Clinical Utility of Synthesized 18-Lead Electrocardiography
Abstract
:1. Introduction
1.1. Aim of This Review
1.2. Methods
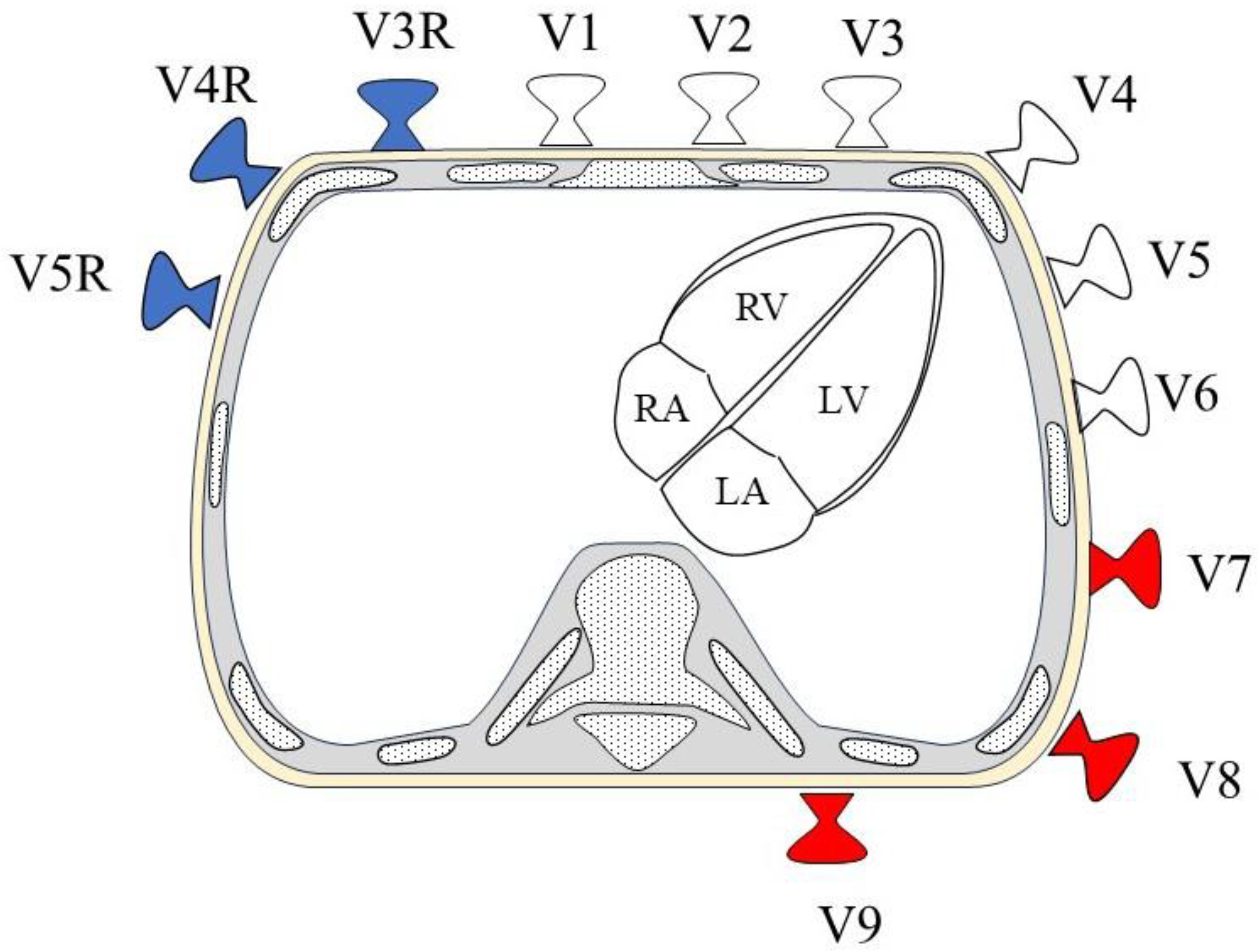
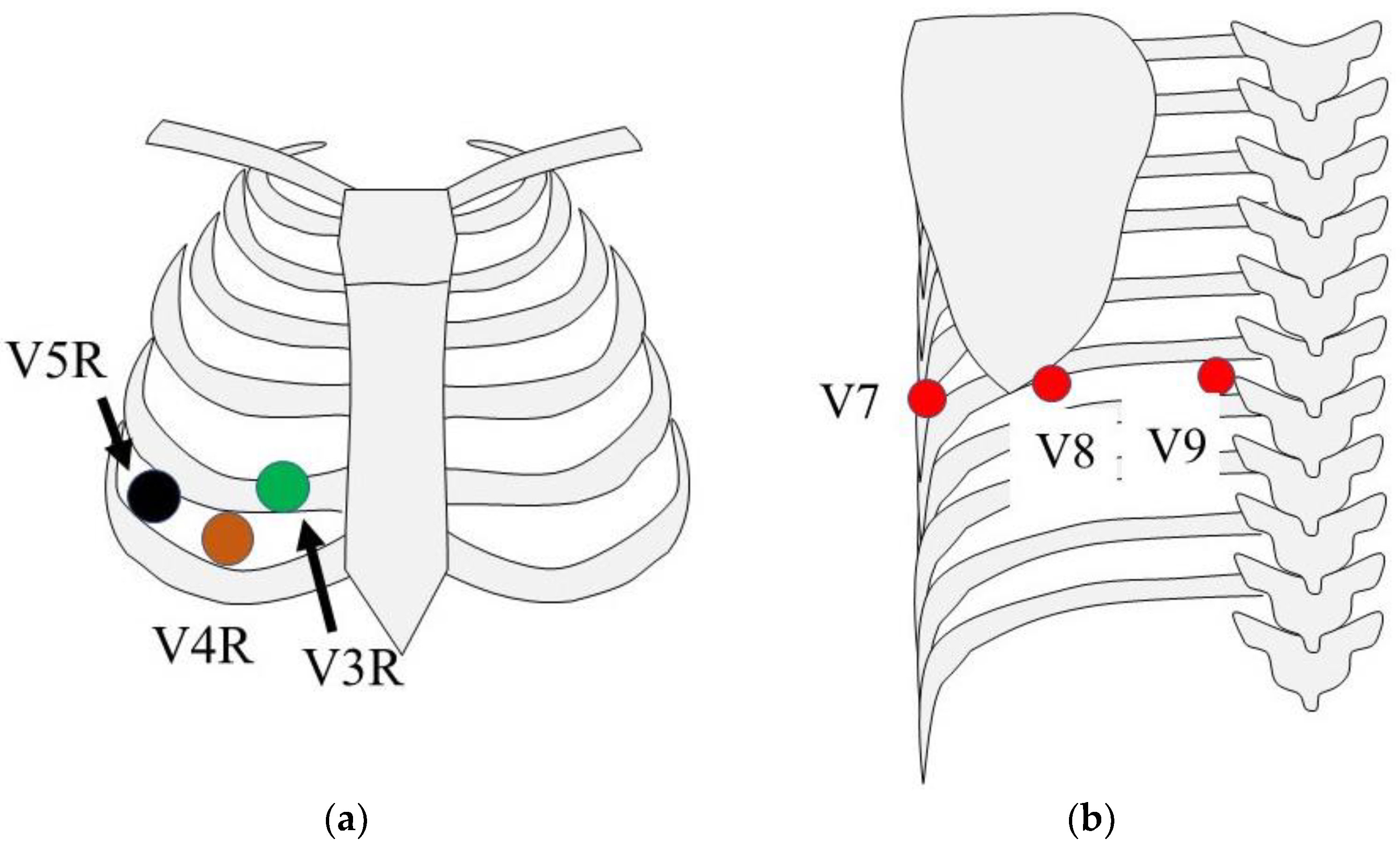
1.3. 18-Lead Electrocardiography
1.4. Syn18-ECG
2. Syn18-ECG and Actual 18-ECG Are Equivalent
3. Acute Coronary Syndromes
4. Arrhythmias
5. Acute Pulmonary Embolism
6. Duchenne Muscular Dystrophy
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef] [PubMed]
- Daming, W. Derived Electrocardiograms on the Posterior Leads from 12-Lead System: Method and Evaluation. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No.03CH37439), Cancun, Mexico, 17–21 September 2003; Volume 1, pp. 74–77. [Google Scholar]
- Nakano, M.; Ueda, M.; Ishimura, M.; Kajiyama, T.; Hashiguchi, N.; Kanaeda, T.; Kondo, Y.; Hiranuma, Y.; Kobayashi, Y. Estimation of the origin of ventricular outflow tract arrhythmia using synthesized right-sided chest leads. Europace 2014, 16, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Ueno, A.; Tanaka, K.; Suto, J.; Wei, D. Clinical significance of synthesized posterior/right-sided chest lead electrocardiograms in patients with acute chest pain. J. Nippon Med. Sch. Nippon Ika Daigaku Zasshi 2011, 78, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ashida, T.; Tani, S.; Nagao, K.; Yagi, T.; Matsumoto, N.; Hirayama, A. Usefulness of synthesized 18-lead electrocardiography in the diagnosis of ST-elevation myocardial infarction: A pilot study. Am. J. Emerg. Med. 2017, 35, 448–457. [Google Scholar] [CrossRef]
- Li, T.; Shinozaki, K.; Brave, M.; Yagi, T.; Becker, L.B. Agreement between actual and synthesized right-sided and posterior electrocardiographic leads in identifying ischemia. Am. J. Emerg. Med. 2020, 38, 1346–1351. [Google Scholar] [CrossRef]
- Takahashi, K.; Enomoto, D.; Morioka, H.; Uemura, S.; Okura, T. Identification of the Vessels Causing Myocardial Ischemia by a Synthesized 18-Lead Electrocardiogram Obtained After the Master Two-Step Exercise Test in a Patient With Effort Angina. Cureus 2023, 15, e47840. [Google Scholar] [CrossRef]
- Nakano, M.; Kondo, Y.; Kajiyama, T.; Miyazawa, K.; Nakano, M.; Hayashi, T.; Ito, R.; Takahira, H.; Kitagawa, M.; Kobayashi, Y. Estimation of the accessory pathway location of the manifest Wolf-Parkinson-White syndrome using synthesized right-sided chest leads. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2020, 59, 43–48. [Google Scholar] [CrossRef]
