdominal compartment syndrome in the critically ill liver cirrhotic patient–prevalence and clinical outcomes. A multicentric retrospective cohort study in intensive care. PLoS ONE 2021, 16, e0251498. [Google Scholar] [CrossRef]
- Pereira, B.; Dorigatti, A.; Melek, M.; Dos Santos, J.; Ferreira, M.; Calderan, T.; Carmona, C.; Fraga, G. Septic shock patients admitted to the intensive care unit with higher SOFA score tend to have higher incidence of abdominal compartment syndrome—A preliminary analysis. Anaesthesiol. Intensive Ther. 2019, 51, 370–372. [Google Scholar] [CrossRef]
- Shaheen, A.W.; Crandall, M.L.; Nicolson, N.G.; Smith-Singares, E.; Merlotti, G.J.; Jalundhwala, Y.; Issa, N.M. Abdominal compartment syndrome in trauma patients: New insights for predicting outcomes. J. Emerg. Trauma Shock 2016, 9, 53–57. [Google Scholar] [PubMed]
- Muturi, A.; Ndaguatha, P.; Ojuka, D.; Kibet, A. Prevalence and predictors of intra-abdominal hypertension and compartment syndrome in surgical patients in critical care units at Kenyatta National Hospital. BMC Emerg. Med. 2016, 17, 10. [Google Scholar] [CrossRef]
- Dalfino, L.; Sicolo, A.; Paparella, D.; Mongelli, M.; Rubino, G.; Brienza, N. Intra-abdominal hypertension in cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 644–651. [Google Scholar] [CrossRef]
- Mao, E.Q.; Tang, Y.Q.; Fei, J.; Qin, S.; Wu, J.; Li, L.; Min, D.; Zhang, S.D. Fluid therapy for severe acute pancreatitis in acute response stage. Chin. Med. J. 2009, 122, 169–173. [Google Scholar] [CrossRef]
- Łagosz, P.; Biegus, J.; Lewandowski, Ł.; Ponikowski, P.; Zymliński, R. Prevalence of increased intra-abdominal pressure and its impact on renal function in acute decompensated heart failure: A prospective pilot study. Kardiol Pol 2024, 82, 292–302. [Google Scholar] [CrossRef]
- Armutcu, F. Organ crosstalk: The potent roles of inflammation and fibrotic changes in the course of organ interactions. Inflamm. Res. 2019, 68, 825–839. [Google Scholar] [CrossRef]
- De Laet, I.E.; Malbrain, M.L.N.G.; De Waele, J.J. A Clinician’s Guide to Management of Intra-abdominal Hypertension and Abdominal Compartment Syndrome in Critically Ill Patients. Crit. Care 2020, 24, 97. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; De Keulenaer, B.L.; Khanna, A.K. Continuous intra-abdominal pressure: Is it ready for prime time? Intensive Care Med. 2022, 48, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, R.; Caregnato, R.C. Intra-abdominal pressure: An integrative review. Einstein 2016, 14, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Iacubovici, L.; Karol, D.; Baar, Y.; Beri, A.; Herzberg, H.; Zarour, S.; Goren, O.; Cohen, B. Assessment of Intra-Abdominal Pressure with a Novel Continuous Bladder Pressure Monitor—A Clinical Validation Study. Life 2023, 13, 384. [Google Scholar] [CrossRef]
- Tayebi, S.; Wise, R.; Pourkazemi, A.; Stiens, J.; Malbrain, M. Pre-Clinical Validation of a Novel Continuous Intra-Abdominal Pressure Measurement Equipment (SERENNO). Life 2022, 12, 1161. [Google Scholar] [CrossRef]
