Performance of a Novel Electronic Nose for the Detection of Volatile Organic Compounds Relating to Starvation or Human Decomposition Post-Mass Disaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
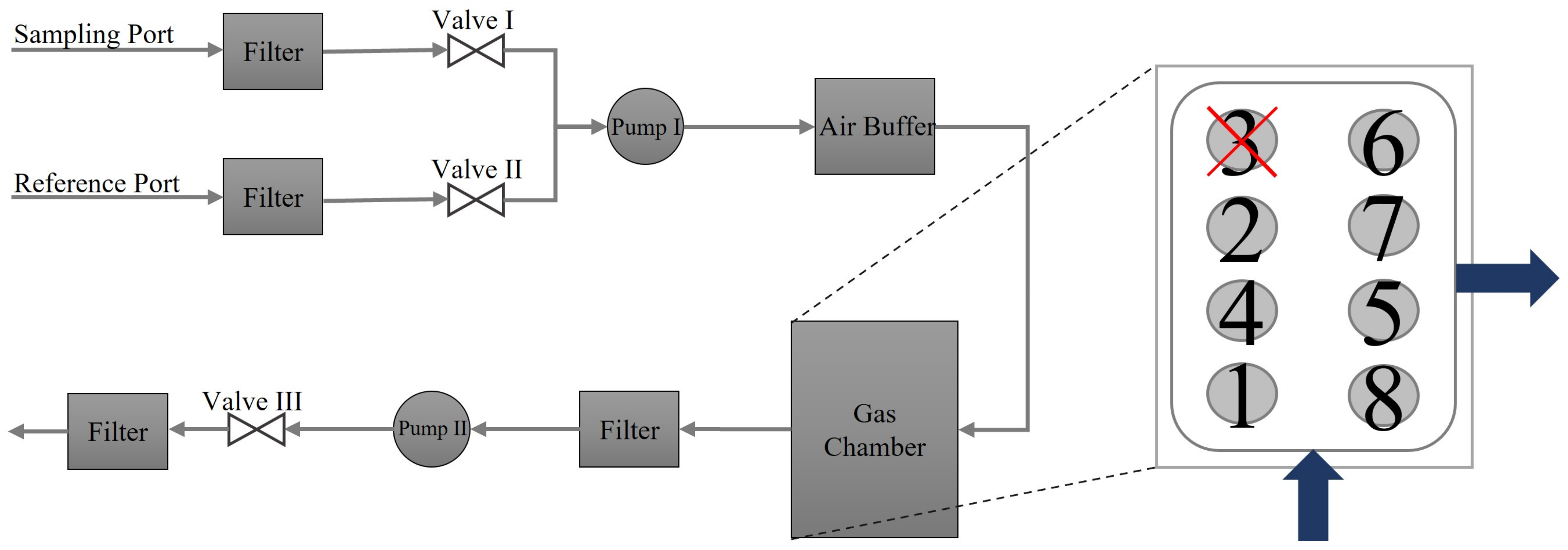
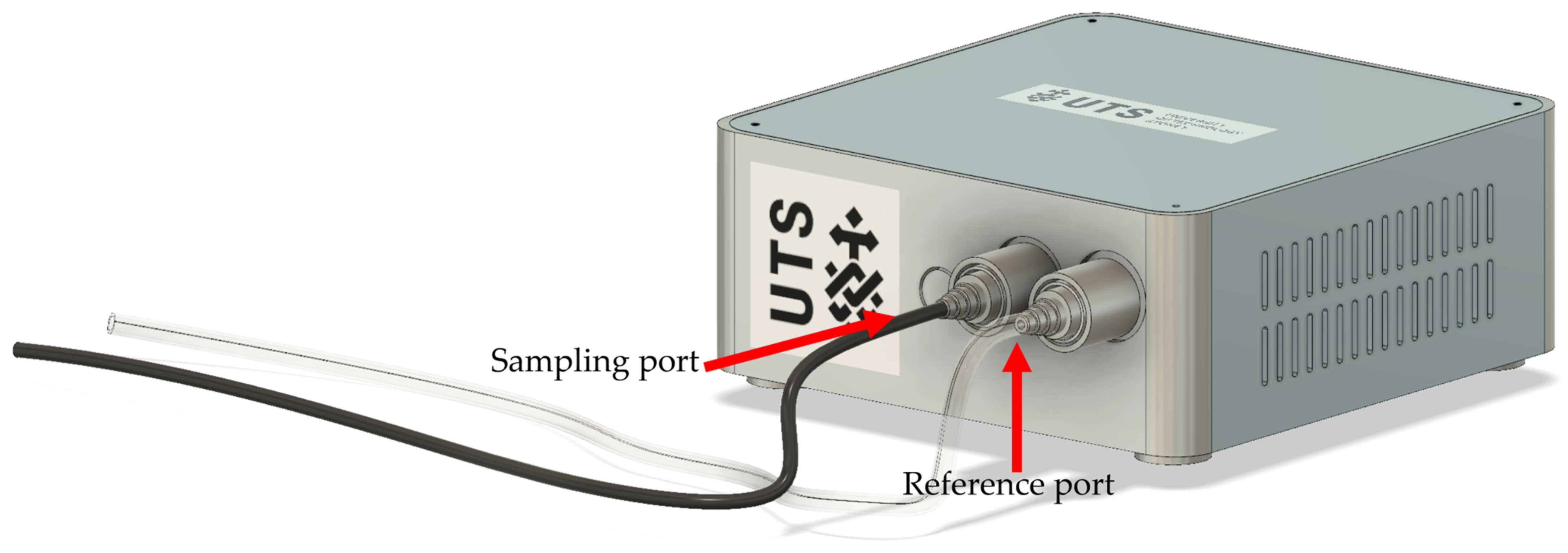
2.3. The NOS.E Test Setup
2.4. Experimental Site
2.5. Data Analysis
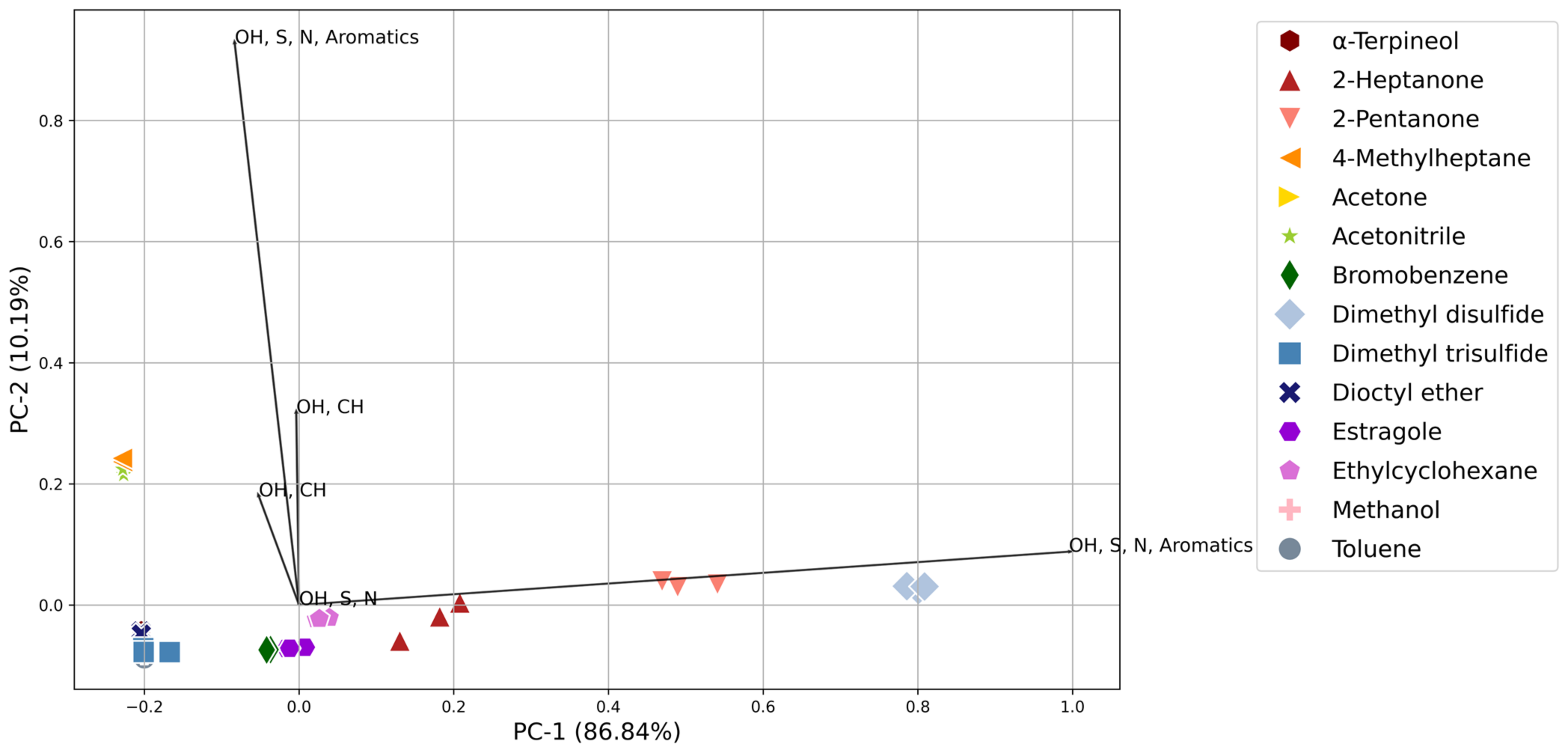
3. Results
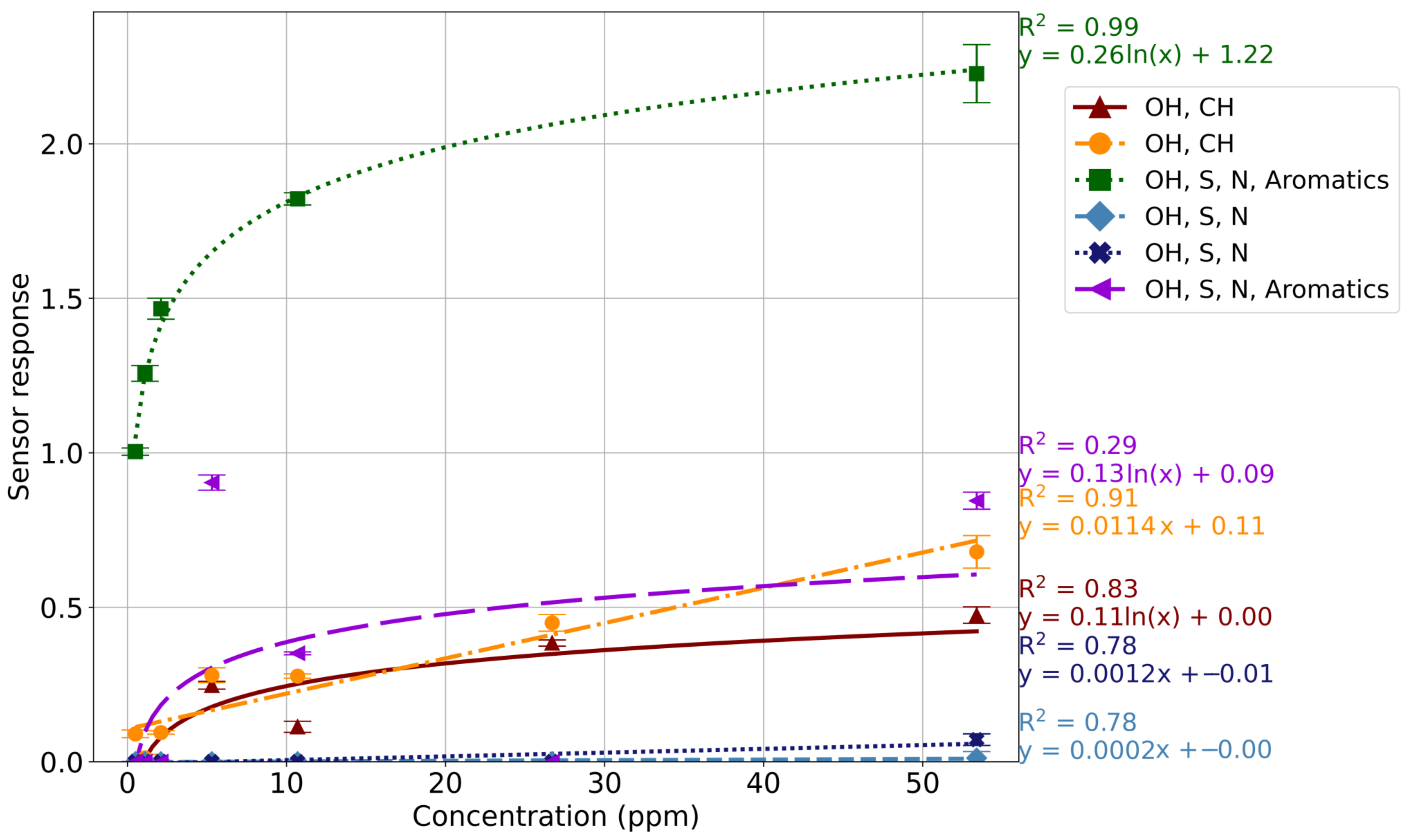
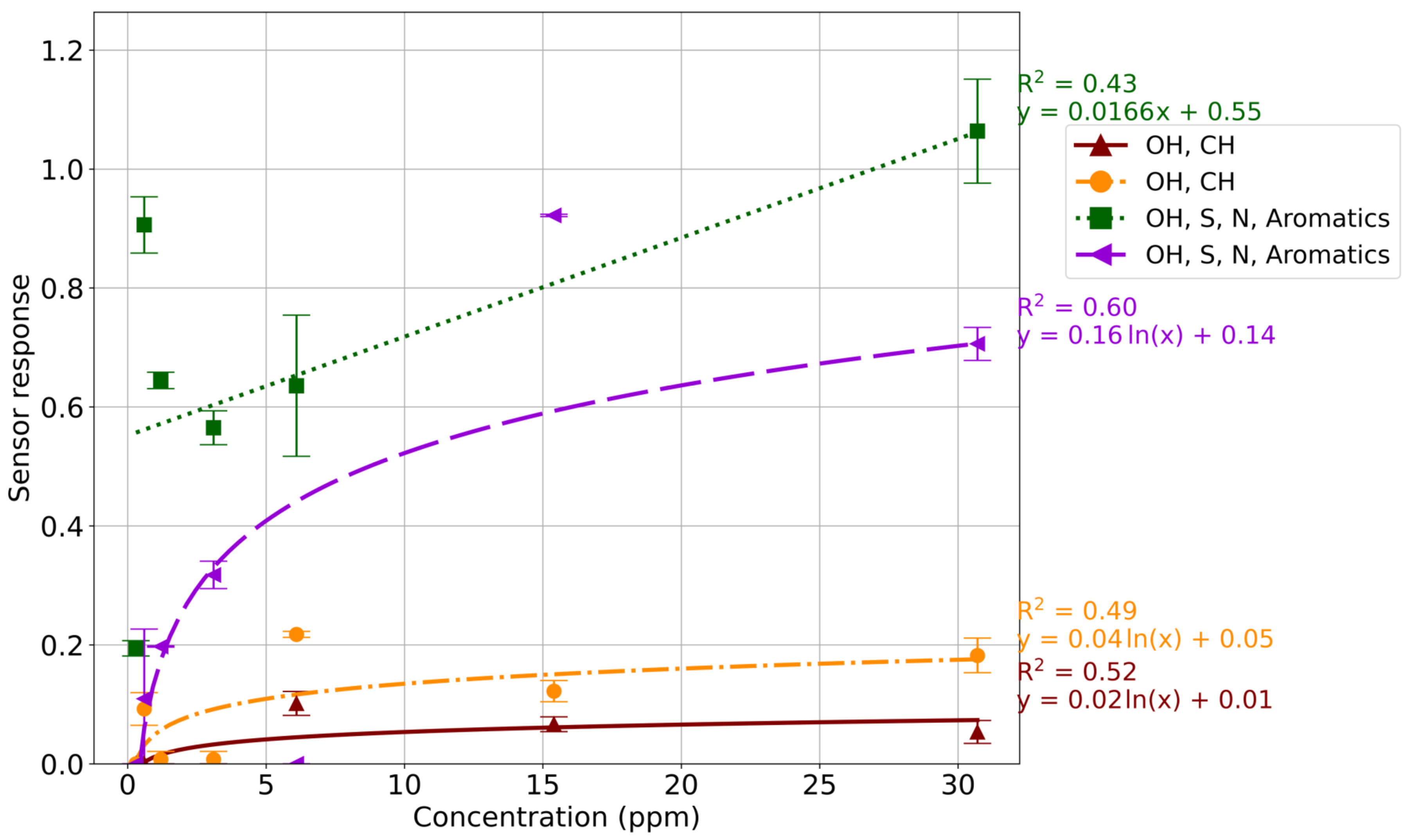
3.1. Sensor Responses
3.2. Limit of Detection
3.3. Compound Detection
3.4. Field Trial
4. Discussion
4.1. Analytes
4.2. Sensor Responses
4.3. Limit of Detection
4.4. Compound Detection
4.5. Field Trial
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brough, A.L.; Morgan, B.; Rutty, G.N. The basics of disaster victim identification. J. Forensic Radiol. Imaging 2015, 3, 29–37. [Google Scholar] [CrossRef]
- Graham, E.A.M. Disaster victim identification. Forensic Sci. Med. Pathol. 2006, 2, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Huo, R.; Agapiou, A.; Bocos-Bintintan, V.; Brown, L.J.; Burns, C.; Creaser, C.S.; Devenport, N.A.; Gao-Lau, B.; Guallar-Hoyas, C.; Hildebrand, L.; et al. The trapped human experiment. J. Breath. Res. 2011, 5, 046006. [Google Scholar] [CrossRef]
- Chiu, W.-T.; Arnold, J.; Shih, Y.-T.; Hsiung, K.-H.; Chi, H.-Y.; Chiu, C.-H.; Tsai, W.-C.; Huang, W.C. A Survey of International Urban Search-and-rescue Teams following the Ji Ji Earthquake. Disasters 2002, 26, 85–94. [Google Scholar] [CrossRef]
- Ueland, M.; Harris, S.; Forbes, S.L. Detecting volatile organic compounds to locate human remains in a simulated collapsed building. Forensic Sci. Int. 2021, 323, 110781. [Google Scholar] [CrossRef]
- Otto, C.M.; Downend, A.B.; Moore, G.E.; Daggy, J.K.; Ranivand, D.L.; Reetz, J.A.; Fitzgerald, S.D. Medical Surveillance of Search Dogs Deployed to the World Trade Center and Pentagon 2001–2006. J. Environ. Health 2010, 73, 12–21. [Google Scholar] [CrossRef]
- Prezant, D.J.; Weiden, M.; Banauch, G.I.; McGuinness, G.; Rom, W.N.; Aldrich, T.K.; Kelly, K.J. Cough and Bronchial Responsiveness in Firefighters at the World Trade Center Site. N. Engl. J. Med. 2002, 347, 806–815. [Google Scholar] [CrossRef]
- Kasnesis, P.; Doulgerakis, V.; Uzunidis, D.; Kogias, D.G.; Funcia, S.I.; González, M.B.; Giannousis, C.; Patrikakis, C.Z. Deep Learning Empowered Wearable-Based Behavior Recognition for Search and Rescue Dogs. Sensors 2022, 22, 993. [Google Scholar] [CrossRef]