- Hisazaki, K.; Miyazaki, S.; Hasegawa, K.; Kaseno, K.; Amaya, N.; Shiomi, Y.; Tama, N.; Ikeda, H.; Fukuoka, Y.; Morishita, T.; et al. The P wave morphology in lead V7 on the synthesized 18-lead ECG is a useful parameter for identifying arrhythmias originating from the right inferior pulmonary vein. Heart Vessel. 2020, 35, 246–251. [Google Scholar] [CrossRef]
- Igarashi, M.; Nogami, A.; Sekiguchi, Y.; Kuroki, K.; Yamasaki, H.; Machino, T.; Yui, Y.; Ogawa, K.; Talib, A.K.; Murakoshi, N.; et al. The QRS morphology pattern in V5R is a novel and simple parameter for differentiating the origin of idiopathic outflow tract ventricular arrhythmias. Europace 2015, 17, 1107–1116. [Google Scholar] [CrossRef]
- Kusayama, T.; Furusho, H.; Kinoshita, M.; Kaneko, S.; Usuda, K.; Takamura, M. Characteristics of synthesized right-sided chest electrocardiograms in patients with acute pulmonary embolism. J. Cardiol. 2019, 73, 313–317. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nambu, Y.; Bo, R.; Morichi, S.; Yanagiya, M.; Matsuo, M.; Awano, H. Electrocardiographic R wave amplitude in V6 lead as a predictive marker of cardiac dysfunction in Duchenne muscular dystrophy. J. Cardiol. 2023, 82, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Konishi, S.; Ohtani, T.; Mizuno, H.; Sera, F.; Nakamoto, K.; Chimura, M.; Sengoku, K.; Miyawaki, H.; Higuchi, R.; Kanzaki, M.; et al. Simple Electrocardiographic Score Can Predict Left Ventricular Reverse Remodeling in Patients With Non-Ischemic Cardiomyopathy. Circ. Rep. 2019, 1, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Torigoe, K.; Goto, Y.; Naono, S.; Shinozaki, K.; Zaizen, H.; Takahashi, N. Reliability of ST-segment shifts in the synthesized V3R-V5R leads after coronary balloon inflations during percutaneous coronary intervention. Am. J. Cardiol. 2014, 114, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, M.; Ishikawa, T.; Morita, S.; Ebina, T.; Hibi, K.; Maejima, N.; Umemura, S.; Kimura, K. Posterior wall involvement attenuates predictive value of ST-segment elevation in lead V4R for right ventricular involvement in inferior acute myocardial infarction. J. Cardiol. 2009, 54, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Melendez, L.J.; Jones, D.T.; Salcedo, J.R. Usefulness of three additional electrocardiographic chest leads (V7, V8, and V9) in the diagnosis of acute myocardial infarction. Can. Med. Assoc. J. 1978, 119, 745–748. [Google Scholar]
- Boden, W.E.; Kleiger, R.E.; Gibson, R.S.; Schwartz, D.J.; Schechtman, K.B.; Capone, R.J.; Roberts, R. Electrocardiographic evolution of posterior acute myocardial infarction: Importance of early precordial ST-segment depression. Am. J. Cardiol. 1987, 59, 782–787. [Google Scholar] [CrossRef]
