First-Principles Investigation on Ru-Doped Janus WSSe Monolayer for Adsorption of Dissolved Gases in Transformer Oil: A Novel Sensing Candidate Exploration
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Analysis of Ru-WSSe Monolayer
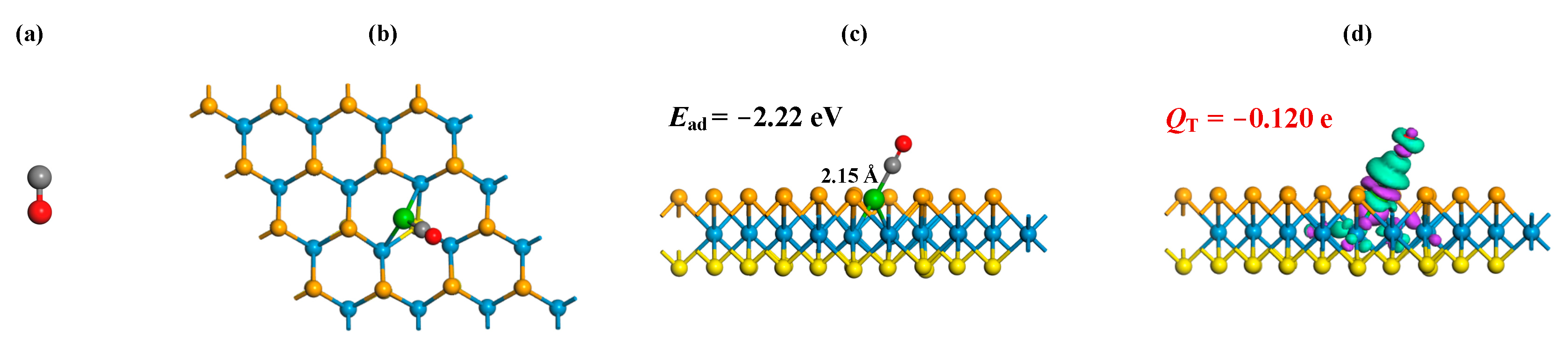
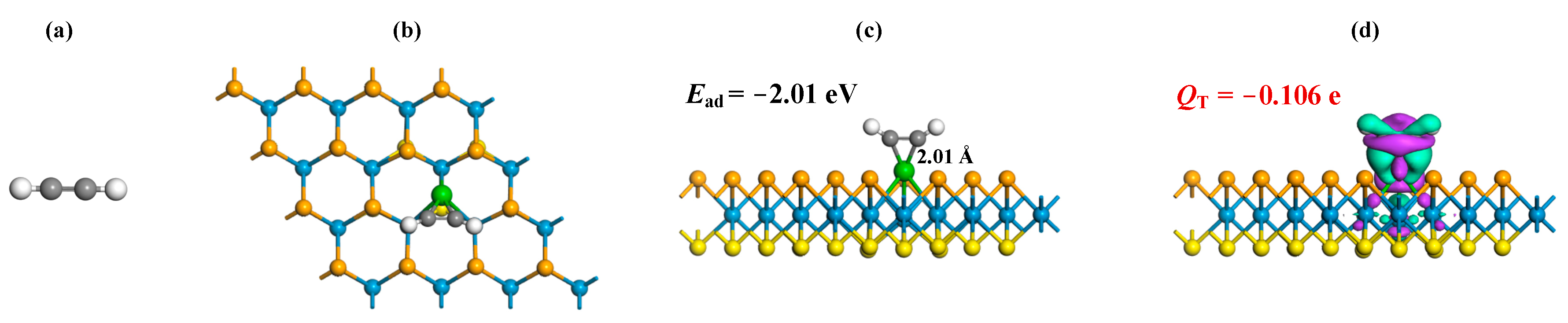
3.2. Gas Adsorptions on Ru-WSSe Monolayer
3.3. Analysis of Electronic Property in Gas Adsorbed Ru-WSSe Systems
3.4. Resistive Gas Sensing Exploration
3.5. Gas Sensing and Operation Mechanisms
4. Conclusions
- (I)
- The Ru dopant is found to preferentially substitute a Se atom within the WSSe monolayer, with an Eform of 0.01 eV, indicating a highly favorable and energetically stable doping process;
- (II)
- The Ru-WSSe monolayer exhibits chemisorption towards the three gas species, with the Ead following the order: CO (−2.22 eV) > C2H2 (−2.01 eV) > C2H4 (−1.70 eV);
- (III)
- The adsorption of these gases significantly increases the energy gap of Ru-WSSe monolayer from its initial value of 0.330 eV to 1.184 eV for CO, 0.723 eV for C2H2, and 0.565 eV for C2H4, respectively, as elucidated by the FMO theory;
- (IV)
- The impact of the O2 molecule on the gas adsorption and sensing performance of the Ru-WSSe monolayer with the three typical gas species is analyzed, revealing a weakened but still remarkable sensing response in the three gas systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, H.; Zhang, X.; Zhang, G.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Liao, R.; Zheng, H.; Grzybowski, S.; Yang, L.; Zhang, Y.; Liao, Y. An Integrated Decision-Making Model for Condition Assessment of Power Transformers Using Fuzzy Approach and Evidential Reasoning. IEEE Trans. Power Deliv. 2011, 26, 1111–1118. [Google Scholar] [CrossRef]
- He, X.; Gui, Y.; Xie, J.; Liu, X.; Wang, Q.; Tang, C. A DFT study of dissolved gas (C2H2, H2, CH4) detection in oil on CuO-modified BNNT. Appl. Surf. Sci. 2020, 500, 144030. [Google Scholar] [CrossRef]
- Yang, F.; Jung, D.; Penner, R.M. Trace Detection of Dissolved Hydrogen Gas in Oil Using a Palladium Nanowire Array. Anal. Chem. 2011, 83, 9472–9477. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.; Westerwaal, R.J.; Slaman, M.; Schreuders, H.; Van Vugt, A.W.; Victoria, M.; Boelsma, C.; Dam, B. Optical fiber sensor for the continuous monitoring of hydrogen in oil. Sens. Actuators B Chem. 2014, 190, 982–989. [Google Scholar] [CrossRef]
- Samsudin, M.; Shee, Y.; Adikan, F.M.; Dahari, M. Fiber Bragg Gratings (FBG) Hydrogen Sensor for Transformer Oil Degradation Monitoring. IEEE Sens. J. 2016, 16, 2993–2999. [Google Scholar] [CrossRef]
- Ding, J.; Li, X.; Cao, J.; Sheng, L.; Yin, L.; Xu, X. New sensor for gases dissolved in transformer oil based on solid oxide fuel cell. Sens. Actuators B Chem. 2014, 202, 232–239. [Google Scholar] [CrossRef]
