Harnessing the Heart’s Magnetic Field for Advanced Diagnostic Techniques
Abstract
1. Introduction
2. Heart Magnetic Field Acquisition
- Hall Effect Sensors
- Principle: They are based on the Hall effect, which occurs when a current-carrying conductor or semiconductor is placed in a perpendicular magnetic field. This results in the generation of a voltage (the Hall voltage) perpendicular to both the current and the magnetic field.
- Applications: They are used in magnetic microscopy [41] and Biomedical Applications [42,43] for detecting magnetic nanoparticles (MNPs) labeled on a biomolecule, monitoring blood pulse wave velocity, characterizing soft biological materials, controlling the syringe injection rate and eye surgery by training systems, and assisting magnetic resonance imaging (MRI) [44].
- Magnetic Field and Frequency Ranges: The sensors may operate effectively in the range of to . The frequency range varies depending on the application from 1 Hz to 10 kHz.
- Distance from the Body: Hall effect sensors used in medical devices are often placed close to the body, typically within a few millimeters to a few centimeters, depending on the application.
- Benefits: Hall effect sensors provide a non-contact high-precision method of measurement, reducing the risk of contamination and wear. These sensors are robust and can operate in various conditions, ensuring consistent performance in medical environments. The small size of Hall effect sensors allows for them to be integrated into a wide range of medical devices, even those with space constraints.
- Limitations: These sensors are sensitive to temperature fluctuations and magnetic interference and have a limited detection range, low signal-to-noise ratio, and high power consumption if required to be used in a portable or wearable device.
- Requires Shielding: Yes, as it is sensitive to magnetic interference.
- Fluxgate Magnetometers
- Magnetic Field and Frequency Ranges: The sensors may operate effectively in the range of to . The frequency range of interest in cardiac magnetic field applications involving fluxgate magnetometers typically spans from 0.05 Hz to 100 Hz.
- Distance from the Body: The distance from the body at which a fluxgate magnetometer operates can vary depending on the specific application. However, it is common for these measurements to be taken at a distance of a few centimeters to several decimeters.
- Benefits: They are capable of detecting very weak magnetic fields, providing accurate measurements of both the magnitude and direction of magnetic fields, and offering a stable and reliable performance over long periods. New fluxgate magnetometers are more robust and significantly easier to handle, making them suitable for some portable applications.
- Limitations: Traditional fluxgate magnetometers can be bulky, which may limit their integration into portable or compact medical devices. They generally consume more power compared to other magnetic field sensors, which can be a drawback for battery-operated medical devices [50]. These sensors can be affected by external magnetic fields and the cost of manufacturing high-precision fluxgate magnetometers can be high.
- Requires Shielding: Yes, as these sensors are affected by the surrounding magnetic fields.
- Superconducting Quantum Interference Devices (SQUIDs)
- Principle: Introduced by Baule and McFee [36], they use superconducting loops containing Josephson junctions to detect extremely small changes in the magnetic flux. The development and refinement of the SQUIDs have significantly advanced the sensitivity and utility of MCG. SQUIDs can detect extremely faint magnetic fields, such as those generated by the heart, making them invaluable in clinical and research settings [51]. High-Tc SQUIDs are a type of Superconducting Quantum Interference Device that operate at relatively high critical temperatures (Tc), where the “high-Tc” refers to the material’s ability to become superconducting at temperatures significantly above those required by traditional superconductors [52]. While classical superconductors used in the first SQUIDs require cooling to temperatures close to absolute zero (typically below 10 Kelvin or −263.15 °C) using liquid helium, high-Tc superconductors can operate at higher temperatures, often above 77 Kelvin (−196 °C), which is the boiling point of liquid nitrogen. They still require cooling but can be made more portable than conventional low-temperature SQUIDs.
- Applications: They are used in medical imaging (MRI), magnetoencephalography (MEG), magnetocardiography (MCG), magnetogastrography (MGG), and the detection of magnetic nanoparticles (MNPs).
- Magnetic Field and Frequency Ranges: The sensors may operate effectively in the range of to . The frequency range of interest in cardiac magnetic field applications involving SQUIDs typically spans from 0.5 Hz to 40 Hz.
- Distance from the Body: SQUIDs are usually placed within a few centimeters of the body to minimize interference and maximize signal strength. This proximity allows for the accurate detection of magnetic fields generated by the brain or heart. SQUID sensors for MCG are positioned close to the chest, usually within a few centimeters.
- Benefits: SQUIDs can detect magnetic fields as small as a few femtoteslas, making them extremely sensitive and capable of picking up subtle physiological signals. Most applications of SQUIDs, such as MEG and MCG, are noninvasive, reducing the risk and discomfort for patients, and SQUIDs provide real-time data with a high temporal resolution, which is crucial for monitoring dynamic physiological processes.
- Limitations: SQUIDs require cooling to very low temperatures using liquid helium, which makes the systems complex and expensive to maintain, and operating SQUID systems requires specialized knowledge and training.
- Requires Shielding: Yes, as these sensors are affected by the surrounding magnetic fields.
- Optically Pumped Magnetometers
- Principle: They are highly sensitive devices used to measure magnetic fields [53]. They operate on the principle of optical pumping, a process that involves the use of light (usually from a laser) to excite electrons in a gas (commonly an alkali metal, such as rubidium, cesium, or potassium) to a higher energy state. The alignment or polarization of these atoms’ spins is then perturbed by external magnetic fields. By monitoring the change in the atoms’ spin states, the magnetic field strength can be deduced with high precision.
- Magnetic Field and Frequency Ranges: The sensors may operate effectively in the range of to . The frequency range of interest in cardiac magnetic field applications involving OPMs typically spans from 0.05 Hz to 150 Hz.
- Distance from the Body: OPMs can operate very close to the body, often within millimeters. This close proximity is advantageous because the magnetic field strength decreases with the square of the distance, so being closer to the heart improves the signal quality.