- Rich, M.W.; Imburgia, M.; King, T.R.; Fischer, K.C.; Kovach, K.L. Electrocardiographic diagnosis of remote posterior wall myocardial infarction using unipolar posterior lead V9. Chest 1989, 96, 489–493. [Google Scholar] [CrossRef]
- Oraii, S.; Maleki, M.; Tavakolian, A.A.; Eftekharzadeh, M.; Kamangar, F.; Mirhaji, P. Prevalence and outcome of ST-segment elevation in posterior electrocardiographic leads during acute myocardial infarction. J. Electrocardiol. 1999, 32, 275–278. [Google Scholar] [CrossRef]
- Brady, W.J.; Hwang, V.; Sullivan, R.; Chang, N.; Beagle, C.; Carter, C.T.; Martin, M.L.; Aufderheide, T.P. A comparison of 12- and 15-lead ECGS in ED chest pain patients: Impact on diagnosis, therapy, and disposition. Am. J. Emerg. Med. 2000, 18, 239–243. [Google Scholar] [CrossRef]
- Aqel, R.A.; Hage, F.G.; Ellipeddi, P.; Blackmon, L.; McElderry, H.T.; Kay, G.N.; Plumb, V.; Iskandrian, A.E. Usefulness of three posterior chest leads for the detection of posterior wall acute myocardial infarction. Am. J. Cardiol. 2009, 103, 159–164. [Google Scholar] [CrossRef]
- Sinha, N.; Ahuja, R.C.; Saran, R.K.; Jain, G.C. Clinical correlates of acute right ventricular infarction in acute inferior myocardial infarction. Int. J. Cardiol. 1989, 24, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Fijewski, T.R.; Pollack, M.L.; Chan, T.C.; Brady, W.J. Electrocardiographic manifestations: Right ventricular infarction. J. Emerg. Med. 2002, 22, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, N.; Pollack, M.L.; Chan, T.C.; Brady, W.J.; Harrigan, R.A. Electrocardiographic manifestations: Acute inferior wall myocardial infarction. J. Emerg. Med. 2004, 26, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.E.; Park, H.C.; Park, J.S.; Nagamoto, Y.; Choi, J.I.; Lim, H.E.; Park, S.W.; Kim, Y.H. Electrocardiographic and electrophysiological characteristics of premature ventricular complexes associated with left ventricular dysfunction in patients without structural heart disease. Europace 2013, 15, 735–741. [Google Scholar] [CrossRef]
- Yokokawa, M.; Good, E.; Crawford, T.; Chugh, A.; Pelosi, F., Jr.; Latchamsetty, R.; Jongnarangsin, K.; Armstrong, W.; Ghanbari, H.; Oral, H.; et al. Recovery from left ventricular dysfunction after ablation of frequent premature ventricular complexes. Heart Rhythm 2013, 10, 172–175. [Google Scholar] [CrossRef]