- Brown, A.; Lamb, E.; Deo, A.; Pasin, D.; Liu, T.; Zhang, W.; Su, S.; Ueland, M. The use of novel electronic nose technology to locate missing persons for criminal investigations. iScience 2023, 26, 106353. [Google Scholar] [CrossRef]
- Agapiou, A.; Mikedi, K.; Karma, S.; Giotaki, Z.K.; Kolostoumbis, D.; Papageorgiou, C.; Zorba, E.; Spiliopoulou, C.; Amann, A.; Statheropoulos, M. Physiology and biochemistry of human subjects during entrapment. J. Breath. Res. 2013, 7, 016004. [Google Scholar] [CrossRef]
- Statheropoulos, M.; Sianos, E.; Agapiou, A.; Georgiadou, A.; Pappa, A.; Tzamtzis, N.; Giotaki, H.; Papageorgiou, C.; Kolostoumbis, D. Preliminary investigation of using volatile organic compounds from human expired air, blood and urine for locating entrapped people in earthquakes. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 822, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Agapiou, A.; Amann, A.; Mochalski, P.; Statheropoulos, M.; Thomas, C.L.P. Trace detection of endogenous human volatile organic compounds for search, rescue and emergency applications. TrAC Trends Anal. Chem. (Regul. Ed.) 2015, 66, 158–175. [Google Scholar] [CrossRef]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 75–88. [Google Scholar] [CrossRef]
- Statheropoulos, M.; Agapiou, A.; Georgiadou, A. Analysis of expired air of fasting male monks at Mount Athos. J. Chromatogr. B 2006, 832, 274–279. [Google Scholar] [CrossRef]
- Huda, A.-K.; de Lacy Costello, B.; Ratcliffe, N. An investigation of volatile organic compounds from the saliva of healthy individuals using headspace-trap/GC-MS. J. Breath. Res. 2013, 7, 036004. [Google Scholar]
- Verheggen, F.; Perrault, K.A.; Megido, R.C.; Dubois, L.M.; Francis, F.; Haubruge, E.; Forbes, S.L.; Focant, J.-F.; Stefanuto, P.-H. The Odor of Death: An Overview of Current Knowledge on Characterization and Applications. Bioscience 2017, 67, 600–613. [Google Scholar] [CrossRef]
- Vass, A.A.; Smith, R.R.; Thompson, C.V.; Burnett, M.N.; Wolf, D.A.; Synstelien, J.A.; Dulgerian, N.A.; Eckenrode, B.A. Decompositional odor analysis database. J. Forensic Sci. 2004, 49, 760–769. [Google Scholar] [CrossRef]