- Gui, Y.; Peng, X.; Liu, K.; Ding, Z. Adsorption of C2H2, CH4 and CO on Mn-doped graphene: Atomic, electronic, and gas-sensing properties. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 119, 113959. [Google Scholar] [CrossRef]
- Ma, G.M.; Li, C.R.; Luo, Y.T.; Mu, R.D.; Wang, L. High sensitive and reliable fiber Bragg grating hydrogen sensor for fault detection of power transformer. Sens. Actuators B Chem. 2012, 169, 195–198. [Google Scholar] [CrossRef]
- Singh, S.; Bandyopadhyay, M.N. Dissolved gas analysis technique for incipient fault diagnosis in power transformers: A bibliographic survey. IEEE Electr. Insul. Mag. 2010, 26, 41–46. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, Y.; Lu, D.; Zhang, J.; Zeng, W.; Zhou, Q. DFT study on adsorption of dissolved gas molecules in the transformer oil on Rh-doped MoTe2 monolayer. Mol. Phys. 2024, 122, e2287127. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Cheng, H.; Tang, J. Ladder-wise calculation method for z-coordinate of transformer PD source based on planar layout UHF antenna sensors. IEEJ Trans. Electr. Electron. Eng. 2019, 15, 340–345. [Google Scholar] [CrossRef]
- Benounis, M.; Aka-Ngnui, T.; Jaffrezic, N.; Dutasta, J.P. NIR and optical fiber sensor for gases detection produced by transformation oil degradation. Sens. Actuators A Phys. 2008, 141, 76–83. [Google Scholar] [CrossRef]
- Kazemi, A.; Rodner, M.; Fadavieslam, M.R.; Kaushik, P.D.; Ivanov, I.G.; Eriksson, J.; Syväjärvi, M.; Yakimova, R.; Yazdi, G.R. The effect of Cl- and N-doped MoS2 and WS2 coated on epitaxial graphene in gas-sensing applications. Surf. Interfaces 2021, 25, 101200. [Google Scholar] [CrossRef]
- Sharma, A.; Anu; Khan, M.S.; Husain, M.; Khan, S.; Srivastava, A. Sensing of CO and NO on Cu-doped MoS2 Monolayer Based Single Electron Transistor: A First Principles Study. IEEE Sens. J. 2018, 18, 2853–2860. [Google Scholar] [CrossRef]
- Cui, H.; Chen, D.; Zhang, Y.; Zhang, X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first-principles theory. Sustain. Mater. Technol. 2019, 20, e00094. [Google Scholar] [CrossRef]
- Cui, H.; Jia, P.; Peng, X. Adsorption of SO2 and NO2 molecule on intrinsic and Pd-doped HfSe2 monolayer: A first-principles study. Appl. Surf. Sci. 2020, 513, 145863. [Google Scholar] [CrossRef]
- Sun, Y.C.; Kim, Y.; Chung, H.S.; Kim, A.R.; Kwon, J.D.; Park, J.; Kim, Y.L.; Kwon, S.H.; Hahm, M.G.; Cho, B. Effect of Nb Doping on Chemical Sensing Performance of Two-Dimensional Layered MoSe2. Acs Appl. Mater. Interfaces 2017, 9, 3817–3823. [Google Scholar]
- Baek, D.H.; Kim, J. MoS2 gas sensor functionalized by Pd for the detection of hydrogen. Sens. Actuators B Chem. 2017, 250, 686–691. [Google Scholar] [CrossRef]
- Wang, D.; Lan, T.; Pan, J.; Liu, Z.; Yang, A.; Yang, M.; Chu, J.; Yuan, H.; Wang, X.; Li, Y.; et al. Janus MoSSe monolayer: A highly strain-sensitive gas sensing material to detect SF6 decompositions. Sens. Actuators A Phys. 2020, 311, 112049. [Google Scholar] [CrossRef]
