Research Progress on Saccharide Molecule Detection Based on Nanopores
Abstract
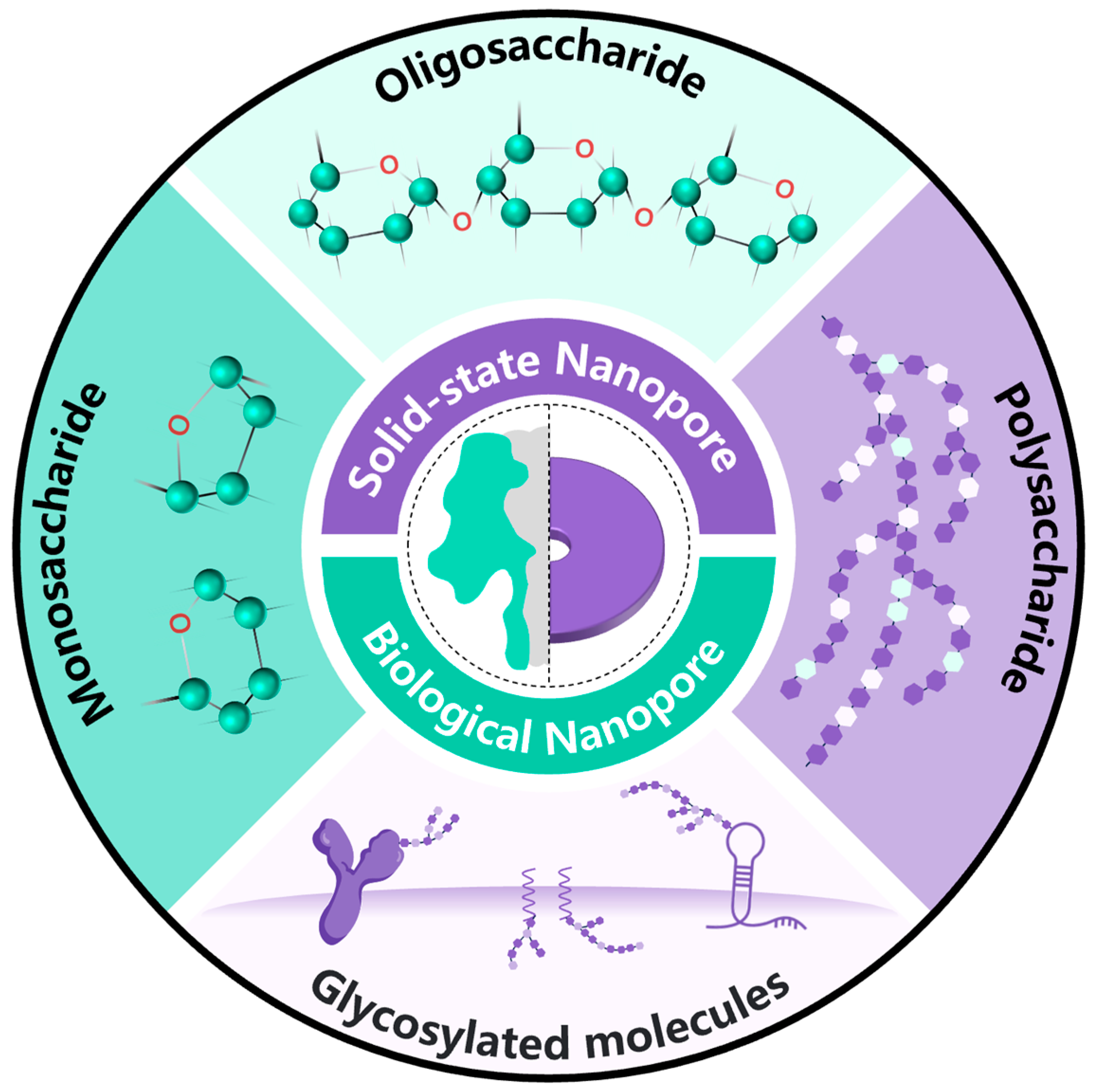
:1. Introduction
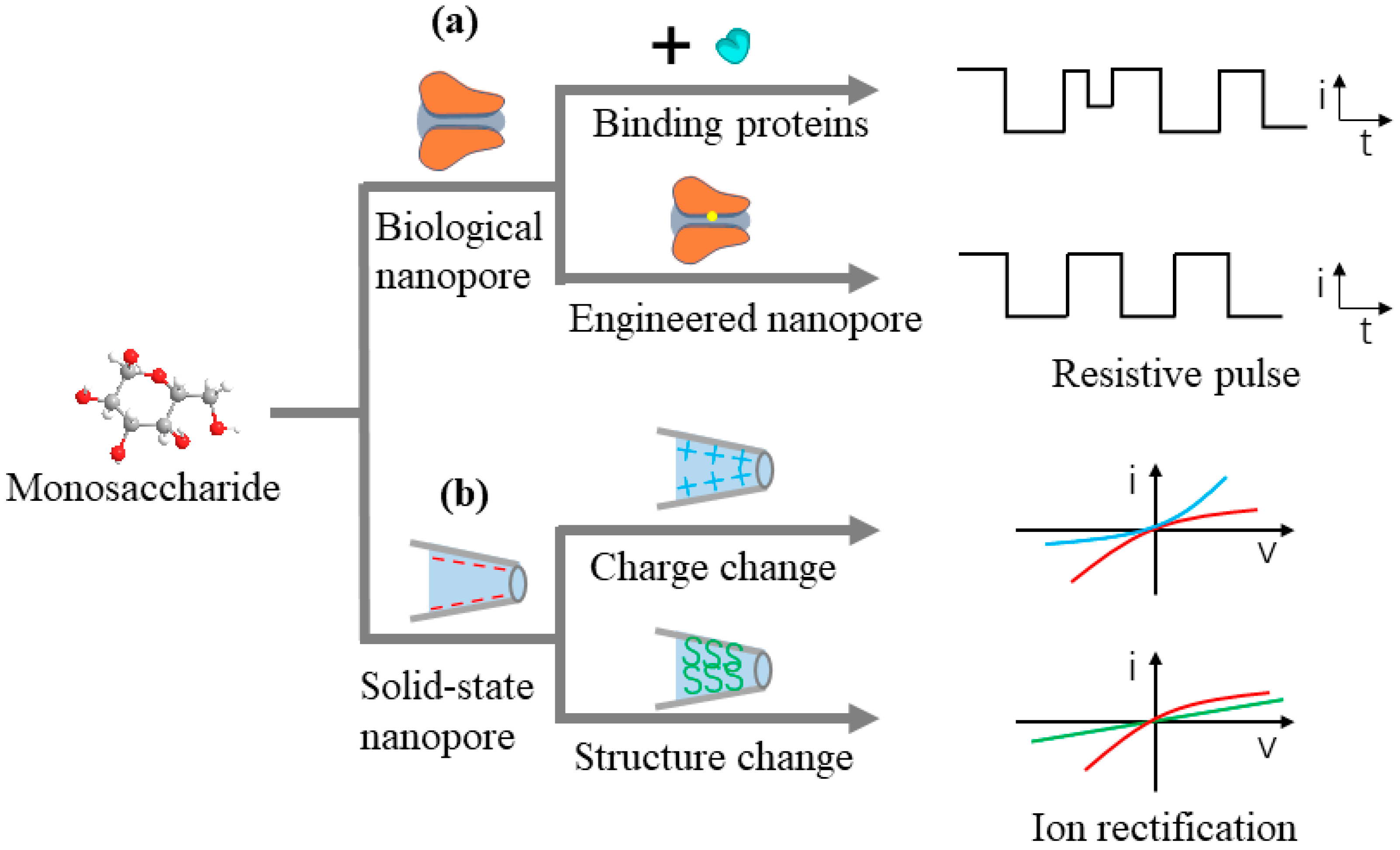
2. Monosaccharide Detection Based on Nanopore Technology
2.1. Research on Monosaccharide Detection Based on Biological Nanopores
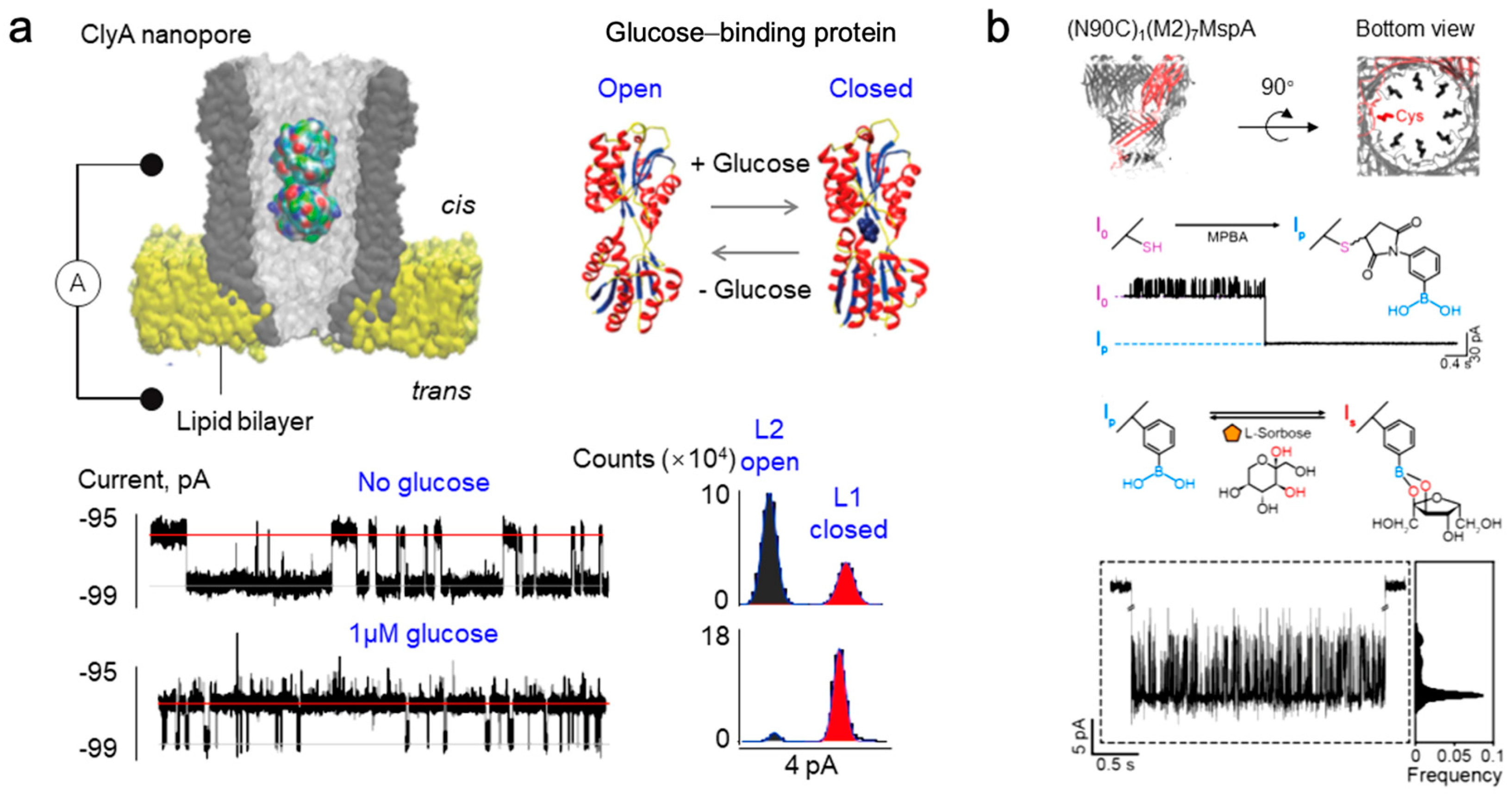
2.2. Monosaccharide Detection Based on Solid-State Nanopores

3. Oligosaccharides Detection Based on Nanopore Technology
3.1. Research on the Detection of Neutral Oligosaccharides
3.2. Research on the Detection of Glycosaminoglycans

4. Polysaccharide Detection Based on Nanopore Technology
4.1. Research on the Detection of Heparin Using Nanopore Technology
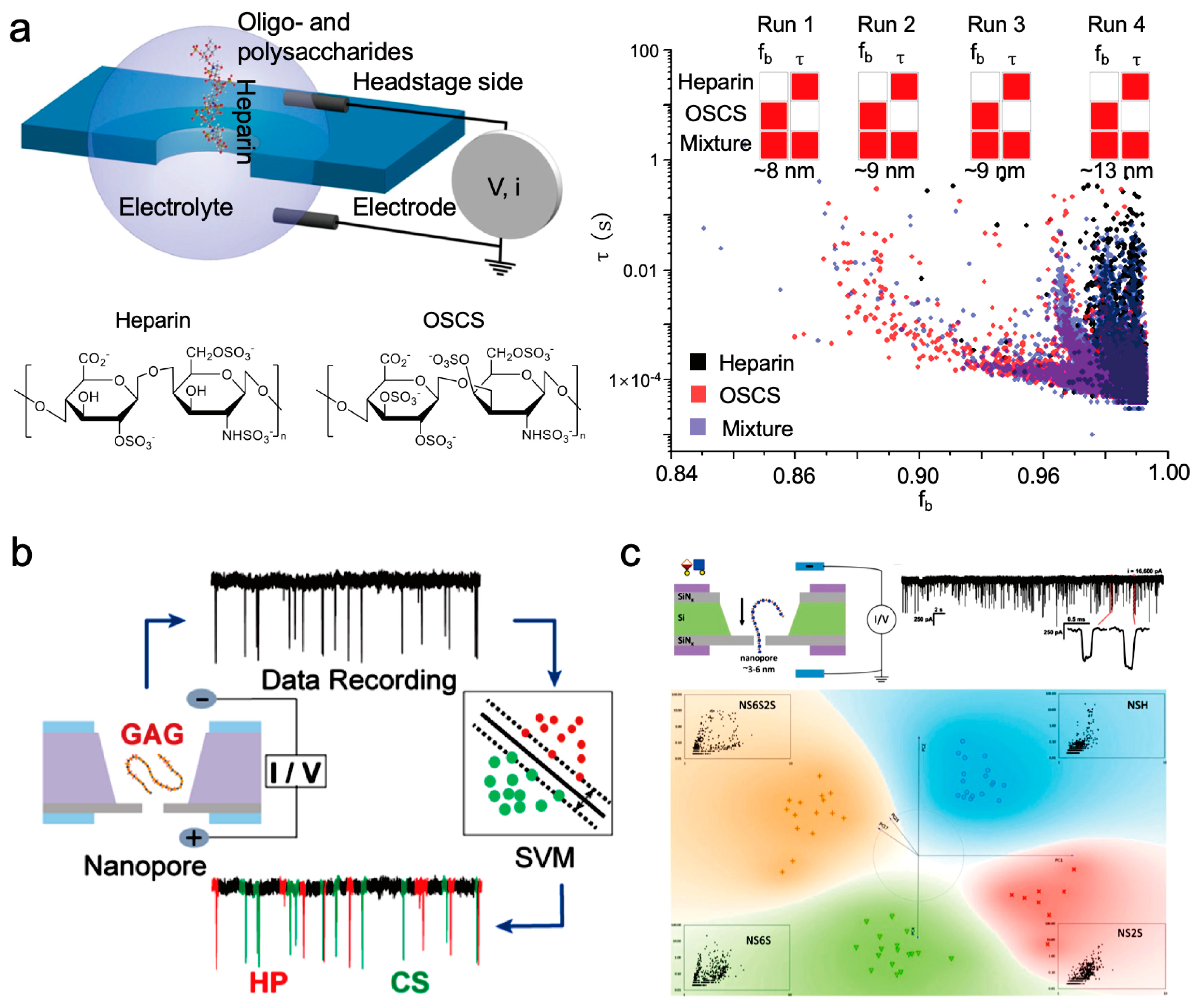
4.2. Research on the Detection of Hyaluronic Acid Using Nanopore Technology
4.3. Detection of Other Polysaccharide Using Nanopore Technology
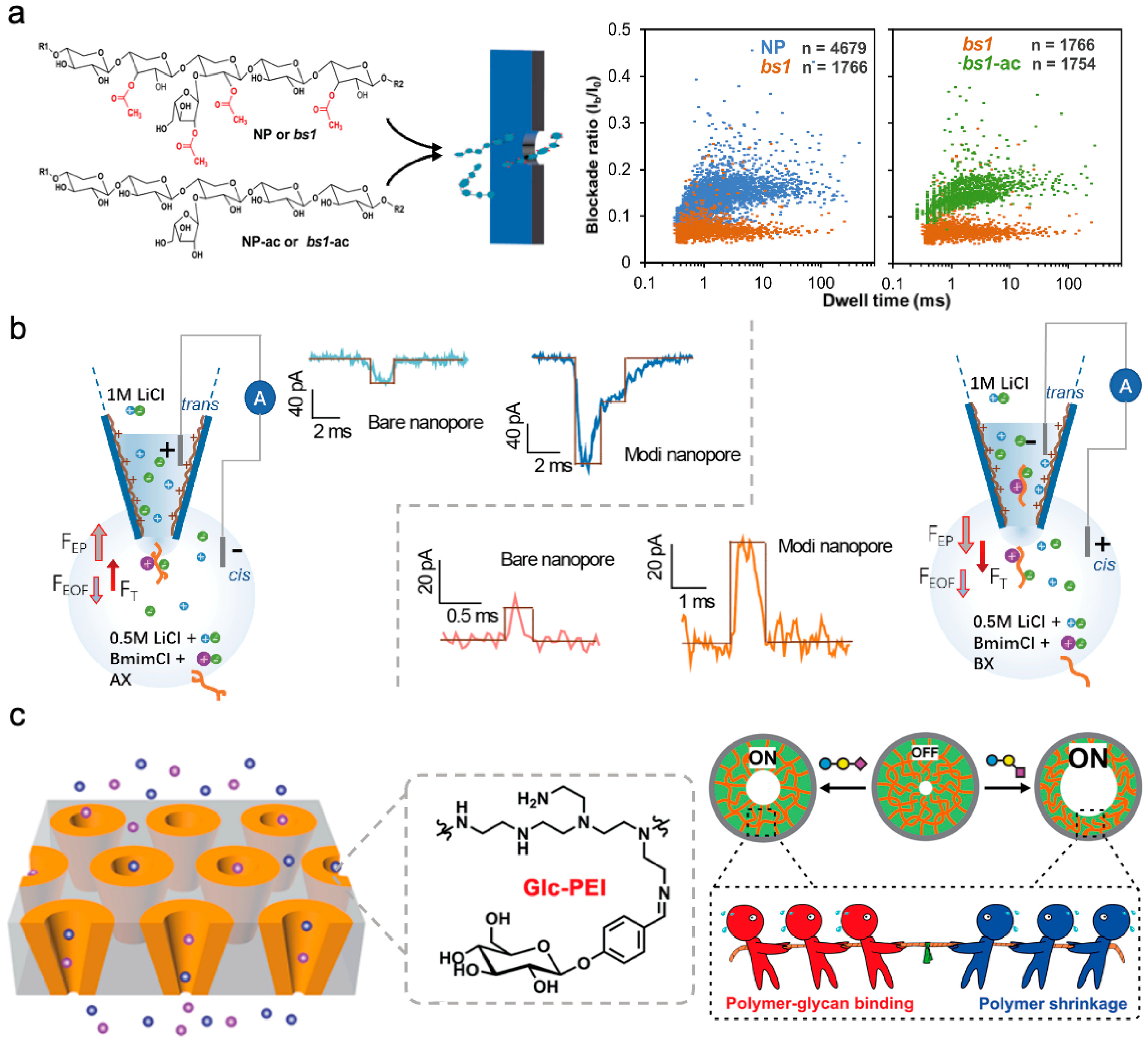
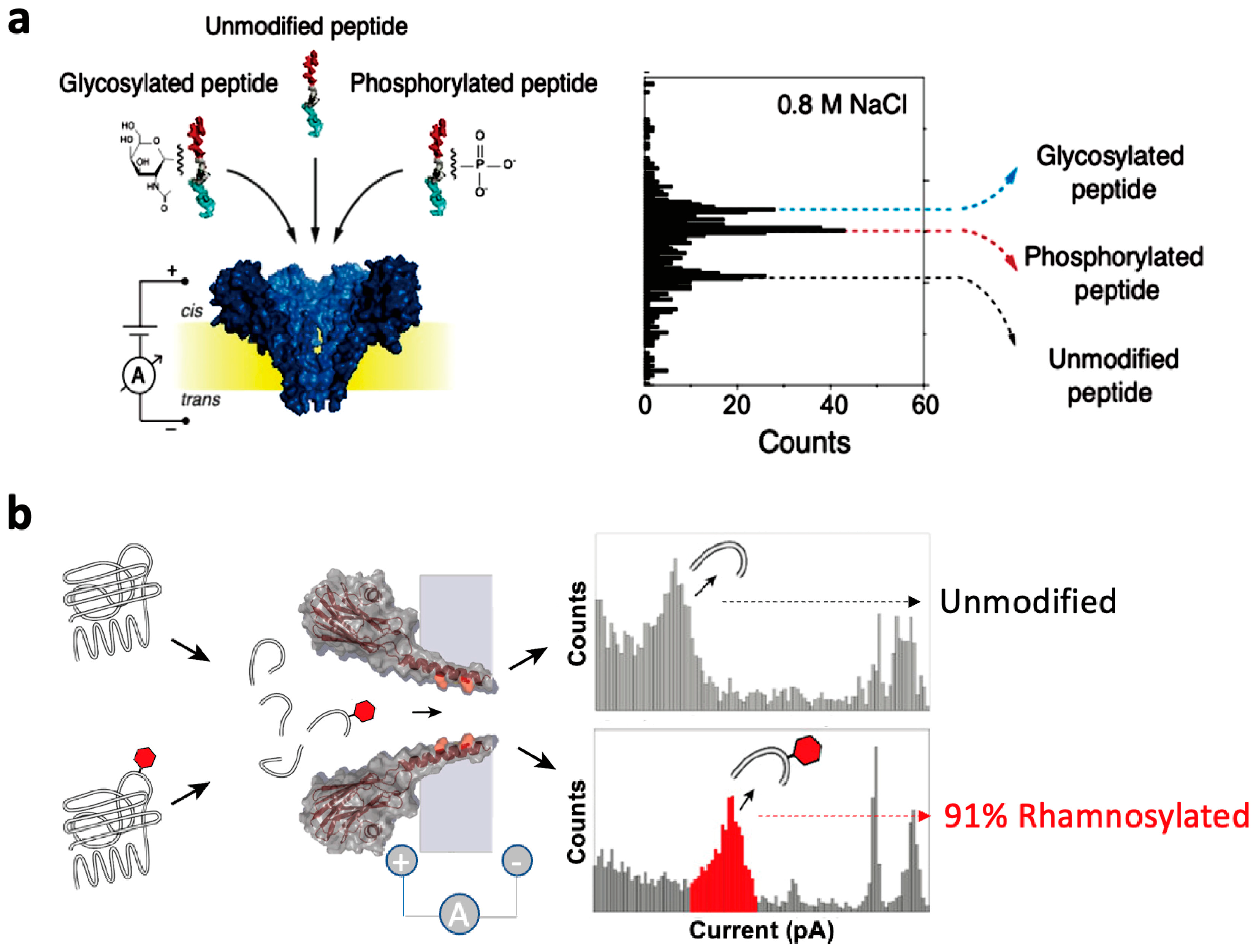
5. Detection of Glycosylation Biomolecules
5.1. Research on Glycosylation Peptide Detection Based on Biological Nanopore Technology
5.2. Research on Glycoprotein Detection Based on Solid-State Nanopore Technology
5.3. Research on Lipopolysaccharide Detection Based on Solid-State Nanopore Technology
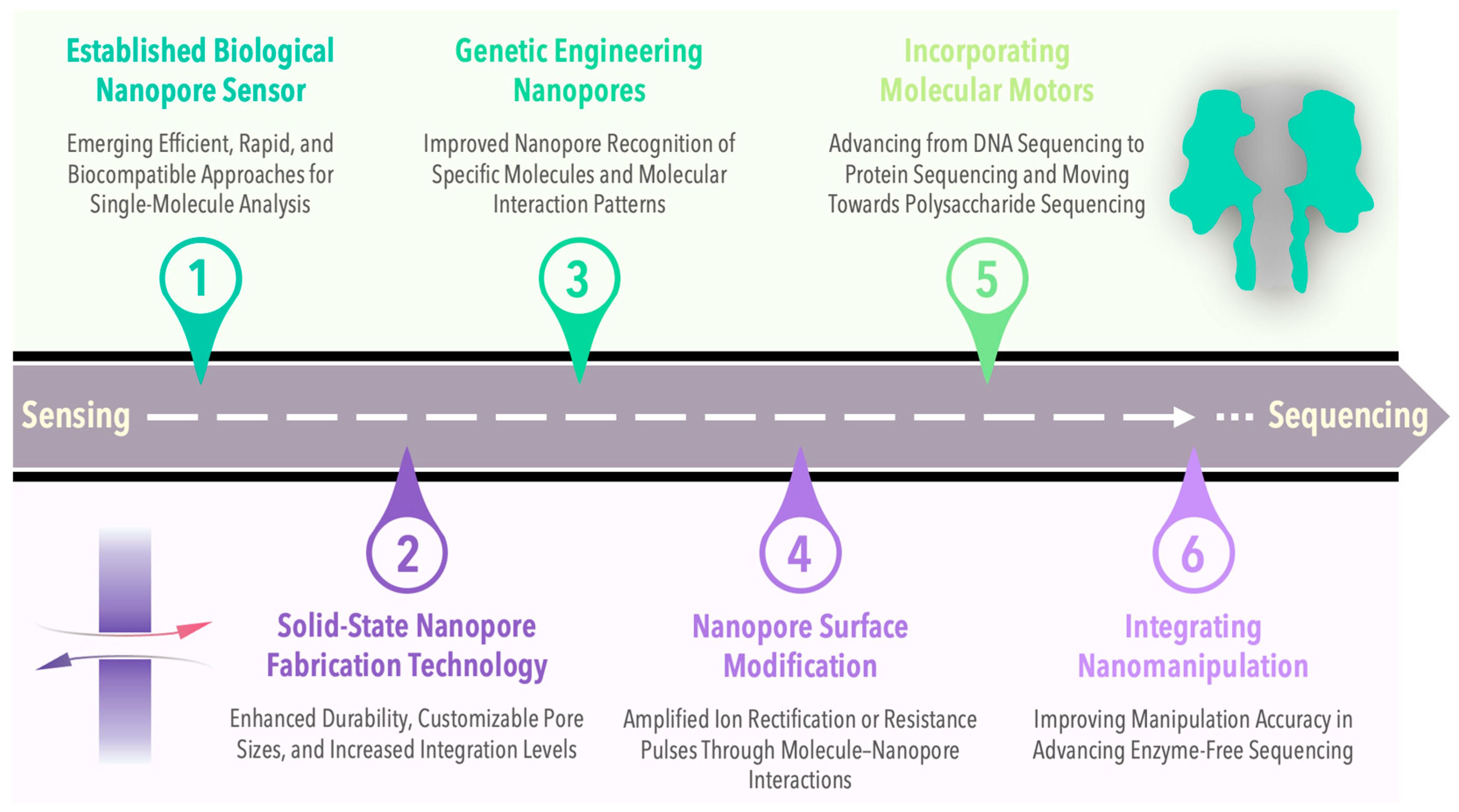
6. Summary and Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, Z. Introduction to Carbohydrates and Glycoconjugates. In Glycosphingolipids in the Central Nervous System; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–30. [Google Scholar]
- Dashty, M. A Quick Look at Biochemistry: Carbohydrate Metabolism. Clin. Biochem. 2013, 46, 1339–1352. [Google Scholar] [CrossRef]
- Zeng, Y.; Himmel, M.E.; Ding, S.-Y. Visualizing Chemical Functionality in Plant Cell Walls. Biotechnol. Biofuels 2017, 10, 263. [Google Scholar] [CrossRef]
- Schmid, T.; Baumann, B.; Himmelsbach, M.; Klampfl, C.W.; Buchberger, W. Analysis of Saccharides in Beverages by HPLC with Direct UV Detection. Anal. Bioanal. Chem. 2016, 408, 1871–1878. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, T.; Tian, C.; Zhu, Y.; Zeng, Y.; Men, Y.; Chen, P.; Sun, Y.; Ma, Y. Multi-Enzyme Systems and Recombinant Cells for Synthesis of Valuable Saccharides: Advances and Perspectives. Biotechnol. Adv. 2019, 37, 107406. [Google Scholar] [CrossRef]
- Sun, X. Glucose detection through surface-enhanced Raman spectroscopy: A review. Anal. Chim. Acta 2022, 1206, 339226. [Google Scholar] [CrossRef]
- Mandpe, P.; Prabhakar, B.; Gupta, H.; Shende, P. Glucose oxidase-based biosensor for glucose detection from biological fluids. Sens. Rev. 2020, 40, 497–511. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef]
- Im, J.; Biswas, S.; Liu, H.; Zhao, Y.; Sen, S.; Biswas, S.; Ashcroft, B.; Borges, C.; Wang, X.; Lindsay, S.; et al. Electronic Single-Molecule Identification of Carbohydrate Isomers by Recognition Tunnelling. Nat. Commun. 2016, 7, 13868. [Google Scholar] [CrossRef]
- Gökalp, F. An Investigation into the Usage of Monosaccharides with GLUT1 and GLUT3 as Prognostic Indicators for Cancer. Nutr. Cancer 2022, 74, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.M.; Carrillo-López, A.; Bello-Perez, L.A. Carbohydrates. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 175–205. [Google Scholar]
- Lu, G.; Crihfield, C.L.; Gattu, S.; Veltri, L.M.; Holland, L.A. Capillary Electrophoresis Separations of Glycans. Chem. Rev. 2018, 118, 7867–7885. [Google Scholar] [CrossRef]