- Dekeirsschieter, J.; Stefanuto, P.H.; Brasseur, C.; Haubruge, E.; Focant, J.F. Enhanced characterization of the smell of death by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GCxGC-TOFMS). PLoS ONE 2012, 7, e39005. [Google Scholar] [CrossRef]
- Armstrong, P.; Nizio, K.D.; Perrault, K.A.; Forbes, S.L. Establishing the volatile profile of pig carcasses as analogues for human decomposition during the early postmortem period. Heliyon 2016, 2, e00070. [Google Scholar] [CrossRef]
- Agapiou, A.; Zorba, E.; Mikedi, K.; McGregor, L.; Spiliopoulou, C.; Statheropoulos, M. Analysis of volatile organic compounds released from the decay of surrogate human models simulating victims of collapsed buildings by thermal desorption–comprehensive two-dimensional gas chromatography–time of flight mass spectrometry. Anal. Chim. Acta 2015, 883, 99–108. [Google Scholar] [CrossRef]
- Dubois, L.M.; Perrault, K.A.; Stefanuto, P.-H.; Koschinski, S.; Edwards, M.; McGregor, L.; Focant, J.-F. Thermal desorption comprehensive two-dimensional gas chromatography coupled to variable-energy electron ionization time-of-flight mass spectrometry for monitoring subtle changes in volatile organic compound profiles of human blood. J. Chromatogr. A 2017, 1501, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Perrault, K.A.; Nizio, K.D.; Forbes, S.L. A Comparison of One-Dimensional and Comprehensive Two-Dimensional Gas Chromatography for Decomposition Odour Profiling Using Inter-Year Replicate Field Trials. Chromatographia 2015, 78, 1057–1070. [Google Scholar] [CrossRef]
- Pearce, T.C.; Schiffman, S.S.; Nagle, H.T.; Gardner, J.W. Handbook of Machine Olfaction: Electronic Nose Technology, 1st ed.; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2003. [Google Scholar]
- Park, S.Y.; Kim, Y.; Kim, T.; Eom, T.H.; Kim, S.Y.; Jang, H.W. Chemoresistive materials for electronic nose: Progress, perspectives, and challenges. InfoMat 2019, 1, 289–316. [Google Scholar] [CrossRef]
- Vass, A.; Thompson, C.V.; Wise, M. A New Forensics Tool: Development of an Advanced Sensor for Detecting Clandestine Graves; National Institute of Justice: Washington, DC, USA, 2010.
- Peters, R.; Beijer, N.; ‘t Hul, B.v.; Bruijns, B.; Munniks, S.; Knotter, J. Evaluation of a Commercial Electronic Nose Based on Carbon Nanotube Chemiresistors. Sensors 2023, 23, 5302. [Google Scholar] [CrossRef]