- Pal Kaur, S.; Hussain, T.; Kumar, T.J.D. Substituted 2D Janus WSSe monolayers as efficient nanosensor toward toxic gases. J. Appl. Phys. 2021, 130, 014501. [Google Scholar] [CrossRef]
- Babariya, B.; Raval, D.; Gupta, S.K.; Gajjar, P.N. Selective and sensitive toxic gas-sensing mechanism in a 2D Janus MoSSe monolayer. Phys. Chem. Chem. Phys. 2022, 24, 15292–15304. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cui, Z.; Li, Q.; Ding, Y. Gas (CO and NO) adsorption and sensing based on transition metals functionalized Janus MoSSe. Appl. Surf. Sci. 2021, 565, 150509. [Google Scholar] [CrossRef]
- Guo, J.-X.; Wu, S.-Y.; Zhong, S.-Y.; Zhang, G.-J.; Shen, G.-Q.; Yu, X.-Y. Janus WSSe monolayer adsorbed with transition-metal atoms (Fe, Co and Ni): Excellent performance for gas sensing and CO catalytic oxidation. Appl. Surf. Sci. 2021, 565, 150558. [Google Scholar] [CrossRef]
- Peng, R.; Zeng, W.; Zhou, Q. Adsorption and gas sensing of dissolved gases in transformer oil onto Ru3-modified SnS2: A DFT study. Appl. Surf. Sci. 2023, 615, 156445. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Zhang, X. Theoretical screening into Ru-doped MoS2 monolayer as a promising gas sensor upon SO2 and SOF2 in SF6 insulation devices. Mol. Phys. 2021, e2018517. [Google Scholar] [CrossRef]
- Zhou, Q.; Ju, W.; Liu, Y.; Li, J.; Zhang, Q. Influence of defects and dopants on the sensitivity of arsenene towards HCN. Appl. Surf. Sci. 2020, 506, 144936. [Google Scholar] [CrossRef]
- Li, P.; Hong, Q.; Wu, T.; Cui, H. SOF2 sensing by Rh-doped PtS2 monolayer for early diagnosis of partial discharge in the SF6 insulation device. Mol. Phys. 2021, 119, e1919774. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, G.; Zhang, X.; Tang, J. Rh-doped MoSe2 as toxic gas scavenger: A first-principles study. Nanoscale Adv. 2019, 2019, 772–780. [Google Scholar] [CrossRef]
- Wu, D.; Lv, P.; Wu, J.; He, B.; Li, X.; Chu, K.; Jia, Y.; Ma, D. Catalytic active centers beyond transition metals: Atomically dispersed alkaline-earth metals for the electroreduction of nitrate to ammonia. J. Mater. Chem. A 2023, 11, 1817–1828. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Di Stasio, R.A., Jr.; Head-Gordon, M.; Scheffler, M. Dispersion-corrected Møller-Plesset second-order perturbation theory. J. Chem. Phys. 2009, 131, 171. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Ran, M.; Peng, X.; Zhang, G. First-principles design of noble metal (Rh and Pd) dispersed Janus WSTe monolayer for toxic gas sensing applications. J. Environ. Chem. Eng. 2024, 112047. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; James, D. Pack. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Gu, M.; Tao, L.; Dastan, D.; Dang, J.; Fang, T.; An, B. Metal-Modified C3N1 Monolayer Sensors for Battery Instability Monitoring. J. Mater. Chem. A, 2024, epub ahead of print.