- Tayebi, S.; McKinney, T.; McKinney, C.; Delvadia, D.; Levine, M.-A.; Spofford, E.S.; Malbrain, L.; Stiens, J.; Dabrowski, W.; Malbrain, M.L.N.G. Evaluation of the TraumaGuard Balloon-in-Balloon Catheter Design for Intra-Abdominal Pressure Monitoring: Insights from Pig and Human Cadaver Studies. Sensors 2023, 23, 8806. [Google Scholar] [CrossRef] [PubMed]
- See, K.C.; Tayebi, S.; Sum, C.L.; Phua, J.; Stiens, J.; Wise, R.; Mukhopadhyay, A.; Malbrain, M. Feasibility analysis of a novel non-invasive ultrasonographic method for the measurement of intra-abdominal pressure in the intensive care unit. J. Clin. Monit. Comput. 2023, 37, 1351–1359. [Google Scholar] [CrossRef]
- Tang, H.; Dai, Y.; Zhao, D.; Sun, Z.; Chen, F.; Zhu, Y.; Liang, H.; Cao, H.; Zhang, L. Deep Domain Adaptation for Predicting Intra-Abdominal Pressure with Multichannel Attention Fusion Radar Chip. Adv. Intell. Syst. 2022, 4, 2100209. [Google Scholar] [CrossRef]
- Tayebi, S.; Pourkazemi, A.; Malbrain, M.; Stiens, J. Non-Invasive Intra-Abdominal Pressure Measurement by Means of Transient Radar Method: In Vitro Validation of a Novel Radar-Based Sensor. Sensors 2021, 21, 5999. [Google Scholar] [CrossRef]
- David, M.; Raviv, A.; Guttel, A.; García Reyes, V.; Simini, F.; Pracca, F. Non-invasive indirect monitoring of intra-abdominal pressure using microwave reflectometry: System design and proof-of-concept clinical trial. J. Clin. Monit. Comput. 2021, 35, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, D.; Guo, Y.; Zhang, H.; Li, Y.; Peng, X.; Wang, Y.; Jiang, D.; Zhang, L.; Wang, Z. A New Device for Measuring Abdominal Wall Tension and Its Value in Screening Abdominal Infection. Med. Devices 2021, 14, 119–131. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Cheatham, M.L.; Malbrain, M.L.; Kirkpatrick, A.W.; Sugrue, M.; Balogh, Z.; Ivatury, R.; De Keulenaer, B.; Kimball, E.J. Recommendations for research from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. Acta Clin. Belg. 2009, 64, 203–209. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Sugrue, M.; McKee, J.L.; Pereira, B.M.; Roberts, D.J.; De Waele, J.J.; Leppaniemi, A.; Ejike, J.C.; Reintam Blaser, A.; D’Amours, S.; et al. Update from the Abdominal Compartment Society (WSACS) on intra-abdominal hypertension and abdominal compartment syndrome: Past, present, and future beyond Banff 2017. Anaesthesiol. Intensive Ther. 2017, 49, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Greffier, J.; Durand, Q.; Frandon, J.; Si-Mohamed, S.; Loisy, M.; de Oliveira, F.; Beregi, J.P.; Dabli, D. Improved image quality and dose reduction in abdominal CT with deep-learning reconstruction algorithm: A phantom study. Eur. Radiol. 2023, 33, 699–710. [Google Scholar] [CrossRef]
- Silva, J.; Milovic, C.; Lambert, M.; Montalba, C.; Arrieta, C.; Irarrazaval, P.; Uribe, S.; Tejos, C. Toward a realistic in silico abdominal phantom for QSM. Magn. Reason. Med. 2023, 89, 2402–2418. [Google Scholar] [CrossRef]