- Leite, L.S.; Visani, V.; Marques, P.C.F.; Seabra, M.A.B.L.; Oliveira, N.C.L.; Gubert, P.; Medeiros, V.W.C.d.; Albuquerque, J.O.d.; Lima Filho, J.L.d. Design and implementation of an electronic nose system for real-time detection of marijuana. Instrum. Sci. Technol. 2021, 49, 471–486. [Google Scholar] [CrossRef]
- Voss, A.; Witt, K.; Kaschowitz, T.; Poitz, W.; Ebert, A.; Roser, P.; Bär, K.-J. Detecting cannabis use on the human skin surface via an electronic nose system. Sensors 2014, 14, 13256–13272. [Google Scholar] [CrossRef]
- Wilson, A.D. Application of electronic-nose technologies and VOC-biomarkers for the noninvasive early diagnosis of gastrointestinal diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef]
- Zhu, L.; Seburg, R.A.; Tsai, E.; Puech, S.; Mifsud, J.-C. Flavor analysis in a pharmaceutical oral solution formulation using an electronic-nose. J. Pharm. Biomed. Anal. 2004, 34, 453–461. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, T.; Brown, A.; Ueland, M.; Forbes, S.L.; Su, S.W. The use of electronic nose for the classification of blended and single malt scotch whisky. IEEE Sens. J. 2022, 22, 7015–7021. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Zeng, M.; Meng, Q.-H. Electronic nose using a bio-inspired neural network modeled on mammalian olfactory system for Chinese liquor classification. Rev. Sci. Instrum. 2019, 90, 025001. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, T.; Ueland, M.; Forbes, S.L.; Wang, R.X.; Su, S.W. Design of an efficient electronic nose system for odour analysis and assessment. Measurement 2020, 165, 108089. [Google Scholar] [CrossRef]
- Vergara, A.; Fonollosa, J.; Mahiques, J.; Trincavelli, M.; Rulkov, N.; Huerta, R. On the performance of gas sensor arrays in open sampling systems using Inhibitory Support Vector Machines. Sens. Actuators B Chem. 2013, 185, 462–477. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, D. Domain Adaptation Extreme Learning Machines for Drift Compensation in E-Nose Systems. IEEE Trans. Instrum. Meas. 2015, 64, 1790–1801. [Google Scholar] [CrossRef]
- Qiao, Y.; Luo, J.; Cui, T.; Liu, H.; Tang, H.; Zeng, Y.; Liu, C.; Li, Y.; Jian, J.; Wu, J.; et al. Soft Electronics for Health Monitoring Assisted by Machine Learning. Nano-Micro Lett. 2023, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-Y.; Ou, L.-X.; Mao, L.-W.; Wu, X.-Y.; Liu, Y.-P.; Lu, H.-L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef]