- Benefits: Optically Pumped Magnetometers offer significant potential in advancing medical diagnostics and research by providing highly sensitive, noninvasive, and cost-effective solutions for measuring biomagnetic fields. Their applications in neuroimaging, cardiac monitoring, muscle activity analysis, and medical imaging are paving the way for new diagnostic techniques and improved patient outcomes. Unlike SQUIDs, which require cryogenic temperatures, OPMs can operate at room temperature, simplifying their use and deployment in various settings with the possibility of creating wearable systems.
- Limitations: OPMs are highly sensitive to magnetic noise from the environment [57], involve complex technology, and can be costly to implement and maintain in clinical settings.
- Requires Shielding: Yes, as these sensors are affected by the surrounding magnetic fields.
- Induction Coil Magnetometers
- Principle: They are based on Faraday’s law of electromagnetic induction, where a changing magnetic field induces a voltage in a coil.
- Applications: Induction coil magnetometers are used to study biomagnetic fields generated by physiological processes in the human body. These magnetometers can measure the magnetic fields associated with brain activity. They can be employed to detect the weak magnetic fields generated by the electrical activity of the heart. This aids in studying cardiac functions and diagnosing heart conditions.
- Magnetic Field and Frequency Ranges: Sensors may operate effectively in the range of to . The frequency range of interest in cardiac magnetic field applications typically spans from 0.05 Hz to 150 Hz.
- Distance from the Body: The magnetometer is placed very close to the chest, often within a few centimeters.
- Benefits: These sensors provide a noninvasive means to study internal body processes. They can also detect extremely weak magnetic fields.
- Requires Shielding: Yes, as these sensors are affected by the surrounding magnetic fields.
- Magnetoresistive Sensors
- Principle: A well-known phenomenon is that the resistance of some material exhibits a change in value when in proximity of a magnetic field. A magnetoresistive sensor uses this property to measure the magnetic field of the heart [59].
- Applications: Magnetoresistive sensors, particularly those utilizing the Giant Magnetoresistance (GMR) effect, have shown significant promise for medical applications due to their unique properties and advantages [60] and show the application of Giant Magnetoresistance-based micro-probes to record biological magnetic fields. GMR sensors exhibit high sensitivity, making them suitable for detecting weak magnetic fields, which is crucial in medical applications, such as magnetic resonance imaging (MRI) machines [61] and magnetoencephalography (MEG) machines [62]. In [63], the authors considered using tunnel magnetoresistance (TMR) sensors that operate at room temperature to measure weak biomagnetic fields. In [64], the authors used hybrid sensors based on Giant Magnetoresistance (GMR) to capture the magnetic signatures of the heart’s electrical activity (magnetocardiography) in healthy volunteers. The P-wave and QRS complex was clearly visible after approximately 1 min of averaging.
- Magnetic Field and Frequency Ranges: The sensors may operate effectively in the range of to . Magnetoresistive sensors are capable of detecting signals across a wide frequency range, typically from near DC (0 Hz) up to several megahertz (MHz). The frequency range of interest in cardiac magnetic field applications typically spans from 0.05 Hz to 150 Hz.
- Distance from the Body: The effectiveness of magnetoresistive sensors depends on their proximity to the source of the magnetic field. For medical applications, these sensors are usually placed very close to the body, often within a few centimeters (typically 1–5 cm).
- Benefits: Magnetoresistive sensors, with their high sensitivity, compact size [61], versatility, compatibility with existing technologies, and noninvasive diagnostic capabilities, hold great promise for advancing medical diagnostics and improving patient care. These benefits make them a valuable tool in the fields of neurology, cardiology, and general medical diagnostics.
- Limitations: Magnetoresistive sensors, while promising in various medical applications, face several significant limitations [65]. One major issue is their sensitivity to external magnetic interference, which can lead to inaccuracies in readings unless adequately shielded. Additionally, their performance can be affected by temperature fluctuations, necessitating complex temperature compensation mechanisms. Furthermore, the high cost of high-precision magnetoresistive sensors can hinder their widespread adoption.
- Requires Shielding: Yes, as these sensors are affected by the surrounding magnetic fields.
- Atomic Magnetometers
- Principle: Similar to OPMs, atomic magnetometers measure magnetic fields by detecting the spin precession of atoms induced by magnetic fields [66]. In [38], the authors presented a detailed review on ultrasensitive magnetic field sensors for Biomedical Applications. Spin-Exchange Relaxation-Free (SERF) magnetometers represent a class of ultra-sensitive atomic magnetometers that can operate at room temperature and have the potential for miniaturization and portability. They are suitable for measuring the very weak magnetic fields [67,68] associated with the human heart [69].
- Magnetic Field and Frequency Ranges: The sensors may operate effectively in the range of down to . Atomic magnetometers are sensitive to a broad range of frequencies, typically from DC (0 Hz) up to several kHz (kilohertz). This range makes them suitable for detecting the low-frequency magnetic fields generated by biological tissues, such as brain waves in the range of 1–100 Hz and cardiac magnetic fields.
- Distance from the Body: The effectiveness of atomic magnetometers depends on their proximity to the source of the magnetic field. For medical applications, especially in neuroimaging, these devices are typically placed within a few millimeters to centimeters from the skin or scalp. For MCG, they need to be placed directly on the chest.
- Benefits: Atomic magnetometers provide a combination of high sensitivity, cost-effectiveness, compactness, noninvasiveness, and precise measurement capabilities, making them highly advantageous for medical applications. These benefits position atomic magnetometers as a valuable tool in the fields of neurology and cardiology, among others, offering the potential for improved diagnostic accuracy and patient outcomes.
- Limitations: Despite their potential, the use of atomic magnetometers in medical applications is constrained by their sensitivity to environmental noise, their operational complexity, and the need for precise temperature control. Sometimes performance is limited due to atomic vapor [66].
- Requires Shielding: To achieve high sensitivity, atomic magnetometers often require operation in magnetically shielded environments to minimize interference from external magnetic sources.
- Search Coil Magnetometers
- Principle: They measure the time derivative of the magnetic field using a coil of wire [38].
- Applications: Search coil magnetometers are highly sensitive and can detect very weak magnetic fields, which is crucial for applications like magnetoencephalography (MEG) and magnetocardiography (MCG).