- Shi, Q.; Yan, J.; Jiang, B.; Chi, X.; Wang, J.; Liang, X.; Ai, X. A General Strategy for the Structural Determination of Carbohydrates by Multi-Dimensional NMR Spectroscopies. Carbohydr. Polym. 2021, 267, 118218. [Google Scholar] [CrossRef] [PubMed]
- Speciale, I.; Notaro, A.; Garcia-Vello, P.; Di Lorenzo, F.; Armiento, S.; Molinaro, A.; Marchetti, R.; Silipo, A.; De Castro, C. Liquid-State NMR Spectroscopy for Complex Carbohydrate Structural Analysis: A Hitchhiker’s Guide. Carbohydr. Polym. 2022, 277, 118885. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Arnold, K.; Liu, J. Using Structurally Defined Oligosaccharides to Understand the Interactions between Proteins and Heparan Sulfate. Curr. Opin. Struct. Biol. 2018, 50, 155–161. [Google Scholar] [CrossRef]
- Bhuniya, S.; Demina, T.S.; Akopova, T.A. Advances in Applications of Polysaccharides and Polysaccharide-Based Materials. Int. J. Mol. Sci. 2024, 25, 6482. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Wong, K.-H. Novel Nanoparticle Materials for Drug/Food Delivery-Polysaccharides. Phys. Sci. Rev. 2016, 1, 20160053. [Google Scholar]
- Chen, Y.; Zhang, N.; Chen, X. Structurally Modified Polysaccharides: Physicochemical Properties, Biological Activities, Structure–Activity Relationship, and Applications. J. Agric. Food Chem. 2024, 72, 3259–3276. [Google Scholar] [CrossRef]
- Shi, L.; He, Q.; Li, J.; Liu, Y.; Cao, Y.; Liu, Y.; Sun, C.; Pan, Y.; Li, X.; Zhao, X. Polysaccharides in Fruits: Biological Activities, Structures, and Structure-Activity Relationships and Influencing Factors-A Review. Food Chem. 2024, 451, 139408. [Google Scholar] [CrossRef]
- Guo, L.; Du, X.; Lan, J.; Liang, Q. Study on Molecular Structural Characteristics of Tea Polysaccharide. Int. J. Biol. Macromol. 2010, 47, 244–249. [Google Scholar] [CrossRef]
- Whitfield, M.B.; Chinn, M.S.; Veal, M.W. Improvement of Acid Hydrolysis Procedures for the Composition Analysis of Herbaceous Biomass. Energy Fuels 2016, 30, 8260–8269. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Buksa, K.; Szumny, A.; Zięba, T.; Gryszkin, A. Analysis of Molecular Structure of Starch Citrate Obtained by a Well-Stablished Method. LWT Food Sci. Technol. 2016, 69, 334–341. [Google Scholar] [CrossRef]
- Li, F.-S.; Phyo, P.; Jacobowitz, J.; Hong, M.; Weng, J.-K. The Molecular Structure of Plant Sporopollenin. Nat. Plants 2018, 5, 41–46. [Google Scholar] [CrossRef]
- Yao, H.-Y.-Y.; Wang, J.-Q.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. A Review of NMR Analysis in Polysaccharide Structure and Conformation: Progress, Challenge and Perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef]
- Huo, J.; Wu, Z.; Zhao, H.; Sun, W.; Wu, J.; Huang, M.; Zhang, J.; Wang, Z.; Sun, B. Structure-Activity Relationship of Antioxidant Polysaccharides from Huangshui Based on the HPLC Fingerprint Combined with Chemometrics Methods. LWT 2022, 159, 113201. [Google Scholar] [CrossRef]
- Gray, C.J.; Migas, L.G.; Barran, P.E.; Pagel, K.; Seeberger, P.H.; Eyers, C.E.; Boons, G.-J.; Pohl, N.L.B.; Compagnon, I.; Widmalm, G.; et al. Advancing Solutions to the Carbohydrate Sequencing Challenge. J. Am. Chem. Soc. 2019, 141, 14463–14479. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Polysaccharides. In Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2019; pp. 75–101. [Google Scholar]