- Subashi, E.; Segars, P.; Veeraraghavan, H.; Deasy, J.; Tyagi, N. A model for gastrointestinal tract motility in a 4D imaging phantom of human anatomy. Med. Phys. 2023, 50, 3066–3075. [Google Scholar] [CrossRef]
- Kovan, B.; Demir, B.; Işık, E.G.; Has Şimşek, D.; Özkan, Z.G.; Kuyumcu, S.; Türkmen, C.; Şanlı, Y. An anthropomorphic body phantom for the determination of calibration factor in radionuclide treatment dosimetry. Radiat. Prot. Dosim. 2023, 199, 1274–1283. [Google Scholar] [CrossRef]
- Makkia, R.; Nelson, K.; Zaidi, H.; Dingfelder, M. Hybrid computational pregnant female phantom construction for radiation dosimetry applications. Biomed. Phys. Eng. Express 2022, 8, 065015. [Google Scholar] [CrossRef]
- Duan, X.; Ananthakrishnan, L.; Guild, J.B.; Xi, Y.; Rajiah, P. Radiation doses and image quality of abdominal CT scans at different patient sizes using spectral detector CT scanner: A phantom and clinical study. Abdom. Radiol. 2020, 45, 3361–3368. [Google Scholar] [CrossRef]
- Li, X.; Yang, K.; Liu, B. Radiation dose dependence on subject size in abdominal computed tomography: Water phantom and patient model comparison. Med. Phys. 2018, 45, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Kroese, L.F.; Harlaar, J.J.; Ordrenneau, C.; Verhelst, J.; Guérin, G.; Turquier, F.; Goossens, R.H.M.; Kleinrensink, G.J.; Jeekel, J.; Lange, J.F. The ‘AbdoMAN’: An artificial abdominal wall simulator for biomechanical studies on laparotomy closure techniques. Hernia 2017, 21, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kokko, M.; Seigne, J.; Van Citters, D.; Halter, R. A novel abdominal phantom model for laparoscopic and robotic exposure of retroperitoneal structures. In Medical Imaging 2022: Image-Guided Procedures, Robotic Interventions, and Modeling; SPIE: Bellingham, WA, USA, 2022; Volume 12034. [Google Scholar]
- Bauer, D.F.; Rosenkranz, J.; Golla, A.K.; Tönnes, C.; Hermann, I.; Russ, T.; Kabelitz, G.; Rothfuss, A.J.; Schad, L.R.; Stallkamp, J.L.; et al. Development of an abdominal phantom for the validation of an oligometastatic disease diagnosis workflow. Med. Phys. 2022, 49, 4445–4454. [Google Scholar] [CrossRef]
- Konovalov, M.E.; Klimov, D.D.; Tohtamir, D.P.; Poduraev, Y.V. Approach to the Development of an Abdominal Phantom with the Function of Respiratory Motion for Robotic Surgery. In Proceedings of the 2020 International Multi-Conference on Industrial Engineering and Modern Technologies (FarEastCon), Vladivostok, Russia, 6–9 October 2020; pp. 1–6. [Google Scholar]
- Filippou, P.; Odisho, A.; Ramaswamy, K.; Usawachintachit, M.; Hu, W.; Li, J.; Chi, T. Using an abdominal phantom to teach urology residentes ultrasound-guided percutaneous needle placement. Int. Braz. J. Urol. 2016, 42, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Al-Zogbi, L.; Bock, B.; Schaffer, S.; Fleiter, T.; Krieger, A. A 3-D-Printed Patient-Specific Ultrasound Phantom for FAST Scan. Ultrasound Med. Biol. 2021, 47, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Uetake, C.; Nakamoto, A.; Suda, T.; Tamano, M. Abdominal ultrasound examination training using an ultrasound phantom and volume navigation system. J. Med. Ultrason. 2016, 43, 381–386. [Google Scholar] [CrossRef]