- Magnetic Field and Frequency Ranges: The sensors may operate effectively in the range of down to . The operational frequency range for search coil magnetometers in medical applications typically spans from a few millihertz to several kilohertz. This range is particularly suited for capturing the low-frequency biomagnetic signals generated by physiological processes, like the cardiac cycle.
- Distance from the Body: The distance from the body at which these magnetometers operate effectively can vary. For applications like magnetocardiography, they are typically placed close to the body, often within a few centimeters, to capture the weak magnetic fields generated by the heart. The exact distance depends on the required spatial resolution and the magnetic field strength.
- Benefits: These magnetometers provide a noninvasive method to monitor and diagnose various medical conditions due to their high sensitivity and capability to detect weak magnetic fields. Compared to other high-sensitivity magnetic field sensors like SQUIDs, search coil magnetometers can be more cost-effective as they do not require cryogenic cooling and are simpler to operate and maintain.
- Limitations: Their limitations include susceptibility to environmental noise [71], temperature sensitivity, complex calibration needs, size, and frequency range restrictions.
- Requires Shielding: Yes, as these sensors are affected by the surrounding magnetic fields.
- Proton Precession Magnetometers
- Principle: The operating principle of a proton magnetometer is based on nuclear magnetic resonance (NMR). In a PPM, the strength of the magnetic field is determined by measuring the precession frequency of hydrogen protons within the device, which aligns with the external magnetic field [72].
- Applications: PPMs can be integrated into MRI systems to improve the measurement of magnetic fields and enhance imaging quality [73].
- Magnetic Field and Frequency Ranges: The sensors may operate effectively in the range of down to . In medical PPM applications where magnetic fields are much weaker, the corresponding frequencies would be in the kilohertz (kHz) range.
- Distance from the Body: PPMs need to be sensitive and placed close to the chest.
- Benefits: Proton Precession Magnetometers hold significant potential in medical applications due to their high sensitivity, noninvasive nature, and portability, making them easy to transport and deploy in various environments.
- Limitations: Their sensitivity to external factors, lower sampling rate, and complex calibration requirements are notable limitations that need to be addressed [72].
- Requires Shielding: Yes, as these sensors are affected by the surrounding magnetic fields.
- Magnetoelectric Sensors
- Principle: These sensors depend on magnetoelectric (ME) composites that show the ME response at room temperature. When a magnetic field is applied to ME composites, an electric charge across the piezoelectric phase is induced due to the piezoelectricity that results from the deformation produced by the ferromagnetic component due to the magnetostriction that is transferred to the ferroelectric component via interfacial bonding [74].
- Distance from the Body: The operational distance varies from a few millimeters (for implantable devices) to several centimeters (for noninvasive measurements, like MEG or MCG).
- Benefits: These sensors consume little power, are highly sensitivities, and have large linear dynamic ranges.
- Limitations: The performance of ME sensors can be affected by temperature changes, which may require compensation or stabilization techniques.
- Requires Shielding: To obtain highly sensitive data readings, the sensors have to be in a shielded area. For example, in [75], the experiments were conducted in a shielded room.
3. Heart Disease Diagnosis Using MCG
- Ischemic Heart Disease Detection: MCG has been found to be particularly useful in the early detection of ischemic heart disease. It can identify ischemic changes in the heart that might not be apparent in a conventional electrocardiogram (ECG), especially in cases of microvascular dysfunction or in patients with non-obstructive coronary artery disease [79]. The basis for using MCG in the detection of ischemic heart disease lies in its ability to detect subtle changes in the cardiac magnetic field patterns associated with ischemia. Ischemia, a condition characterized by reduced blood flow to the heart muscle, can lead to alterations in the myocardial electrical properties, which in turn affects the heart’s magnetic field. MCG is sensitive to these changes, even in the early stages of ischemia. There are many advantages for using MCG over conventional methods. MCG has been shown to have a higher sensitivity in detecting ischemic changes, particularly in patients with non-obstructive coronary artery disease or microvascular dysfunction [80]. Unlike angiography or other invasive diagnostic methods, MCG is completely noninvasive and does not involve exposure to radiation, making it safer for repeated use. Several studies have highlighted the effectiveness of MCG in detecting ischemic heart disease [81]. The future of ischemic heart disease detection using MCG looks promising, with ongoing research focused on enhancing the technology’s sensitivity and specificity. Studies indicate that MCG can differentiate between patients with CAD (coronary artery disease) and healthy controls, offering diagnostic accuracy superior to traditional tests like an EKG in certain scenarios. MCG’s ability to detect myocardial ischemia in patients with a normal EKG and biomarker has been highlighted in [80].
- Cardiac Arrhythmias: Cardiac arrhythmias, which are irregular heartbeats caused by abnormal electrical conduction or the firing of electrical impulses in the heart, produce distinct magnetic field patterns. MCG can detect these patterns, enabling the diagnosis and analysis of arrhythmias with a high degree of sensitivity. The technology’s ability to noninvasively map the heart’s magnetic field in detail allows for the identification of arrhythmic sources and pathways [36] that may not be apparent, with ECG contributing to more accurate diagnosis and treatment planning for conditions, such as atrial fibrillation [85], ventricular tachycardia, and others [86]. Research into MCG’s application in arrhythmia diagnosis is ongoing, with studies focusing on improving the technology’s accuracy, portability, and cost-effectiveness.
- Risk Stratification: MCG technology has shown potential in risk stratification among patients with heart failure, identifying those at higher risk of arrhythmias or sudden cardiac death. By analyzing the heart’s magnetic signals, MCG can detect subtle abnormalities in cardiac function that are indicators of a poor prognosis, such as heterogeneities in repolarization, which are not easily detectable with conventional electrocardiography (ECG) [87,88].
- Fetal Cardiac Monitoring: One of the most promising applications of MCG is in fetal cardiac monitoring [89]. MCG can be used to assess the fetal heart’s electrophysiological properties, detecting congenital heart defects and arrhythmias in early pregnancy without the risks associated with invasive procedures [90,91].
- Pharmacological Studies: MCG has been employed in pharmacological studies to evaluate the effects of drugs on cardiac electrophysiology, particularly for monitoring the QT interval, the time taken for ventricular depolarization and repolarization. This application is crucial for both clinical trials and post-market surveillance of medications [92].