- Anggara, K.; Sršan, L.; Jaroentomeechai, T.; Wu, X.; Rauschenbach, S.; Narimatsu, Y.; Clausen, H.; Ziegler, T.; Miller, R.L.; Kern, K. Direct Observation of Glycans Bonded to Proteins and Lipids at the Single-Molecule Level. Science 2023, 382, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Delbianco, M.; Anggara, K.; Michnowicz, T.; Pardo-Vargas, A.; Bharate, P.; Sen, S.; Pristl, M.; Rauschenbach, S.; Schlickum, U.; et al. Imaging Single Glycans. Nature 2020, 582, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xie, W.; Fang, S.; He, S.; Yin, B.; Wang, Y.; Hou, C.; Huo, D.; Wang, D. Nanopore Electrochemical Sensors for Emerging Hazardous Pollutants Detection. Electrochim. Acta 2024, 475, 143678. [Google Scholar] [CrossRef]
- Kasianowicz, J.J.; Brandin, E.; Branton, D.; Deamer, D.W. Characterization of Individual Polynucleotide Molecules Using a Membrane Channel. Proc. Natl. Acad. Sci. USA 1996, 93, 13770–13773. [Google Scholar] [CrossRef]
- Bayley, H.; Cremer, P.S. Stochastic Sensors Inspired by Biology. Nature 2001, 413, 226–230. [Google Scholar] [CrossRef]
- Ying, Y.-L.; Cao, C.; Hu, Y.-X.; Long, Y.-T. A Single Biomolecule Interface for Advancing the Sensitivity, Selectivity and Accuracy of Sensors. Natl. Sci. Rev. 2018, 5, 450–452. [Google Scholar] [CrossRef]
- Bayley, H. Designed Membrane Channels and Pores. Curr. Opin. Biotechnol. 1999, 10, 94–103. [Google Scholar] [CrossRef]
- Li, J.; Stein, D.; McMullan, C.; Branton, D.; Aziz, M.J.; Golovchenko, J.A. Ion-Beam Sculpting at Nanometre Length Scales. Nature 2001, 412, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Storm, A.J.; Chen, J.H.; Ling, X.S.; Zandbergen, H.W.; Dekker, C. Fabrication of Solid-State Nanopores with Single-Nanometre Precision. Nat. Mater. 2003, 2, 537–540. [Google Scholar] [CrossRef]
- Yin, B.; Fang, S.; Zhou, D.; Liang, L.; Wang, L.; Wang, Z.; Wang, D.; Yuan, J. Nanopore Fabrication via Transient High Electric Field Controlled Breakdown and Detection of Single RNA Molecules. ACS Appl. Bio Mater. 2020, 3, 6368–6375. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Takei, H.; Tomiyasu, K.; Sakamoto, O.; Naono, N. Sensing the Performance of Artificially Intelligent Nanopores Developed by Integrating Solid-State Nanopores with Machine Learning Methods. J. Phys. Chem. C 2022, 126, 12197–12209. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Q.; Wang, W.; Fang, F.; Zhang, J. Solid-State Nanopore Array: Manufacturing and Applications. Small 2023, 19, 2205680. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, Z.; Zhang, X.; Wu, D.; Wu, Y.; Li, G. Recent Advances in Nanopore-Based Analysis for Carbohydrates and Glycoconjugates. Anal. Methods 2024, 16, 1454–1467. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Ke, W.; Xia, B.; Gao, Z. Nanopore-Based Glycan Sequencing: State of the Art and Future Prospects. Chem. Sci. 2024, 15, 6229–6243. [Google Scholar] [CrossRef]
- Casey, A.K.; Miller, B.G. Kinetic Basis of Carbohydrate-Mediated Inhibition of Human Glucokinase by the Glucokinase Regulatory Protein. Biochemistry 2016, 55, 2899–2902. [Google Scholar] [CrossRef]
- Li, C.; Ma, X.; Lu, J.; Tao, R.; Yu, X.; Mo, Y.; Lu, W.; Bao, Y.; Zhou, J.; Jia, W. Decreasing Complexity of Glucose Time Series Derived from Continuous Glucose Monitoring Is Correlated with Deteriorating Glucose Regulation. Front. Med. 2023, 17, 68–74. [Google Scholar] [CrossRef]