- Redfearn, D.P.; Hill, J.D.; Keal, R.; Toff, W.D.; Stafford, P.J. Left ventricular dysfunction resulting from frequent unifocal ventricular ectopics with resolution following radiofrequency ablation. Europace 2003, 5, 247–250. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. J. Arrhythm. 2017, 33, 369–409. [Google Scholar] [CrossRef]
- Yamane, T.; Shah, D.C.; Peng, J.T.; Jaïs, P.; Hocini, M.; Deisenhofer, I.; Choi, K.J.; Macle, L.; Clémenty, J.; Haïssaguerre, M. Morphological characteristics of P waves during selective pulmonary vein pacing. J. Am. Coll. Cardiol. 2001, 38, 1505–1510. [Google Scholar] [CrossRef]
- Kuo, J.Y.; Tai, C.T.; Tsao, H.M.; Hsieh, M.H.; Tsai, C.F.; Lin, W.S.; Lin, Y.K.; Ding, Y.A.; Hou, C.J.; Tsai, C.H.; et al. P wave polarities of an arrhythmogenic focus in patients with paroxysmal atrial fibrillation originating from superior vena cava or right superior pulmonary vein. J. Cardiovasc. Electrophysiol. 2003, 14, 350–357. [Google Scholar] [CrossRef]
- Kistler, P.M.; Roberts-Thomson, K.C.; Haqqani, H.M.; Fynn, S.P.; Singarayar, S.; Vohra, J.K.; Morton, J.B.; Sparks, P.B.; Kalman, J.M. P-wave morphology in focal atrial tachycardia: Development of an algorithm to predict the anatomic site of origin. J. Am. Coll. Cardiol. 2006, 48, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Kukla, P.; Długopolski, R.; Krupa, E.; Furtak, R.; Szełemej, R.; Mirek-Bryniarska, E.; Jastrzębski, M.; Nowak, J.; Wańczura, P.; Bryniarski, L. Electrocardiography and prognosis of patients with acute pulmonary embolism. Cardiol. J. 2011, 18, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Dalen, J.E.; McIntyre, K.M.; Sasahara, A.A.; Wenger, N.K.; Willis, P.W., 3rd. The electrocardiogram in acute pulmonary embolism. Prog. Cardiovasc. Dis. 1975, 17, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Norwood, F.L.; Harling, C.; Chinnery, P.F.; Eagle, M.; Bushby, K.; Straub, V. Prevalence of genetic muscle disease in Northern England: In-depth analysis of a muscle clinic population. Brain 2009, 132 Pt 11, 3175–3186. [Google Scholar] [CrossRef]
- Passamano, L.; Taglia, A.; Palladino, A.; Viggiano, E.; D’Ambrosio, P.; Scutifero, M.; Rosaria Cecio, M.; Torre, V.; De Luca, F.; Picillo, E.; et al. Improvement of survival in Duchenne Muscular Dystrophy: Retrospective analysis of 835 patients. Acta Myol. 2012, 31, 121–125. [Google Scholar]
- Rall, S.; Grimm, T. Survival in Duchenne muscular dystrophy. Acta Myologica 2012, 31, 117–120. [Google Scholar]