- Szulczyński, B.; Armiński, K.; Namieśnik, J.; Gębicki, J. Determination of Odour Interactions in Gaseous Mixtures Using Electronic Nose Methods with Artificial Neural Networks. Sensors 2018, 18, 519. [Google Scholar] [CrossRef]
- Galanga, M.G.K.; Zarrab, T.; Naddeob, V.; Belgiornob, V. Artificial neural network in the measurement of environmental odours by e-nose. Chem. Eng. 2018, 68, 247–252. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, W.; Yuwono, M.; Zhang, M.; Ueland, M.; Forbes, S.L.; Su, S.W. A data-driven meat freshness monitoring and evaluation method using rapid centroid estimation and hidden Markov models. Sens. Actuators B Chem. 2020, 311, 127868. [Google Scholar] [CrossRef]
- Wentian, Z.; Taoping, L.; Miao, Z.; Yi, Z.; Huiqi, L.; Ueland, M.; Forbes, S.L.; Rosalind Wang, X.; Su, S.W. NOS.E: A New Fast Response Electronic Nose Health Monitoring System. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; Volume 2018, pp. 4977–4980. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, T.; Ye, L.; Ueland, M.; Forbes, S.L.; Su, S.W. A novel data pre-processing method for odour detection and identification system. Sens. Actuators A Phys. 2019, 287, 113–120. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, W.; Ye, L.; Ueland, M.; Forbes, S.L.; Su, S.W. A novel multi-odour identification by electronic nose using non-parametric modelling-based feature extraction and time-series classification. Sens. Actuators B Chem. 2019, 298, 126690. [Google Scholar] [CrossRef]
- Paul Pickering, S.T. Metal Oxide Gas Sensing Material and MEMS Process. Available online: https://www.fierceelectronics.com/components/metal-oxide-gas-sensing-material-and-mems-process#:~:text=MOS%20sensors%20detect%20concentration%20of,of%20the%20metal%20oxide%20material (accessed on 26 April 2024).
- Miller, J.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson Education: Harlow, UK, 2018. [Google Scholar]
- Xia, J.; Wishart, D.S. Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. Curr. Protoc. Bioinform. 2011, 34, 14.10.11–14.10.48. [Google Scholar] [CrossRef] [PubMed]
- Figaro USA Inc. TGS 2602—For the Detection of Air Contaminants. Available online: https://www.figarosensor.com/product/docs/TGS2602-B00%20%280615%29.pdf (accessed on 30 September 2023).
- Rosier, E.; Loix, S.; Develter, W.; Van de Voorde, W.; Tytgat, J.; Cuypers, E. The search for a volatile human specific marker in the decomposition process. PLoS ONE 2015, 10, e0137341. [Google Scholar] [CrossRef] [PubMed]