- Ge, X.; Tolosa, L.; Simpson, J.; Rao, G. Genetically Engineered Binding Proteins as Biosensors for Fermentation and Cell Culture. Biotechnol. Bioeng. 2003, 84, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Berntsson, R.P.-A.; Smits, S.H.J.; Schmitt, L.; Slotboom, D.-J.; Poolman, B. A Structural Classification of Substrate-binding Proteins. FEBS Lett. 2010, 584, 2606–2617. [Google Scholar] [CrossRef]
- Galenkamp, N.S.; Soskine, M.; Hermans, J.; Wloka, C.; Maglia, G. Direct Electrical Quantification of Glucose and Asparagine from Bodily Fluids Using Nanopores. Nat. Commun. 2018, 9, 4085. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.-K.; Kim, J.-S.; Lee, M.-K.; Ryu, K.-S.; Chi, S.-W. Probing the Neuraminidase Activity of Influenza Virus Using a Cytolysin A Protein Nanopore. Anal. Chem. 2020, 92, 14303–14308. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Chen, X.-X.; Fossey, J.S.; James, T.D.; Jiang, Y.-B. Selective Sensing of Saccharides Using Simple Boronic Acids and Their Aggregates. Chem. Soc. Rev. 2013, 42, 8032. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, W.J.; Bayley, H. Single-Molecule Determination of the Isomers of d -Glucose and d -Fructose That Bind to Boronic Acids. Angew. Chem. 2018, 130, 2891–2895. [Google Scholar] [CrossRef]
- Sun, X.; James, T.D. Glucose Sensing in Supramolecular Chemistry. Chem. Rev. 2015, 115, 8001–8037. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, Z.; Fan, P.; Wang, Y.; Jia, W.; Wang, L.; Wang, K.; Liu, Y.; Du, X.; Hu, C.; et al. A Nanopore-Based Saccharide Sensor. Angew. Chem. Int. Ed. 2022, 61, e202203769. [Google Scholar] [CrossRef]
- He, S.; Liu, Y.; Fang, S.; Li, Y.; Weng, T.; Tian, R.; Yin, Y.; Zhou, D.; Yin, B.; Wang, Y.; et al. Solid-State Nanopore DNA Sequencing: Advances, Challenges and Prospects. Coord. Chem. Rev. 2024, 510, 215816. [Google Scholar] [CrossRef]
- Liu, L.; Ma, X.; Chang, Y.; Guo, H.; Wang, W. Biosensors with Boronic Acid-Based Materials as the Recognition Elements and Signal Labels. Biosensors 2023, 13, 785. [Google Scholar] [CrossRef]
- Deuel, H.; Neukom, H.; Weber, F. Reaction of boric acid with polysaccharides. Nature 1948, 161, 96. [Google Scholar] [CrossRef]
- Williams, G.; Kedge, J.L.; Fossey, J.S. Molecular Boronic Acid-Based Saccharide Sensors. ACS Sens. 2021, 6, 1508–1528. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Han, C.; Wen, L.; Tian, D.; Li, H.; Jiang, L. pH Gated Glucose Responsive Biomimetic Single Nanochannels. Chem. Commun. 2012, 48, 3282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zheng, Y.-B.; Cai, S.-L.; Weng, Y.-H.; Cao, S.-H.; Yang, J.-L.; Li, Y.-Q. Sugar-Stimulated Robust Nanodevice: 4-Carboxyphenylboronic Acid Modified Single Glass Conical Nanopores. Electrochem. Commun. 2013, 36, 71–74. [Google Scholar] [CrossRef]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The Relationship among pKa, pH, and Binding Constants in the Interactions between Boronic Acids and Diols—It Is Not as Simple as It Appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Zheng, Y.-B.; Zhao, S.; Cao, S.-H.; Cai, S.-L.; Cai, X.-H.; Li, Y.-Q. A Temperature, pH and Sugar Triple-Stimuli-Responsive Nanofluidic Diode. Nanoscale 2017, 9, 433–439. [Google Scholar] [CrossRef]
- Vilozny, B.; Wollenberg, A.L.; Actis, P.; Hwang, D.; Singaram, B.; Pourmand, N. Carbohydrate-Actuated Nanofluidic Diode: Switchable Current Rectification in a Nanopipette. Nanoscale 2013, 5, 9214. [Google Scholar] [CrossRef]