- Zhai, S.; Jiang, X.; Wu, D.; Chen, L.; Su, Y.; Cui, H.; Wu, F. Single Rh atom decorated pristine and S-defected PdS2 monolayer for sensing thermal runaway gases in a lithium-ion battery: A first-principles study. Surf. Interfaces 2023, 37, 102735. [Google Scholar] [CrossRef]
- Zhu, H.; Cui, H.; He, D.; Cui, Z.; Wang, X. Rh-doped MoTe2 Monolayer as a Promising Candidate for Sensing and Scavenging SF6 Decomposed Species: A DFT Study. Nanoscale Res. Lett. 2020, 15, 129. [Google Scholar] [CrossRef]
- Pyykkö, P. M Atsumi. Molecular single-bond covalent radii for elements 1-118. Chemistry 2009, 15, 186–197. [Google Scholar] [CrossRef]
- Chaurasiya, R.; Dixit, A.; Pandey, R. Strain-mediated stability and electronic properties of WS2, Janus WSSe and WSe2 monolayers. Superlattices Microstruct. 2018, 122, 268–279. [Google Scholar] [CrossRef]
- Petrić, M.M.; Kremser, M.; Barbone, M.; Qin, Y.; Sayyad, Y.; Shen, Y.; Tongay, S.; Finley, J.J.; Botello-Méndez, A.R.; Müller, K. Raman spectrum of Janus transition metal dichalcogenide monolayers WSSe and MoSSe. Phys. Rev. B 2021, 103, 035414. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Liu, L.; Jia, Y. Electrocatalytic nitrogen reduction on the transition-metal dimer anchored N-doped graphene: Performance prediction and synergetic effect. Phys. Chem. Chem. Phys. 2021, 23, 4018–4029. [Google Scholar] [CrossRef]
- Chen, F.; Hong, C.; Jiang, J.; Zhang, Z.; Zhou, Q. A comparative DFT study on the adsorption properties of lithium batteries thermal runaway gases CO, CO2, CH4 and C2H4 on pristine and Au doped CdS monolayer. Surf. Interfaces 2024, 46, 104200. [Google Scholar] [CrossRef]
- Wu, H.; Fang, J.; Yuan, S.; Liu, Y.; Zeng, J.; Jiang, T. Adsorption and gas sensing properties of Cun and Pdn (n = 1–3) clusters modified MoSe2 for lithium battery thermal runaway gases. Appl. Surf. Sci. 2024, 648, 158963. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Li, L.; Sakthive, T.; Guo, Z.; Dai, Z. Asymmetric Bond Delta-Polarization at the Interfacial Se–Ru–O Bridge for Efficient pH-Robust Water Electrolysis. Adv. Funct. Mater. 2024, 2406587, early view. [Google Scholar] [CrossRef]
- Tantardini, C.; Oganov, A.R. Thermochemical electronegativities of the elements. Nat. Commun. 2021, 12, 2087. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, D.; Wang, D.; Deng, J.; Kong, D.; Zhang, H. Performance prediction of 2D vertically stacked MoS2-WS2 heterostructures base on first-principles theory and Pearson correlation coefficient. Appl. Surf. Sci. 2022, 596, 153498. [Google Scholar] [CrossRef]
- Cui, H.; Guo, Y.; Zhao, Q.; Zhang, G. Pd-doped PtSe2 monolayer with strain-modulated effect for sensing SF6 decomposed species: A first-principles study. J. Mater. Res. Technol. 2022, 18, 629–636. [Google Scholar] [CrossRef]
- Huang, J.; Chu, J.; Wang, Z.; Zhang, J.; Yang, A.; Li, X.; Gao, C.; Huang, H.; Wang, X.; Cheng, Y.; et al. Chemisorption of NO2 to MoS2 Nanostructures and its Effects for MoS2 Sensors. ChemNanoMat 2019, 5, 1123–1130. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Qiu, Y.; Zhu, J.; Zhang, Y.; Hu, G. A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption. Comput. Mater. Sci. 2017, 138, 255–266. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Gui, Y.; Hu, W. First-principles study of SF6 decomposed gas adsorbed on Au-decorated graphene. Appl. Surf. Sci. 2016, 367, 259–269. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Huang, L.; Zeng, W.; Zhou, Q. Anti-interference detection of mixed NOX via In2O3-based sensor array combining with neural network model at room temperature. J. Hazard. Mater. 2024, 463, 132857. [Google Scholar] [CrossRef]