- Sakumura, Y.; Koyama, Y.; Tokutake, H.; Hida, T.; Sato, K.; Itoh, T.; Akamatsu, T.; Shin, W. Diagnosis by Volatile Organic Compounds in Exhaled Breath from Lung Cancer Patients Using Support Vector Machine Algorithm. Sensors 2017, 17, 287. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Zlatkis, A.; Liebich, H.M. Profile of volatile metabolites in human urine. Clin. Chem. 1971, 17, 592–594. [Google Scholar] [CrossRef]
- Kusano, M.; Mendez, E.; Furton, K.G. Development of headspace SPME method for analysis of volatile organic compounds present in human biological specimens. Anal. Bioanal. Chem. 2011, 400, 1817–1826. [Google Scholar] [CrossRef]
- Mochalski, P.; Krapf, K.; Ager, C.; Wiesenhofer, H.; Agapiou, A.; Statheropoulos, M.; Fuchs, D.; Ellmerer, E.; Buszewski, B.; Amann, A. Temporal profiling of human urine VOCs and its potential role under the ruins of collapsed buildings. Toxicol. Mech. Methods 2012, 22, 502–511. [Google Scholar] [CrossRef]
- Rosier, E.; Cuypers, E.; Dekens, M.; Verplaetse, R.; Develter, W.; Van de Voorde, W.; Maes, D.; Tytgat, J. Development and validation of a new TD-GC/MS method and its applicability in the search for human and animal decomposition products. Anal. Bioanal. Chem. 2014, 406, 3611–3619. [Google Scholar] [CrossRef]
- Dubois, L.M.; Stefanuto, P.-H.; Perrault, K.A.; Delporte, G.; Delvenne, P.; Focant, J.-F. Comprehensive approach for monitoring human tissue degradation. Chromatographia 2019, 82, 857–871. [Google Scholar] [CrossRef]
- Itoh, T.; Miwa, T.; Tsuruta, A.; Akamatsu, T.; Izu, N.; Shin, W.; Park, J.; Hida, T.; Eda, T.; Setoguchi, Y. Development of an exhaled breath monitoring system with semiconductive gas sensors, a gas condenser unit, and gas chromatograph columns. Sensors 2016, 16, 1891. [Google Scholar] [CrossRef]
- Drabińska, N.; Flynn, C.; Ratcliffe, N.; Belluomo, I.; Myridakis, A.; Gould, O.; Fois, M.; Smart, A.; Devine, T.; Costello, B.D.L. A literature survey of all volatiles from healthy human breath and bodily fluids: The human volatilome. J. Breath Res. 2021, 15, 034001. [Google Scholar] [CrossRef] [PubMed]
- Figaro USA Inc. TGS 2600—For the Detection of Air Contaminants. Available online: https://www.figarosensor.com/product/docs/TGS2600B00%20%280913%29.pdf (accessed on 30 September 2023).