- He, L.; Herzig, N.; Lusignan, S.d.; Scimeca, L.; Maiolino, P.; Iida, F.; Nanayakkara, T. An Abdominal Phantom with Tunable Stiffness Nodules and Force Sensing Capability for Palpation Training. IEEE Trans. Robot. 2021, 37, 1051–1064. [Google Scholar] [CrossRef]
- Arpaia, P.; Cuneo, D.; Grassini, S.; Mancino, F.; Minucci, S.; Moccaldi, N.; Sannino, I. A finite element model of abdominal human tissue for improving the accuracy in insulin absorption assessment: A feasibility study. Meas. Sens. 2021, 18, 100218. [Google Scholar] [CrossRef]
- Abrahams, A.C.; Dendooven, A.; van der Veer, J.W.; Wientjes, R.; Toorop, R.J.; Bleys, R.; Hendrickx, A.P.A.; van Leeuwen, M.S.; de Lussanet, Q.G.; Verhaar, M.C.; et al. Direct Comparison of the Thickness of the Parietal Peritoneum Using Peritoneal Biopsy and Ultrasonography of the Abdominal Wall in Patients Treated with Peritoneal Dialysis. Perit. Dial. Int. 2019, 39, 455–464. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Peeters, Y.; Wise, R. The neglected role of abdominal compliance in organ-organ interactions. Crit. Care 2016, 20, 67. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Hiraishi, M.; Tanioka, K.; Shimokawa, T. Concordance rate of a four-quadrant plot for repeated measurements. BMC Med. Res. Methodol. 2021, 21, 270. [Google Scholar] [CrossRef]
- Tayebi, S.; Wise, R.; Zarghami, A.; Malbrain, L.; Khanna, A.K.; Dabrowski, W.; Stiens, J.; Malbrain, M.L.N.G. In Vitro Validation of a Novel Continuous Intra-Abdominal Pressure Measurement System (TraumaGuard). J. Clin. Med. 2023, 12, 6260. [Google Scholar] [CrossRef]
- Yu, T.; Cheng, Y.; Wang, X.; Tu, B.; Cheng, N.; Gong, J.; Bai, L. Gases for establishing pneumoperitoneum during laparoscopic abdominal surgery. Cochrane Database Syst. Rev. 2017, 6, Cd009569. [Google Scholar] [CrossRef]
- Blaser, A.R.; Björck, M.; De Keulenaer, B.; Regli, A. Abdominal compliance: A bench-to-bedside review. J. Trauma Acute Care Surg. 2015, 78, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Pfortmueller, C.A.; Dabrowski, W.; Wise, R.; van Regenmortel, N.; Malbrain, M.L.N.G. Fluid accumulation syndrome in sepsis and septic shock: Pathophysiology, relevance and treatment—A comprehensive review. Ann. Intensive Care. 2024, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ. A New Artificial Urine Protocol to Better Imitate Human Urine. Sci. Rep. 2019, 9, 20159. [Google Scholar] [CrossRef]


| Phantom | Intra-Gastric | Intra-Bladder | ||||
|---|---|---|---|---|---|---|
| Sampled IAP (mmHg) | Ventra (mmHg) | Gauge (mmHg) | CiMON (mmHg) | Spiegelberg (mmHg) | TraumaGuard (mmHg) | Accuryn (mmHg) |
| 0 (n = 12) | 0.34 ± 0.21 | 0.00 ± 0.00 | 1.75 ± 0.29 | 0.03 ± 0.08 | 0.83 ± 0.72 | 0.14 ± 0.23 |