4. Recommendations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Federation, W.H. World Heart Report 2023. Available online: https://world-heart-federation.org/wp-content/uploads/World-Heart-Report-2023.pdf (accessed on 18 June 2024).
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 18 June 2024).
- Kim, H.C. Epidemiology of cardiovascular disease and its risk factors in Korea. Glob. Health Med. 2021, 3, 134–141. [Google Scholar] [CrossRef] [PubMed]
- England, P.H. Health Matters: Preventing Cardiovascular Disease. Available online: https://www.gov.uk/government/publications/health-matters-preventing-cardiovascular-disease/health-matters-preventing-cardiovascular-disease (accessed on 25 June 2024).
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 23 May 2024).
- Rafie, N.; Kashou, A.H.; Noseworthy, P.A. ECG Interpretation: Clinical Relevance, Challenges, and Advances. Hearts 2021, 2, 505–513. [Google Scholar] [CrossRef]
- Asch, F.M.; Shah, S.; Rattin, C.; Swaminathan, S.; Fuisz, A.; Lindsay, J. Lack of sensitivity of the electrocardiogram for detection of old myocardial infarction: A cardiac magnetic resonance imaging study. Am. Heart J. 2006, 152, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Roguin, A. Rene Theophile Hyacinthe Laënnec (1781–1826): The man behind the stethoscope. Clin. Med. Res. 2006, 4, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Goyal, A. The origin of echocardiography: A tribute to Inge Edler. Tex. Heart Inst. J. 2007, 34, 431–438. [Google Scholar]
- Utility and Limitations of the Surface ECG. In Clinical Electrocardiography; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; Chapter 3; pp. 17–22. [CrossRef]
- Barold, S.S. Willem Einthoven and the birth of clinical electrocardiography a hundred years ago. Card. Electrophysiol. Rev. 2003, 7, 99–104. [Google Scholar] [CrossRef]
- West, J.J.; Simpson, A.G. What are the Limitations of the ECG in Clinical Practice? In Critical Decisions in Emergency and Acute Care Electrocardiography; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; Chapter 3; pp. 19–23. [Google Scholar] [CrossRef]
- Patel, V.; Danish, M.; Monteleone, C. All Roads “Lead” to Anaphylaxis: Hypersensitivity to Electrocardiogram Leads. Ann. Allergy Asthma Immunol. 2018, 121, S78–S79. [Google Scholar] [CrossRef]
- Mubarik, I.A. Holter Monitor. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538203/ (accessed on 25 July 2024).
- Galli, A.; Ambrosini, F.; Lombardi, F. Holter Monitoring and Loop Recorders: From Research to Clinical Practice. Arrhythmia Electrophysiol. Rev. 2016, 5, 136–143. [Google Scholar] [CrossRef]
- Krish Tangella, A.E.; Warren, A. Holter Monitor: Exploring the Applications, Procedure, and Analysis of Continuous ECG Monitoring. Available online: https://www.dovemed.com/health-topics/focused-health-topics/holter-monitor-exploring-applications-procedure-and-analysis-continuous-ecg-monitoring (accessed on 25 July 2024).
- Pohost, G.M. The History of Cardiovascular Magnetic Resonance. JACC Cardiovasc. Imaging 2008, 1, 672–678. [Google Scholar] [CrossRef]
- Potter, A.; Pearce, K.; Hilmy, N. The benefits of echocardiography in primary care. Br. J. Gen. Pract. 2019, 69, 358–359. [Google Scholar] [CrossRef]
- Lancellotti, P.; Price, S.; Edvardsen, T.; Cosyns, B.; Neskovic, A.N.; Dulgheru, R.; Flachskampf, F.A.; Hassager, C.; Pasquet, A.; Gargani, L.; et al. The use of echocardiography in acute cardiovascular care: Recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur. Heart J. Cardiovasc. Imaging 2014, 16, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H. The Role of Echocardiography in Heart Failure. J. Nucl. Med. 2015, 56, 31S–38S. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.S.; François, C.J.; Leiner, T. Cardiac MRI: State of the Art. Radiology 2023, 307, e223008. [Google Scholar] [CrossRef] [PubMed]
- Seetharam, K.; Lerakis, S. Cardiac magnetic resonance imaging: The future is bright. F1000Research 2019, 8, 1636. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.A.; Chamarti, K.S.; Tou, L.C.; Demirjian, G.A.; Noorani, S.; Zink, S.; Umair, M. The Merits, Limitations, and Future Directions of Cost-Effectiveness Analysis in Cardiac MRI with a Focus on Coronary Artery Disease: A Literature Review. J. Cardiovasc. Dev. Dis. 2022, 9, 357. [Google Scholar] [CrossRef]
- Nikolaou, K.; Alkadhi, H.; Bamberg, F.; Leschka, S. MRI and CT in the diagnosis of coronary artery disease: Indications and applications. Insights Imaging 2011, 2, 9–24. [Google Scholar] [CrossRef]
- Ritman, E.L. Cardiac computed tomography imaging: A history and some future possibilities. Cardiol. Clin. 2003, 21, 491–513. [Google Scholar] [CrossRef]
- Clinic, C. Cardiac Computed Tomography (CT) Scan. Available online: https://my.clevelandclinic.org/health/diagnostics/16834-cardiac-computed-tomography (accessed on 15 June 2023).