- Cui, H.; Liu, T.; Zhang, Y.; Zhang, X. Ru-InN Monolayer as a Gas Scavenger to Guard the Operation Status of SF6 Insulation Devices: A First-Principles Theory. IEEE Sens. J. 2019, 19, 5249–5255. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Liu, C.; Wei, M.; Xu, X. CuO-Modified PtSe2 Monolayer as a Promising Sensing Candidate toward C2H2 and C2H4 in Oil-Immersed Transformers: A Density Functional Theory Study. ACS Omega 2022, 7, 45590–45597. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Chen, X.; Gui, Y. First-Principles Insight into a Ru-Doped SnS2 Monolayer as a Promising Biosensor for Exhale Gas Analysis. ACS Omega 2020, 5, 8919–8926. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhao, P.; Wang, L.; Zhu, L.; Wu, Z.; Zhang, D. Density Functional Theory Study of Adsorption of Dissolved Gas in Transformer Oil on a Metal (Ag, Pd, and Pt)-Doped NbSe2 Monolayer. ACS Appl. Nano Mater. 2023, 6, 5517–5526. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, J.; Zeng, W.; Zhou, Q. Adsorption and Sensing Performances of MoTe2 Monolayers Doped with Pd, Ni, and Pt for SO2 and NH3: A DFT Investigation. Langmuir 2023, 39, 4125–4139. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, H.; Gui, Y.; Long, Y.; Wang, Q.; Yang, P. First-principles calculations of gas-sensing properties of Pd clusters decorated AlNNTs to dissolved gases in transformer oil. IEEE Access 2020, 8, 162692–162700. [Google Scholar] [CrossRef]
- Li, F.; Chen, F.; Cui, H.; Jiang, X. Pristine and Ni-doped WTe2 monolayer for adsorption and sensing of C2H2 and C2H4 in oil-immersed transformers: A DFT study. Comput. Theor. Chem. 2023, 1226, 114187. [Google Scholar] [CrossRef]
- Srivastava, R.; Suman, H.; Shrivastava, S.; Srivastava, A. DFT analysis of pristine and functionalized zigzag CNT: A case of H2S sensing. Chem. Phys. Lett. 2019, 731, 136575. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X.; Zhang, Y. Fabrication of polypyrrole/Zn2SnO4 nanofilm for ultra-highly sensitive ammonia sensing application. Sens. Actuators B Chem. 2018, 274, 575–586. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Ma, R.; Ran, M.; Cui, H. First-Principles Investigation on Ru-Doped Janus WSSe Monolayer for Adsorption of Dissolved Gases in Transformer Oil: A Novel Sensing Candidate Exploration. Sensors 2024, 24, 5967. https://doi.org/10.3390/s24185967
Cao L, Ma R, Ran M, Cui H. First-Principles Investigation on Ru-Doped Janus WSSe Monolayer for Adsorption of Dissolved Gases in Transformer Oil: A Novel Sensing Candidate Exploration. Sensors. 2024; 24(18):5967. https://doi.org/10.3390/s24185967
Chicago/Turabian StyleCao, Liang, Ruilong Ma, Mingxin Ran, and Hao Cui. 2024. "First-Principles Investigation on Ru-Doped Janus WSSe Monolayer for Adsorption of Dissolved Gases in Transformer Oil: A Novel Sensing Candidate Exploration" Sensors 24, no. 18: 5967. https://doi.org/10.3390/s24185967