- Figaro USA Inc. TGS 2610—For the Detection of LP Gas. Available online: https://www.figarosensor.com/product/docs/TGS2610CD%200114.pdf (accessed on 30 September 2023).
- Figaro USA Inc. TGS 2612—For the Detection of Methane and LP Gas. Available online: https://www.figaro.co.jp/en/product/docs/TGS2612_Product%20Infomation_rev02.pdf (accessed on 30 September 2023).
- Figaro USA Inc. TGS2603—For Detection of Odor and Air Contaminants. Available online: https://www.figarosensor.com/product/docs/tgs2603_product%20information%28fusa%29_rev08.pdf (accessed on 30 September 2023).
- Sorocki, J.; Rydosz, A. A Prototype of a Portable Gas Analyzer for Exhaled Acetone Detection. Appl. Sci. 2019, 9, 2605. [Google Scholar] [CrossRef]
- Chao, L.K.; Hua, K.-F.; Hsu, H.-Y.; Cheng, S.-S.; Liu, J.-Y.; Chang, S.-T. Study on the antiinflammatory activity of essential oil from leaves of Cinnamomum osmophloeum. J. Agric. Food Chem. 2005, 53, 7274–7278. [Google Scholar] [CrossRef]
- Stejskal, S.M. Death, Decomposition, and Detector Dogs: From Science to Scene; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Sasamoto, K.; Ochiai, N.; Heim, J.; Libarondi, M. Trace-Level Organochlorine and Organophosphorus Pesticides Analysis by SBSE–GC–TOFMS and SBSE–GCxGC–TOFMS. LCGC Suppl. 2008, 31–35. Available online: https://www.chromatographyonline.com/view/trace-level-organochlorine-and-organophosphorus-pesticides-analysis-sbse-gc-tofms-and-sbse-gcxgc-t-0 (accessed on 30 September 2023).
- Kim, S.-J.; Choi, S.-J.; Jang, J.-S.; Kim, N.-H.; Hakim, M.; Tuller, H.L.; Kim, I.-D. Mesoporous WO3 Nanofibers with Protein-Templated Nanoscale Catalysts for Detection of Trace Biomarkers in Exhaled Breath. ACS Nano 2016, 10, 5891–5899. [Google Scholar] [CrossRef]



| Air Volume (L) | 1 | 10 | 10 | 10 | 50 | 100 |
|---|---|---|---|---|---|---|
| Analyte Volume (µL) | 0.2 | 1.0 | 0.4 | 0.2 | 0.2 | 0.2 |
| α-Terpineol | 34.6 | 17.3 | 6.9 | 3.5 | 0.7 | 0.3 |
| 2-Heptanone | 45.2 | 22.6 | 9.0 | 4.5 | 0.9 | 0.5 |
| 2-Pentanone | 29.7 | 14.8 | 5.9 | 3.0 | 0.6 | 0.3 |
| 4-Methylheptane | 65.5 | 32.8 | 13.1 | 6.6 | 1.3 | 0.7 |
| Acetone | 91.6 | 45.8 | 18.3 | 9.2 | 1.8 | 0.9 |
| Acetonitrile | 45.7 | 22.8 | 9.1 | 4.6 | 0.9 | 0.5 |
| Bromobenzene | 16.0 | 8.0 | 3.2 | 1.6 | 0.3 | 0.2 |
| DMDS | 53.4 | 26.7 | 10.7 | 5.3 | 1.1 | 0.5 |
| DMTS | 45.6 | 22.8 | 9.1 | 4.6 | 0.9 | 0.5 |
| Dioctyl ether | 30.7 | 15.4 | 6.1 | 3.1 | 0.6 | 0.3 |
| Estragole | 33.8 | 16.9 | 6.8 | 3.4 | 0.7 | 0.3 |
| Ethylcyclohexane | 29.0 | 14.5 | 5.8 | 2.9 | 0.6 | 0.3 |
| Methanol | 118.8 | 59.4 | 23.8 | 11.9 | 2.4 | 1.2 |
| Toluene | 45.2 | 22.6 | 9.0 | 4.5 | 0.9 | 0.5 |
| Sensor Number (Air Dilution) | Sensor # (Field Trial) | Sensor Type | Target Chemical Class |
|---|---|---|---|
| 1 | 1 | TGS 2610 | Alcohols, alkanes, and hydrogen |
| 2 | 3 | TGS 2600 | Alcohols, alkanes, and hydrogen |
| 4, 7 | 2 | TGS 2602 | Alcohols, hydrogen, sulfur, nitrogen, and toluene |
| 5, 6 | 5 | TGS 2603 | Alcohols, hydrogen, sulfur, and nitrogen |
| 8 | 4 | TGS 2612 | Alkanes |
| Parameter | Time (s) | Phase |
|---|---|---|
| Chamber Wash I | 300 | Pre-conditioning |
| Vacuum Time I | 10 | |
| Baseline Setup | 60 | |
| Vacuum Time II | 10 | Sampling |
| Sampling Time | 60 | |
| Baseline Recovery | 60 | Recovery and cleaning |
| Chamber Wash II | 300 |