- Yang, M.; Ma, C.; Ding, S.; Zhu, Y.; Shi, G.; Zhu, A. Rational Design of Stimuli-Responsive Polymers Modified Nanopores for Selective and Sensitive Determination of Salivary Glucose. Anal. Chem. 2019, 91, 14029–14035. [Google Scholar] [CrossRef]
- Kullman, L.; Winterhalter, M.; Bezrukov, S.M. Transport of Maltodextrins through Maltoporin: A Single-Channel Study. Biophys. J. 2002, 82, 803–812. [Google Scholar] [CrossRef]
- Li, X.; Lee, K.H.; Shorkey, S.; Chen, J.; Chen, M. Different Anomeric Sugar Bound States of Maltose Binding Protein Resolved by a Cytolysin A Nanopore Tweezer. ACS Nano 2020, 14, 1727–17375. [Google Scholar] [CrossRef]
- Bacri, L.; Oukhaled, A.; Hémon, E.; Bassafoula, F.B.; Auvray, L.; Daniel, R. Discrimination of Neutral Oligosaccharides through a Nanopore. Biochem. Biophys. Res. Commun. 2011, 412, 561–5641. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiong, Y.; Cao, Y.; Zhang, C.; Li, Y.; Ning, H.; Liu, F.; Zhou, H.; Li, X.; Ye, X.; et al. Identification of Tagged Glycans with a Protein Nanopore. Nat. Commun. 2023, 14, 1737. [Google Scholar] [CrossRef]
- Vikraman, D.; Satheesan, R.; Rajendran, M.; Kumar, N.A.; Johnson, J.B.; Krishnan R, S.; Mahendran, K.R. Selective Translocation of Cyclic Sugars through Dynamic Bacterial Transporter. ACS Sens. 2022, 7, 1766–1776. [Google Scholar] [CrossRef]
- Xia, B.; Fang, J.; Ma, S.; Ma, M.; Yao, G.; Li, T.; Cheng, X.; Wen, L.; Gao, Z. Mapping the Acetylamino and Carboxyl Groups on Glycans by Engineered α-Hemolysin Nanopores. J. Am. Chem. Soc. 2023, 145, 18812–18824. [Google Scholar] [CrossRef]
- Yao, G.; Tian, Y.; Ke, W.; Fang, J.; Ma, S.; Li, T.; Cheng, X.; Xia, B.; Wen, L.; Gao, Z. Direct Identification of Complex Glycans via a Highly Sensitive Engineered Nanopore. J. Am. Chem. Soc. 2024, 146, 13356–13366. [Google Scholar] [CrossRef]
- Fennouri, A.; Przybylski, C.; Pastoriza-Gallego, M.; Bacri, L.; Auvray, L.; Daniel, R. Single Molecule Detection of Glycosaminoglycan Hyaluronic Acid Oligosaccharides and Depolymerization Enzyme Activity Using a Protein Nanopore. ACS Nano 2012, 6, 9672–9678. [Google Scholar] [CrossRef]
- Fennouri, A.; Ramiandrisoa, J.; Bacri, L.; Mathé, J.; Daniel, R. Comparative biosensing of glycosaminoglycan hyaluronic acid oligo-and polysaccharides using aerolysin and α-hemolysin nanopores. Eur. Phys. J. E 2018, 41, 127. [Google Scholar] [CrossRef] [PubMed]
- Oukhaled, G.; Bacri, L.; Mathé, J.; Pelta, J.; Auvray, L. Effect of Screening on the Transport of Polyelectrolytes through Nanopores. Europhys. Lett. 2008, 82, 48003. [Google Scholar] [CrossRef]
- Guo, L.; Han, Y.; Yang, H.; Fu, J.; Li, W.; Xie, R.; Zhang, Y.; Wang, K.; Xia, X.-H. Single-Molecule Discrimination of Saccharides Using Carbon Nitride Nanopores. Nano Lett. 2024, 24, 5639–5646. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Qiu, M.; Huang, S.; Luo, C.; Wu, Z.; Liang, B.; Huang, H.; Ci, Z.; Zhang, D.; Han, L.; Lin, J. Pharmacological and Clinical Application of Heparin Progress: An Essential Drug for Modern Medicine. Biomed. Pharmacother. 2021, 139, 111561. [Google Scholar] [CrossRef]
- Karawdeniya, B.I.; Bandara, Y.M.N.D.Y.; Nichols, J.W.; Chevalier, R.B.; Dwyer, J.R. Surveying Silicon Nitride Nanopores for Glycomics and Heparin Quality Assurance. Nat. Commun. 2018, 9, 3278. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Lindsay, S.; Wang, X.; Zhang, P. Single Molecule Identification and Quantification of Glycosaminoglycans Using Solid-State Nanopores. ACS Nano 2019, 13, 6308–6318. [Google Scholar] [CrossRef]