- Prat-Gonzalez, S.; Sanz, J.; Garcia, M.J. Cardiac CT: Indications and Limitations. J. Nucl. Med. Technol. 2008, 36, 18–24. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.; Underwood, R. Nuclear cardiology. Clin. Med. 2012, 12, 373–377. [Google Scholar] [CrossRef]
- Malek, H. Chapter 9—Nuclear Cardiology. In Practical Cardiology, 2nd ed.; Maleki, M., Alizadehasl, A., Haghjoo, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 185–192. [Google Scholar] [CrossRef]
- Werner, R.A.; Thackeray, J.T.; Diekmann, J.; Weiberg, D.; Bauersachs, J.; Bengel, F.M. The Changing Face of Nuclear Cardiology: Guiding Cardiovascular Care Toward Molecular Medicine. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 951–961. [Google Scholar] [CrossRef]
- Underwood, S.R.; de Bondt, P.; Flotats, A.; Marcasa, C.; Pinto, F.; Schaefer, W.; Verberne, H.J. The current and future status of nuclear cardiology: A consensus report. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.S.U.D.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review. Biomed Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef] [PubMed]
- Rotundo, S.; Brizi, D.; Flori, A.; Giovannetti, G.; Menichetti, L.; Monorchio, A. Shaping and Focusing Magnetic Field in the Human Body: State-of-the Art and Promising Technologies. Sensors 2022, 22, 5132. [Google Scholar] [CrossRef]
- Tenforde, T.S. Magnetically induced electric fields and currents in the circulatory system. Prog. Biophys. Mol. Biol. 2005, 87, 279–288. [Google Scholar] [CrossRef]
- Verywellhealth. The Heart’s Electrical System: Anatomy and Function. Available online: https://www.verywellhealth.com/cardiac-electrical-system-how-the-heart-beats-1746299 (accessed on 2 December 2023).
- Baule, G.M.; McFee, R. The magnetic heart vector. Am. Heart J. 1970, 79, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Turutin, A.V.; Kubasov, I.V.; Kislyuk, A.M.; Kuts, V.V.; Malinkovich, M.D.; Parkhomenko, Y.N.; Sobolev, N.A. Ultra-Sensitive Magnetoelectric Sensors of Magnetic Fields for Biomedical Applications. Nanobiotechnol. Rep. 2022, 17, 261–289. [Google Scholar] [CrossRef]
- Murzin, D.; Mapps, D.J.; Levada, K.; Belyaev, V.; Omelyanchik, A.; Panina, L.; Rodionova, V. Ultrasensitive Magnetic Field Sensors for Biomedical Applications. Sensors 2020, 20, 1569. [Google Scholar] [CrossRef] [PubMed]
- Baule, G. Detection of the magnetic field of the heart. Am. Heart J. 1963, 66, 95–96. [Google Scholar] [CrossRef]
- Plonsey, R. Bioelectricity: A Quantitative Approach; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Sinimbu, L.I.; Gutierrez, F.V.; Lima, C.D.; Sommer, R.L.; Silva, B.G.; Serna, J.D.P.; Luz-Lima, C.; Bruno, A.C.; Araújo, J.F. Magnetic microscopy using Hall effect sensors biased with pulsed currents. J. Magn. Magn. Mater. 2024, 596, 171959. [Google Scholar] [CrossRef]
- Uddin, S.M.; Sayad, A.; Chan, J.; Skafidas, E.; Kwan, P. Design and Optimisation of Elliptical-Shaped Planar Hall Sensor for Biomedical Applications. Biosensors 2022, 12, 108. [Google Scholar] [CrossRef]
- Lapicki, A.; Sanbonsugi, H.; Yamamura, T.; Matsushita, N.; Abe, M.; Narimatsu, H.; Handa, H.; Sandhu, A. Functionalization of micro-Hall effect sensors for biomedical applications utilizing superparamagnetic beads. IEEE Trans. Magn. 2005, 41, 4134–4136. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Feng, Q.; Hu, Q.; Zuo, S.; Nabaei, V.; Heidari, H. Detection techniques of biological and chemical Hall sensors. RSC Adv. 2021, 11, 7257–7270. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.T.; Lu, C.C.; Ku, H.W.; Huang, B.R.; Chia, M.H.; Trinh, X.T. Three-Axis Micofluxgate With a Fluxguide. IEEE Trans. Magn. 2019, 55, 2002304. [Google Scholar] [CrossRef]
- Miles, D.M.; Ciurzynski, M.; Barona, D.; Narod, B.B.; Bennest, J.R.; Kale, A.; Lessard, M.; Milling, D.K.; Larson, J.; Mann, I.R. Low-noise permalloy ring cores for fluxgate magnetometers. Geosci. Instrum. Methods Data Syst. 2019, 8, 227–240. [Google Scholar] [CrossRef]
- Elrefai, A.L.; Sasada, I.; Harada, S. Gradiometer and Magnetometer Integration Using a Pair of Fundamental Mode Orthogonal Fluxgate Sensor Heads. IEEE Trans. Magn. 2015, 51, 4005604. [Google Scholar] [CrossRef]
- Janosek, M. Parallel Fluxgate Magnetometers. In High Sensitivity Magnetometers; Grosz, A., Haji-Sheikh, M.J., Mukhopadhyay, S.C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 41–61. [Google Scholar] [CrossRef]
- Sengottuvel, S.; Sharma, A.; Biswal, D.; Khan, P.F.; Swain, P.P.; Patel, R.; Gireesan, K. Feasibility study on measurement of magnetocardiography (MCG) using fluxgate magnetometer. AIP Conf. Proc. 2018, 1942, 060018. [Google Scholar]
- Lu, C.C.; Huang, J.; Chiu, P.K.; Chiu, S.L.; Jeng, J.T. High-Sensitivity Low-Noise Miniature Fluxgate Magnetometers Using a Flip Chip Conceptual Design. Sensors 2014, 14, 13815–13829. [Google Scholar] [CrossRef]
- Cohen, D. Magnetoencephalography: Evidence of magnetic fields produced by alpha-rhythm currents. Science 1968, 161, 784–786. [Google Scholar] [CrossRef]
- Erné, S.N.; Lehmann, J. Magnetocardiography, an introduction. In SQUID Sensors: Fundamentals, Fabrication and Applications; Weinstock, H., Ed.; Springer: Dordrecht, The Netherlands, 1996; pp. 395–412. [Google Scholar] [CrossRef]
- Budker, D. Optical magnetometry. Nat. Phys. 2007, 3, 227–234. [Google Scholar] [CrossRef]
- Wittevrongel, B.; Holmes, N.; Boto, E.; Hill, R.; Rea, M.; Libert, A.; Khachatryan, E.; Van Hulle, M.M.; Bowtell, R.; Brookes, M.J. Practical real-time MEG-based neural interfacing with optically pumped magnetometers. BMC Biol. 2021, 19, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Zahran, S.; Mahmoudzadeh, M.; Wallois, F.; Betrouni, N.; Derambure, P.; Le Prado, M.; Palacios-Laloy, A.; Labyt, E. Performance Analysis of Optically Pumped 4He Magnetometers vs. Conventional SQUIDs: From Adult to Infant Head Models. Sensors 2022, 22, 3093. [Google Scholar] [CrossRef] [PubMed]
- Sometti, D.; Semeia, L.; Baek, S.; Chen, H.; Righetti, G.; Dax, J.; Kronlage, C.; Kirchgässner, M.; Romano, A.; Heilos, J.; et al. Muscle Fatigue Revisited—Insights From Optically Pumped Magnetometers. Front. Physiol. 2021, 12, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Yu, X.; Bonnette, S.; Anand, M.; Riehm, C.D.; Schlink, B.; Diekfuss, J.A.; Myer, G.D.; Jiang, Y. Improved Biomagnetic Signal-To-Noise Ratio and Source Localization Using Optically Pumped Magnetometers with Synthetic Gradiometers. Brain Sci. 2023, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Marmugi, L.; Renzoni, F. Electromagnetic Induction Imaging with Atomic Magnetometers: Progress and Perspectives. Appl. Sci. 2020, 10, 6370. [Google Scholar] [CrossRef]
- Sensors, R. Magnetoresistive Sensors. Available online: https://www.rechner-sensors.com/en/documentations/knowledge/magnetoresistive-sensors (accessed on 30 June 2024).