- Ishikawa, Y.; Miura, T.; Ishikawa, Y.; Aoyagi, T.; Ogata, H.; Hamada, S.; Minami, R. Duchenne muscular dystrophy: Survival by cardio-respiratory interventions. Neuromuscular disorders 2011, 21, 47–51. [Google Scholar] [CrossRef]
- Nigro, G.; Comi, L.I.; Politano, L.; Bain, R.J. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int. J. Cardiol. 1990, 26, 271–277. [Google Scholar] [CrossRef]
- Yamamoto, T.; Awano, H.; Zhang, Z.; Sakuma, M.; Kitaaki, S.; Matsumoto, M.; Nagai, M.; Sato, I.; Imanishi, T.; Hayashi, N.; et al. Cardiac Dysfunction in Duchenne Muscular Dystrophy Is Less Frequent in Patients With Mutations in the Dystrophin Dp116 Coding Region Than in Other Regions. Circ. Genom. Precis. Med. 2018, 11, e001782. [Google Scholar] [CrossRef]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Tang, L.; Shao, S.; Wang, C. Electrocardiographic features of children with Duchenne muscular dystrophy. Orphanet J. Rare Dis. 2022, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Ergul, Y.; Ekici, B.; Nisli, K.; Tatli, B.; Binboga, F.; Acar, G.; Ozmen, M.; Omeroglu, R.E. Evaluation of the North Star Ambulatory Assessment scale and cardiac abnormalities in ambulant boys with Duchenne muscular dystrophy. J. Paediatr. Child Health 2012, 48, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Takami, Y.; Takeshima, Y.; Awano, H.; Okizuka, Y.; Yagi, M.; Matsuo, M. High incidence of electrocardiogram abnormalities in young patients with duchenne muscular dystrophy. Pediatr. Neurol. 2008, 39, 399–403. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Kinnett, K.; Wang, Y.; Ittenbach, R.F.; Benson, D.W.; Cripe, L. Electrocardiographic abnormalities in very young Duchenne muscular dystrophy patients precede the onset of cardiac dysfunction. Neuromuscular disorders 2011, 21, 462–467. [Google Scholar] [CrossRef]
- Mori, K.; Hayabuchi, Y.; Inoue, M.; Suzuki, M.; Sakata, M.; Nakagawa, R.; Kagami, S.; Tatara, K.; Hirayama, Y.; Abe, Y. Myocardial strain imaging for early detection of cardiac involvement in patients with Duchenne’s progressive muscular dystrophy. Echocardiography 2007, 24, 598–608. [Google Scholar] [CrossRef]
- Ogata, H.; Nakatani, S.; Ishikawa, Y.; Negishi, A.; Kobayashi, M.; Ishikawa, Y.; Minami, R. Myocardial strain changes in Duchenne muscular dystrophy without overt cardiomyopathy. Int. J. Cardiol. 2007, 115, 190–195. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tanaka, H.; Matsumoto, K.; Lee, T.; Awano, H.; Yagi, M.; Imanishi, T.; Hayashi, N.; Takeshima, Y.; Kawai, H.; et al. Utility of transmural myocardial strain profile for prediction of early left ventricular dysfunction in patients with Duchenne muscular dystrophy. Am. J. Cardiol. 