- Winters-Hilt, S.; Akeson, M. Nanopore Cheminformatics. DNA Cell Biol. 2004, 23, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Hagan, J.T.; Fu, L.; Sheetz, B.S.; Bhattacharya, S.; Zhang, F.; Dwyer, J.R.; Linhardt, R.J. Synthetic Heparan Sulfate Standards and Machine Learning Facilitate the Development of Solid-State Nanopore Analysis. Proc. Natl. Acad. Sci. USA 2021, 118, e2022806118. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Balanzat, E.; Janot, J.-M.; Balme, S. Single Conical Track-Etched Nanopore for a Free-Label Detection of OSCS Contaminants in Heparin. Biosens. Bioelectron. 2019, 137, 207–212. [Google Scholar] [CrossRef]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its Nature, Distribution, Functions and Turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Cowman, M.K.; Lee, H.-G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front. Immunol. 2015, 6, 261. [Google Scholar] [CrossRef]
- Fennouri, A.; Daniel, R.; Pastoriza-Gallego, M.; Auvray, L.; Pelta, J.; Bacri, L. Kinetics of enzymatic degradation of high molecular weight polysaccharides through a nanopore: Experiments and data-modeling. Anal. Chem. 2013, 85, 8488–8492. [Google Scholar] [CrossRef]
- Bayat, P.; Rambaud, C.; Priem, B.; Bourderioux, M.; Bilong, M.; Poyer, S.; Pastoriza-Gallego, M.; Oukhaled, A.; Mathé, J.; Daniel, R. Comprehensive structural assignment of glycosaminoglycan oligo- and polysaccharides by protein nanopore. Nat. Commun. 2022, 13, 5113. [Google Scholar] [CrossRef]
- Rivas, F.; Zahid, O.K.; Reesink, H.L.; Peal, B.T.; Nixon, A.J.; DeAngelis, P.L.; Skardal, A.; Rahbar, E.; Hall, A.R. Label-Free Analysis of Physiological Hyaluronan Size Distribution with a Solid-State Nanopore Sensor. Nat. Commun. 2018, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Janot, J.-M.; Balme, S. Dynamics of Long Hyaluronic Acid Chains through Conical Nanochannels for Characterizing Enzyme Reactions in Confined Spaces. Nanoscale 2020, 12, 7231–7239. [Google Scholar] [CrossRef]
- Vorwerk, S.; Somerville, S.; Somerville, C. The Role of Plant Cell Wall Polysaccharide Composition in Disease Resistance. Trends Plant Sci. 2004, 9, 203–2095. [Google Scholar] [CrossRef]
- Höfte, H.; Voxeur, A. Plant Cell Walls. Curr. Biol. 2017, 27, R865–R870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The Plant Cell Wall: Biosynthesis, Construction, and Functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, B.; Liang, L.; Wang, S.; Zhang, L.; Wang, L.; Cui, H.-L.; Zhou, Y.; Wang, D. A Solid-State Nanopore-Based Single-Molecule Approach for Label-Free Characterization of Plant Polysaccharides. Plant Commun. 2021, 2, 100106. [Google Scholar] [CrossRef]
- Xie, W.; Fang, S.; Yin, B.; Tian, R.; Liang, L.; He, S.; Wang, D. Application of Nanopore Single Molecule Detection Technology in Analysis of Xylan Dissolved in Ionic Liquid. Chin. J. Chem. 2023, 41, 1720–1726. [Google Scholar] [CrossRef]
- Xie, W.; He, S.; Fang, S.; Yin, B.; Tian, R.; Wang, Y.; Wang, D. Analysis of Starch Dissolved in Ionic Liquid by Glass Nanopore at Single Molecular Level. Int. J. Biol. Macromol. 2023, 239, 124271. [Google Scholar] [CrossRef]
- Xie, W.; He, S.; Fang, S.; Tian, R.; Liang, L.; Wang, D. Phenylboronic Acid-Modified Polyethyleneimine Assisted Neutral Polysaccharide Detection and Weight-Resolution Analysis with a Nanopipette. Nanoscale 2023, 15, 7147–7153. [Google Scholar] [CrossRef]