- Barbieri, F.; Trauchessec, V.; Caruso, L.; Trejo-Rosillo, J.; Telenczuk, B.; Paul, E.; Bal, T.; Destexhe, A.; Fermon, C.; Pannetier-Lecoeur, M.; et al. Local recording of biological magnetic fields using Giant Magneto Resistance-based micro-probes. Sci. Rep. 2016, 6, 39330. [Google Scholar] [CrossRef]
- Electricity Magnetism.org. Magnetoresistive Sensors. Available online: https://www.electricity-magnetism.org/magnetoresistive-sensors/ (accessed on 30 June 2024).
- Kanno, A.; Nakasato, N.; Oogane, M.; Fujiwara, K.; Nakano, T.; Arimoto, T.; Matsuzaki, H.; Ando, Y. Scalp attached tangential magnetoencephalography using tunnel magneto-resistive sensors. Sci. Rep. 2022, 12, 6106. [Google Scholar] [CrossRef]
- Fujiwara, K.; Oogane, M.; Kanno, A.; Imada, M.; Jono, J.; Terauchi, T.; Okuno, T.; Aritomi, Y.; Morikawa, M.; Tsuchida, M.; et al. Magnetocardiography and magnetoencephalography measurements at room temperature using tunnel magneto-resistance sensors. Appl. Phys. Express 2018, 11, 023001. [Google Scholar] [CrossRef]
- Pannetier-Lecoeur, M.; Parkkonen, L.; Sergeeva-Chollet, N.; Polovy, H.; Fermon, C.; Fowley, C. Magnetocardiography with sensors based on giant magnetoresistance. Appl. Phys. Lett. 2011, 98, 153705. [Google Scholar] [CrossRef]
- Cubells-Beltran, M.; Reig, C.; Martos, J.; Torres, J.; Soret, J. Limitations of Magnetoresistive Current Sensors in Industrial Electronics Applications. Int. Rev. Electr. Eng. 2011, 6, 423–429. [Google Scholar]
- Bai, X.; Wen, K.; Peng, D.; Liu, S.; Luo, L. Atomic magnetometers and their application in industry. Front. Phys. 2023, 11, 1212368. [Google Scholar] [CrossRef]
- Ma, Y.; Qiao, Z.; Yu, M.; Wang, Y.; Chen, Y.; Luo, G.; Yang, P.; Lin, Q.; Zhao, L.; Zhang, Y.; et al. Single-beam integrated hybrid optical pumping spin exchange relaxation free magnetometer for biomedical applications. Appl. Phys. Lett. 2022, 121, 114001. [Google Scholar] [CrossRef]
- Savukov, I.M. Spin Exchange Relaxation Free (SERF) Magnetometers. In High Sensitivity Magnetometers; Grosz, A., Haji-Sheikh, M.J., Mukhopadhyay, S.C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 451–491. [Google Scholar] [CrossRef]
- Wyllie, R.; Kauer, M.; Smetana, G.S.; Wakai, R.T.; Walker, T.G. Magnetocardiography with a modular spin-exchange relaxation-free atomic magnetometer array. Phys. Med. Biol. 2012, 57, 2619. [Google Scholar] [CrossRef] [PubMed]
- Wakai, R.T. The atomic magnetometer: A new era in biomagnetism. AIP Conf. Proc. 2014, 1626, 46–54. [Google Scholar] [CrossRef]
- Nourmohammadi Abadchi, A.; Feiz, S.; Asteraki, M. Investigation of Noise Reduction and SNR Enhancement in Search Coil Magnetometers at Low Frequencies. arXiv 2014, arXiv:1409.7267. [Google Scholar]
- Electricity Magnetism.org. Proton Precession Magnetometer. Available online: https://www.electricity-magnetism.org/proton-precession-magnetometer/ (accessed on 22 July 2024).