| 5 (n = 12) | 5.28 ± 0.35 | 4.89 ± 0.29 | 6.41 ± 0.67 | 5.45 ± 0.43 | 5.67 ± 1.15 | 5.68 ± 0.84 |
| 10 (n = 12) | 10.39 ± 0.43 | 10.01 ± 0.35 | 11.73 ± 0.56 | 11.00 ± 0.49 | 10.58 ± 1.16 | 10.46 ± 1.38 |
| 15 (n = 12) | 15.18 ± 0.43 | 15.00 ± 0.44 | 16.82 ± 0.64 | 16.24 ± 0.62 | 16.25 ± 1.49 | 15.44 ± 1.28 |
| 20 (n = 12) | 19.98 ± 0.42 | 20.21 ± 0.47 | 22.25 ± 0.53 | 21.54 ± 0.55 | 21.16 ± 0.94 | 21.40 ± 1.66 |
| 25 (n = 12) | 25.20 ± 0.32 | 25.63 ± 0.47 | 27.63 ± 0.76 | 27.15 ± 0.41 | 26.51 ± 1.46 | 26.23 ± 1.43 |
| Average IAP | ||||||
| 12.5 (n = 72) | 12.73 ± 9.25 p = 0.94 | 12.62 ± 9.58 - | 14.43 ± 9.73 p = 0.26 | 13.57 ± 10.12 p = 0.56 | 13.50 ± 8.89 p = 0.57 | 13.25 ± 8.99 p = 0.69 |
| Device | Mean IAP (mmHg) | Bias (mmHg) | Precision (mmHg) | ULA (mmHg) | LLA (mmHg) | PE (%) |
|---|---|---|---|---|---|---|
| Ventra | 13.33 | 0.11 | 0.49 | 1.07 | −0.85 | 7 |
| CiMON | 15.03 | 1.81 | 0.57 | 2.93 | +0.69 | 8 |
| Spiegelberg | 14.23 | 1.01 | 0.63 | 2.24 | −0.22 | 9 |
| TraumaGuard | 14.16 | 0.94 | 1.16 | 3.21 | −1.33 | 16 |
| Accuryn | 13.85 | 0.62 | 1.52 | 3.60 | −2.36 | 21 |
| At 5 mmHg | At 10 mmHg | At 15 mmHg | At 20 mmHg | At 25 mmHg | |
|---|---|---|---|---|---|
| Low compliance (n = 5) (mL/mmHg) | 154.39 ± 15.43 | 98.25 ± 16.46 | 84.33 ± 4.37 | 73.38 ± 4.71 | 74.33 ± 2.31 |
| Medium compliance (n = 5) (mL/mmHg) | 184.29 ± 34.03 | 123.61 ± 8.91 | 102.65 ± 7.11 | 92.89 ± 5.42 | 100.46 ± 8.74 |
| High compliance (n = 5) (mL/mmHg) | 272.12 ± 23.99 | 144.67 ± 11.92 | 108.32 ± 4.27 | 115.82 ± 10.13 | 139.49 ± 8.21 |
| IAPee (mmHg) | IAPei (mmHg) | IAPmean (mmHg) | ΔIAP (mmHg) | APV (%) | RR (rpm) | tins (s) | texp (s) | I/E (-) | |
|---|---|---|---|---|---|---|---|---|---|
| 1st simulation | 10.71 ± 0.08 | 12.15 ± 0.08 | 11.38 ± 0.08 | 1.27 ± 0.11 | 11.16 ± 0.97 | 13.48 ± 2.24 | 2.32 ± 0.42 | 2.13 ± 0.61 | 1.09 ± 0.37 |
| 2nd simulation | 10.61 ± 0.07 | 13.21 ± 0.16 | 11.91 ± 0.12 | 2.78 ± 0.17 | 23.34 ± 1.45 | 12.17 ± 1.32 | 2.52 ± 0.12 | 2.41 ± 0.52 | 1.05 ± 0.23 |
| 3rd simulation | 10.13 ± 0.05 | 14.02 ± 0.13 | 12.07 ± 0.09 | 3.91 ± 0.14 | 32.39 ± 1.18 | 11.56 ± 0.75 | 2.61 ± 0.20 | 2.58 ± 0.27 | 1.01 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayebi, S.; Wise, R.; Zarghami, A.; Dabrowski, W.; Malbrain, M.L.N.G.; Stiens, J. An Introduction to Ventra: A Programmable Abdominal Phantom for Training, Educational, Research, and Development Purposes. Sensors 2024, 24, 5431. https://doi.org/10.3390/s24165431
Tayebi S, Wise R, Zarghami A, Dabrowski W, Malbrain MLNG, Stiens J. An Introduction to Ventra: A Programmable Abdominal Phantom for Training, Educational, Research, and Development Purposes. Sensors. 2024; 24(16):5431. https://doi.org/10.3390/s24165431
Chicago/Turabian StyleTayebi, Salar, Robert Wise, Ashkan Zarghami, Wojciech Dabrowski, Manu L. N. G. Malbrain, and Johan Stiens. 2024. "An Introduction to Ventra: A Programmable Abdominal Phantom for Training, Educational, Research, and Development Purposes" Sensors 24, no. 16: 5431. https://doi.org/10.3390/s24165431