- Atik, M. Dextran 40 and Dextran 70: A Review. Arch. Surg. 1967, 94, 664. [Google Scholar] [CrossRef]
- Kam, P.C.A.; Liou, J.P.C.; Yang, K.X.F. In Vitro Evaluation of the Effect of Haemodilution with Dextran 40 on Coagulation Profile as Measured by Thromboelastometry and Multiple Electrode Aggregometry. Anaesth. Intensive Care 2017, 45, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Glycan-Based Interactions Involving Vertebrate Sialic-Acid-Recognizing Proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, H.; Xiong, Y.; Ju, H. Quantitative Screening of Cell-Surface Gangliosides by Nondestructive Extraction and Hydrophobic Collection. Angew. Chem. 2018, 130, 793–797. [Google Scholar] [CrossRef]
- Li, M.; Xiong, Y.; Wang, D.; Liu, Y.; Na, B.; Qin, H.; Liu, J.; Liang, X.; Qing, G. Biomimetic Nanochannels for the Discrimination of Sialylated Glycans via a Tug-of-War between Glycan Binding and Polymer Shrinkage. Chem. Sci. 2020, 11, 748–756. [Google Scholar] [CrossRef]
- Session 27: Post-Translational Modifications-Glycosylation and Glycoproteomics. Mol. Cell. Proteom. 2005, 4, S262–S267. [CrossRef]
- Illiano, A.; Pinto, G.; Melchiorre, C.; Carpentieri, A.; Faraco, V.; Amoresano, A. Protein Glycosylation Investigated by Mass Spectrometry: An Overview. Cells 2020, 9, 1986. [Google Scholar] [CrossRef]
- Khoury, G.A.; Baliban, R.C.; Floudas, C.A. Proteome-Wide Post-Translational Modification Statistics: Frequency Analysis and Curation of the Swiss-Prot Database. Sci. Rep. 2011, 1, 90. [Google Scholar] [CrossRef]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef]
- Restrepo-Pérez, L.; Wong, C.H.; Maglia, G.; Dekker, C.; Joo, C. Label-Free Detection of Post-Translational Modifications with a Nanopore. Nano Lett. 2019, 19, 7957–7964. [Google Scholar] [CrossRef]
- Versloot, R.C.A.; Lucas, F.L.R.; Yakovlieva, L.; Tadema, M.J.; Zhang, Y.; Wood, T.M.; Martin, N.I.; Marrink, S.J.; Walvoort, M.T.C.; Maglia, G. Quantification of Protein Glycosylation Using Nanopores. Nano Lett. 2022, 22, 5357–5364. [Google Scholar] [CrossRef]
- Tang, H.; Wang, H.; Zhao, D.; Cao, M.; Zhu, Y.; Li, Y. Nanopore-Based Single-Entity Electrochemistry for the Label-Free Monitoring of Single-Molecule Glycoprotein–Boronate Affinity Interaction and Its Sensing Application. Anal. Chem. 2022, 94, 5715–5722. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Ali, M.; Neumann, R.; Ensinger, W. Saccharide/Glycoprotein Recognition inside Synthetic Ion Channels Modified with Boronic Acid. Sens. Actuators B Chem. 2012, 162, 216–222. [Google Scholar] [CrossRef]
- Okuda, S.; Sherman, D.J.; Silhavy, T.J.; Ruiz, N.; Kahne, D. Lipopolysaccharide Transport and Assembly at the Outer Membrane: The PEZ Model. Nat. Rev. Microbiol. 2016, 14, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Di Guida, R.; Casillo, A.; Tomás, J.M.; Merino, S.; Corsaro, M.M. Complete Characterization of the O-Antigen from the LPS of Aeromonas bivalvium. Int. J. Mol. Sci. 2022, 23, 1204. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Joshi, H.; Zhu, H.; Wang, F.; Xu, Y.; Gao, J.; Huang, F.; Aksimentiev, A.; Feng, J. Synthetic Macrocycle Nanopore for Potassium-Selective Transmembrane Transport. J. Am. Chem. Soc. 2021, 143, 15975–15983. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, H.; Deng, K.; Wu, S.; Xue, J.; Shu, L.; Yu, Y.; Geng, Y.; Li, P.; Yang, Y.; et al. Peptide Recognition by Functional Supramolecular Nanopores with Complementary Size and Binding Sites. Nano Res. 2016, 9, 1452–1459. [Google Scholar] [CrossRef]