- Ayon, A.I. Proton Precession Magnetometer. Bachelor’s Thesis, Macquarie University, Sydney, Australia, 2018. [Google Scholar]
- Wang, Y.; Li, J.; Viehland, D. Magnetoelectrics for magnetic sensor applications: Status, challenges and perspectives. Mater. Today 2014, 17, 269–275. [Google Scholar] [CrossRef]
- Elzenheimer, E.; Hayes, P.; Thormählen, L.; Engelhardt, E.; Zaman, A.; Quandt, E.; Frey, N.; Höft, M.; Schmidt, G. Investigation of Converse Magnetoelectric Thin-Film Sensors for Magnetocardiography. IEEE Sens. J. 2023, 23, 5660–5669. [Google Scholar] [CrossRef]
- Reermann, J.; Durdaut, P.; Salzer, S.; Demming, T.; Piorra, A.; Quandt, E.; Frey, N.; Höft, M.; Schmidt, G. Evaluation of magnetoelectric sensor systems for cardiological applications. Measurement 2018, 116, 230–238. [Google Scholar] [CrossRef]
- Bichurin, M.; Petrov, R.; Sokolov, O.; Leontiev, V.; Kuts, V.; Kiselev, D.; Wang, Y. Magnetoelectric Magnetic Field Sensors: A Review. Sensors 2021, 21, 6232. [Google Scholar] [CrossRef]
- Viehland, D.; Wuttig, M.; McCord, J.; Quandt, E. Magnetoelectric magnetic field sensors. MRS Bull. 2018, 43, 834–840. [Google Scholar] [CrossRef]
- Yamada, S.; Yamaguchi, I. agnetocardiograms in clinical medicine: Unique information on cardiac ischemia, arrhythmias, and fetal diagnosis. Intern. Med. 2005, 44, 1–19. [Google Scholar] [CrossRef]
- Her, A.Y.; Dischl, D.; Kim, Y.H.; Kim, S.W.; Shin, E.S. Magnetocardiography for the detection of myocardial ischemia. Front. Cardiovasc. Med. 2023, 10, 1242215. [Google Scholar] [CrossRef] [PubMed]
- Brisinda, D.; Caristo, M.E.; Fenici, R. Longitudinal study of cardiac electrical activity in anesthetized guinea pigs by contactless magnetocardiography. Physiol. Meas. 2007, 28, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, G.; Singh, S.; Pathania, M.; Gosavi, S.; Abhishek, S.; Parchani, A.; Dhar, M. Artificial intelligence in clinical medicine: Catalyzing a sustainable global healthcare paradigm. Front. Artif. Intell. 2023, 6, 1227091. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef]
- Khalifa, M.; Albadawy, M. AI in diagnostic imaging: Revolutionising accuracy and efficiency. Comput. Methods Programs Biomed. Update 2024, 5, 100146. [Google Scholar] [CrossRef]
- Yoshida, K.; Ogata, K.; Inaba, T.; Nakazawa, Y.; Ito, Y.; Yamaguchi, I.; Kandori, A.; Aonuma, K. Ability of magnetocardiography to detect regional dominant frequencies of atrial fibrillation. J. Arrhythmia 2015, 31, 345–351. [Google Scholar] [CrossRef]
- Brisinda, D.; Fenici, P.; Fenici, R. Clinical magnetocardiography: The unshielded bet—Past, present, and future. Front. Cardiovasc. Med. 2023, 10, 1232882. [Google Scholar] [CrossRef]
- Grimm, W.; Glaveris, C.; Hoffmann, J.; Menz, V.; Müller, H.H.; Hufnagel, G.; Maisch, B. RArrhythmia risk stratification in idiopathic dilated cardiomyopathy based on echocardiography and 12-lead, signal-averaged, and 24-hour holter electrocardiography. Am. Heart J. 2000, 140, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, P.; Väänänen, H.; Mäkijärvi, M.; Katila, T.; Toivonen, L. Repolarization abnormalities detected by magnetocardiography in patients with dilated cardiomyopathy and ventricular arrhythmias. J. Cardiovasc. Electrophysiol. 2001, 12, 772–777. [Google Scholar] [CrossRef]
- Strand, S.A.; Strasburger, J.F.; Wakai, R.T. Fetal magnetocardiogram waveform characteristics. Physiol. Meas. 2019, 40, 035002. [Google Scholar] [CrossRef]
- Strasburger, J. Agnetocardiography for fetal arrhythmias. Heart Rhythm. 2008, 5, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Comani, S.; Liberati, M.; Mantini, D.; Gabriele, E.; Brisinda, D.; Di Luzio, S.; Fenici, R.; Luca Romani, G. Characterization of Fetal Arrhythmias by Means of Fetal Magnetocardiography in Three Cases of Difficult Ultrasonographic Imaging. Pacing Clin. Electrophysiol. 2004, 27, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Fenici, R.; Brisinda, D.; Meloni, A.M. Clinical application of magnetocardiography. Expert Rev. Mol. Diagn. 