2013, 111, 902–907. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tanaka, H.; Takeshima, Y.; Hayashi, N.; Hirata, K.; Kawano, S. Combining passive leg-lifting with transmural myocardial strain profile for enhanced predictive capability for subclinical left ventricular dysfunction in Duchenne muscular dystrophy. J. Cardiol. 2015, 66, 212–217. [Google Scholar] [CrossRef]
- Kamdar, F.; Garry, D.J. Dystrophin-Deficient Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2533–2546. [Google Scholar] [CrossRef]
| 12-ECG | 18-ECG | |
|---|---|---|
| Additional chest electrode | V3R–V5R and V7–V9 | |
| Chest electrode for evaluation of the right ventricle | V1–V2 | In addition to left, V3R–V5R |
| Chest electrode for evaluation of the left ventricular posterior | Nothing | V7–V9 |
| Actual 18-ECG | syn18-ECG | |
|---|---|---|
| Reattachment | Necessary | Not necessary |
| Lateral decubitus | Necessary | Not necessary |
| Additional recording time after 12-ECG recording | Necessary | Not necessary |
| Electrocardiograph used | Most common electrocardiograph | Dedicated special electrocardiograph |
| Waveform comparison | Measured waveform | Synthesized waveform |
| 12-ECG | syn18-ECG | |
|---|---|---|
| Diagnosis of acute coronary syndrome | ||
| Right ventricular | Little ST elevation | ST elevation in V3R–V5R |
| Left ventricular posterior | Little ST elevation | ST elevation in V7–V9 |
| Diagnosis of arrhythmia | ||
| Accessory pathway in the septum of the WPW | Difficulty | QS or Qr in V4R |
| Atrial tachycardia of right inferior pulmonary vein origin | Difficulty | Isoelectric P wave in V7 |
| Others | ||
| Diagnosis of acute pulmonary embolism | Low sensitivity and nonspecificity | Negative T wave in V3R |
| Cardiac dysfunction in Duchenne muscular dystrophy | Not related | Sharp decrease R amplitude in V7–V9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, T.; Awano, H.; Ogawa, S.; Matsuo, M. Clinical Utility of Synthesized 18-Lead Electrocardiography. Sensors 2024, 24, 5947. https://doi.org/10.3390/s24185947
Yamamoto T, Awano H, Ogawa S, Matsuo M. Clinical Utility of Synthesized 18-Lead Electrocardiography. Sensors. 2024; 24(18):5947. https://doi.org/10.3390/s24185947
Chicago/Turabian StyleYamamoto, Tetsushi, Hiroyuki Awano, Shuichiro Ogawa, and Masafumi Matsuo. 2024. "Clinical Utility of Synthesized 18-Lead Electrocardiography" Sensors 24, no. 18: 5947. https://doi.org/10.3390/s24185947