- Xing, X.-L.; Liao, Q.-B.; Ahmed, S.A.; Wang, D.; Ren, S.; Qin, X.; Ding, X.L.; Xi, K.; Ji, L.N.; Wang, K.; et al. Single Molecule DNA Analysis Based on Atomic-Controllable Nanopores in Covalent Organic Frameworks. Nano Lett. 2022, 22, 1358–1365. [Google Scholar] [CrossRef]
- Xing, X.-L.; He, Z.-C.; Ahmed, S.A.; Liao, Q.; Guo, L.R.; Ren, S.; Xi, K.; Ji, L.N.; Wang, K.; Xia, X.H. High Spatial Resolution of Ultrathin Covalent Organic Framework Nanopores for Single-Molecule DNA Sensing. Anal. Chem. 2022, 94, 9851–9855. [Google Scholar] [CrossRef]


| Nanopore Types | Sensing Strategy | Analyte Types | Pros | Cons | Ref. | ||
|---|---|---|---|---|---|---|---|
| Biological nanopore | ClyA | Resistive pulse sensing | Protein assist | Monosaccharide | Specific recognition | Cannot determine the structure | [46,47] |
| Oligosaccharides | Identifies the bond types | Not universal | [63] | ||||
| α-HL, MspA | Engineered nanopore | Monosaccharides | Identifies multiple polysaccharides | Nanopore needs engineering | [49,51] | ||
| α-HL | Oligosaccharides | [68] | |||||
| AeL | Incorporate labels | Oligosaccharides | Oligosaccharides with low charge density | Analytes need to be labeled | [65] | ||
| AeL, α-HL | Direct detection | Oligo/Polysaccharides | Simple and direct | For high-charge linear molecules | [69,70,71] | ||
| CymA | Needs specific membrane pore proteins | [66] | |||||
| FraC | Glycosylation peptide | Simple and direct | [102,103] | ||||
| Solid-state nanopore | PET, Glass | Ion rectification | Charge change | Mono/Polysaccharides | High sensitivity | Nanopore modification, unable to perform structural analysis | [56,57,59,79] |
| Glass | Structure change | Monosaccharides | [61] | ||||
| CN | Resistive pulse sensing | Direct detection | Oligosaccharides | Directly distinguishable structure | Multi- nanopore structure | [72] | |
| SiNx | Polysaccharides Lipopolysaccharide | Multi- condition testing | [89,106] | ||||
| SiNx | Detection through algorithms | Direct detection | Suitable for high molecular weight, charge and linear molecules | [75,76,78,84] | |||
| Glass | Enzyme modification | Real-time reaction monitoring | [85] | ||||
| Chemical modification | Glycoprotein | High sensitivity | Nanopore modification | [104] | |||
| SiNx, Glass | Detection via ionic liquids | Poly- saccharide | Polysaccharides with low charge density | Electrolyte contains multiple solvents | [90,91] | ||
| Glass | Chemical molecules assistant | Introduction of other molecules | [96] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, B.; Xie, W.; Fang, S.; He, S.; Ma, W.; Liang, L.; Yin, Y.; Zhou, D.; Wang, Z.; Wang, D. Research Progress on Saccharide Molecule Detection Based on Nanopores. Sensors 2024, 24, 5442. https://doi.org/10.3390/s24165442
Yin B, Xie W, Fang S, He S, Ma W, Liang L, Yin Y, Zhou D, Wang Z, Wang D. Research Progress on Saccharide Molecule Detection Based on Nanopores. Sensors. 2024; 24(16):5442. https://doi.org/10.3390/s24165442
Chicago/Turabian StyleYin, Bohua, Wanyi Xie, Shaoxi Fang, Shixuan He, Wenhao Ma, Liyuan Liang, Yajie Yin, Daming Zhou, Zuobin Wang, and Deqiang Wang. 2024. "Research Progress on Saccharide Molecule Detection Based on Nanopores" Sensors 24, no. 16: 5442. https://doi.org/10.3390/s24165442