| Donor Number | Age | Sex | Cause of Death (CoD) |
|---|---|---|---|
| D1 | 33 | Female | Coroner’s case |
| D2 | 94 | Female | Aspiration Pneumonia, Cerebral Infarct—Left Posterior, Inferior |
| D3 | 88 | Female | Bronchiectasis, Aspiration Pneumonia |
| D4 | 89 | Female | Large Bowel Obstruction, Sigmoid Colon Cancer, Heart Failure |
| D5 | 77 | Male | Multiple Organ Shutdown, Pancreatic Cancer, Liver Metastases |
| D6 | 67 | Male | Cardiac arrest due to acute myocardial infarction, Diabetes Mellitus—Type 2, Hypertension |
| Compound | Model | Average R2 of All Sensors |
|---|---|---|
| α-Terpineol | Log and linear | 0.8 |
| 2-Heptanone | Log | 0.82 |
| 2-Pentanone | Log | 0.67 |
| 4-Methylheptane | Log and linear | 0.88 |
| Acetone | Log and linear | 0.97 |
| ACN | Log and linear | 0.87 |
| Bromobenzene | Log and linear | 0.66 |
| Dioctyl ether | Log and linear | 0.09 |
| DMDS | Log and linear | 0.69 |
| DMTS | Log and linear | 0.88 |
| Estragole | Log and linear | 0.50 |
| Ethylcyclohexane | Log | 0.62 |
| Methanol | Log and linear | 0.90 |
| Toluene | Log and linear | 0.77 |
| Sensor Number | Sensor Type | Lowest LOD | Number of Analytes | Classes Reacted to |
|---|---|---|---|---|
| 1 | TGS 2610 | 2.0 | 14 | Alcohols, ethers, halogens, hydrocarbons, ketones, nitrogen, and sulfur |
| 2 | TGS 2600 | 1.3 | 14 | |
| 4 | TGS 2602 | 1.3 | 11 | |
| 7 | TGS 2602 | 0.6 | 10 | |
| 5 | TGS 2603 | 10.4 | 6 | Hydrocarbons, alcohols, sulfur, halogens, nitrogen |
| 6 | TGS 2603 | 10.9 | 7 |
| Compound | Class | LOD (ppm) | Sensor Number | Target Chemical Class |
|---|---|---|---|---|
| α-Terpineol | Alcohols | 67.3 | 4 | OH, S, N, Aromatics |
| Methanol | 1.6 | 2 | OH, CH | |
| Dioctyl ether | Ethers | 10.8 | 2 | OH, CH |
| Estragole | 4.4 | 7 | OH, S, N, Aromatics | |
| Bromobenzene | Halogen | 2.4 | 2 | OH, CH |
| 4-Methylheptane | Hydrocarbons | 0.6 | 7 | OH, S, N, Aromatics |
| Ethylcyclohexane | 3.0 | 2 | OH, CH | |
| Toluene | 2.5 | 2 | OH, CH | |
| 2-Heptanone | Ketones | 1.3 | 2 | OH, S, N, Aromatics |
| 2-Pentanone | 3.6 | 2 | OH, S, N, Aromatics | |
| Acetone | 8.0 | 1 | OH, S, N, Aromatics | |
| ACN | Nitrogen-containing | 1.9 | 2 | OH, CH |
| DMDS | Sulfur-containing | 2.5 | 1 | OH, S, N, Aromatics |
| DMTS | 1.0 | 7 | OH, S, N, Aromatics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunnucks, E.J.; Thurn, B.; Brown, A.O.; Zhang, W.; Liu, T.; Forbes, S.L.; Su, S.; Ueland, M. Performance of a Novel Electronic Nose for the Detection of Volatile Organic Compounds Relating to Starvation or Human Decomposition Post-Mass Disaster. Sensors 2024, 24, 5918. https://doi.org/10.3390/s24185918
Sunnucks EJ, Thurn B, Brown AO, Zhang W, Liu T, Forbes SL, Su S, Ueland M. Performance of a Novel Electronic Nose for the Detection of Volatile Organic Compounds Relating to Starvation or Human Decomposition Post-Mass Disaster. Sensors. 2024; 24(18):5918. https://doi.org/10.3390/s24185918
Chicago/Turabian StyleSunnucks, Emily J., Bridget Thurn, Amber O. Brown, Wentian Zhang, Taoping Liu, Shari L. Forbes, Steven Su, and Maiken Ueland. 2024. "Performance of a Novel Electronic Nose for the Detection of Volatile Organic Compounds Relating to Starvation or Human Decomposition Post-Mass Disaster" Sensors 24, no. 18: 5918. https://doi.org/10.3390/s24185918