2005, 5, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Dekie, L.; Kleiman, R.B. False Negative ECG Device Results May Increase the Risk of Adverse Events in Clinical Oncology Trials. Ther. Innov. Regul. Sci. 2022, 55, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Sakib, S.; Fouda, M.M.; Al-Mahdawi, M.; Mohsen, A.; Oogane, M.; Ando, Y.; Fadlullah, Z.M. Noise-Removal from Spectrally-Similar Signals Using Reservoir Computing for MCG Monitoring. In Proceedings of the ICC 2021—IEEE International Conference on Communications, Montreal, QC, Canada, 14–23 June 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Mohsen, A.; Al-Mahdawi, M.; Fouda, M.M.; Oogane, M.; Ando, Y.; Fadlullah, Z.M. AI Aided Noise Processing of Spintronic Based IoT Sensor for Magnetocardiography Application. In Proceedings of the ICC 2020—2020 IEEE International Conference on Communications (ICC), Dublin, Ireland, 7–11 June 2020; pp. 1–6. [Google Scholar] [CrossRef]
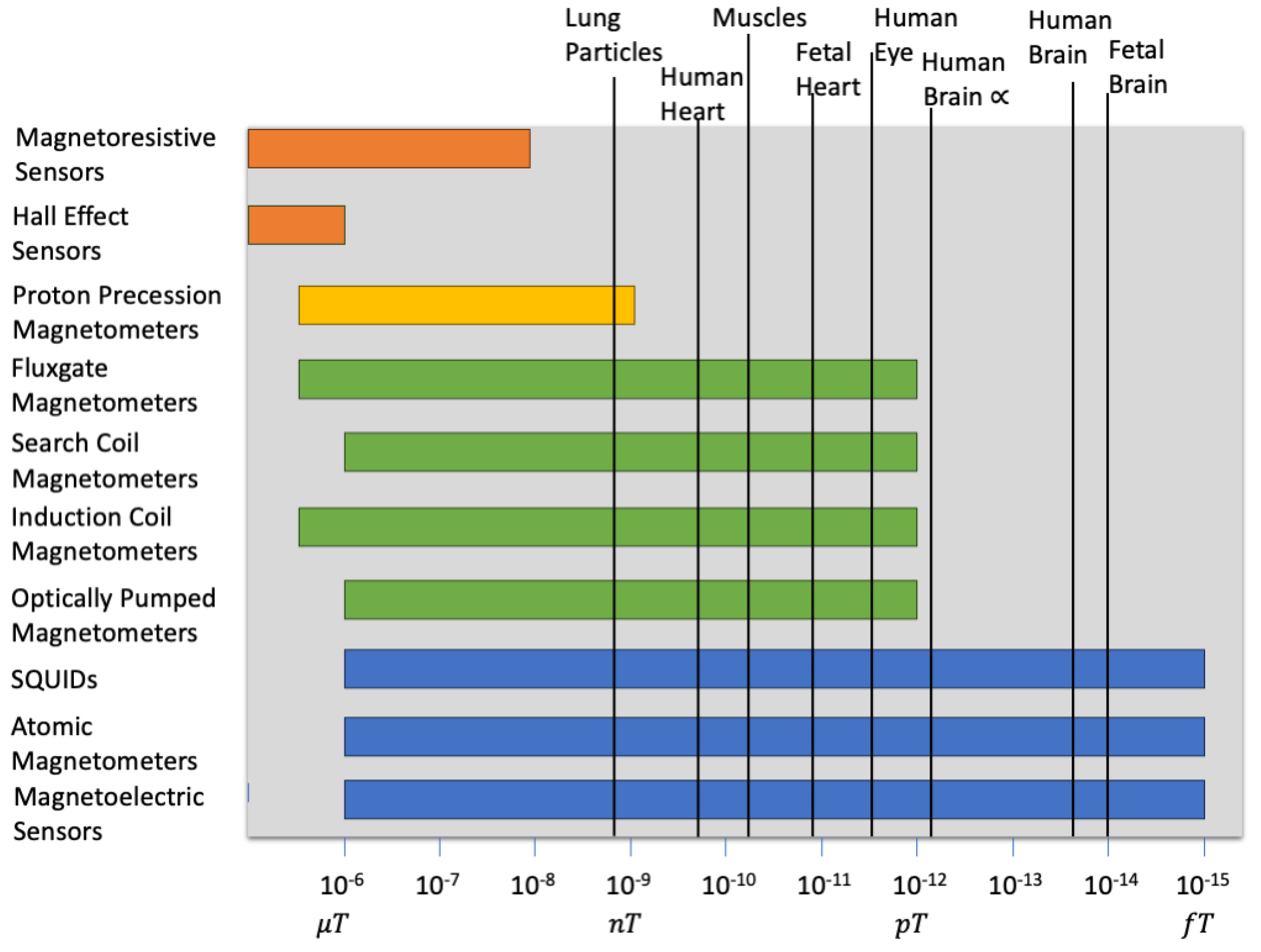
| Magnetic Sensor Technology | Measurement Range | Medical Applications |
|---|---|---|
| Hall Effect Sensors | to | Magnetic microscopy [41], detecting magnetic nanoparticles (MNPs) |
| Fluxgate Magnetometers | to | Biomagnetic field detection, magnetoencephalography (MEG) |
| Superconducting Quantum Interference Devices (SQUIDs) | to | Magnetoencephalography (MEG), magnetocardiography (MCG), MRI |
| Optically Pumped Magnetometers | to | Magnetoencephalography (MEG), magnetocardiography (MCG) |
| Induction Coil Magnetometers | to | Detection of neural and cardiac magnetic fields |
| Magnetoresistive Sensors | to | Magnetic resonance imaging (MRI), biosensors |
| Atomic Magnetometers | to | Magnetoencephalography (MEG), magnetocardiography (MCG) |
| Search Coil Magnetometers | to | Magnetic field mapping in medical research |
| Proton Precession Magnetometers | to | Magnetocardiography (MCG), fetal monitoring, MRI |
| Magnetoelectric Sensors | [77,78] to [77] | Magnetocardiography (MCG) |
| Feature | Magnetocardiography (MCG) | Electrocardiography (ECG) |
|---|---|---|
| Principle of operation | Measures the magnetic fields generated by the heart’s electrical activity [92]. | Measures the electrical activity of the heart via skin electrodes. |
| Sensitivity | High sensitivity to small, intracardiac electrical events [80,85]. | Lower sensitivity to small, intracardiac signals compared to MCG. |
| Spatial resolution | High; can provide detailed mapping of cardiac electrical activity. | Relatively lower spatial resolution. |
| Invasiveness | Noninvasive and does not require any type of contact to the patient’s body. | Noninvasive, but some patients find it uncomfortable as it touches their skin. |
| Equipment | Requires specialized equipment, such as SQUID sensors, often housed in magnetically shielded rooms [92]. | Standardized and widely available equipment. |
| Cost | Generally more expensive due to specialized equipment and facility requirements. | Less expensive and more widely accessible. |
| Use cases | Particularly useful in detecting and analyzing arrhythmias, ischemic heart disease, and fetal heart conditions. | Broadly used for routine cardiac assessments and diagnosing arrhythmias, myocardial infarction, and other heart conditions. |
| Signal interference | Less susceptible to muscle and movement artifacts due to the nature of magnetic field measurement. | More susceptible to noise from muscle movements and electrode displacement. |
| Availability | Less widely available; mainly used in research settings and specialized clinical centers. | Widely available in hospitals, clinics, and even as portable devices for home use. |
| Patient comfort | Generally comfortable, but requires lying still in a magnetically shielded room. | Generally comfortable, but adhesive electrodes may cause minor skin irritation for some patients. |
| Magnetic Sensor Technology | Problems |
|---|---|
| Hall Effect Sensors | Sensitive to temperature fluctuations, magnetic interference (requires shielding), has a limited detection range, low signal-to-noise ratio, high power consumption. |
| Fluxgate Magnetometers | Susceptible to temperature fluctuations, requires complex calibration, bulkiness can limit portability. |
| Superconducting Quantum Interference Devices (SQUIDs) | Requires cryogenic cooling, high operational costs, complex setup and maintenance. |
| Optically Pumped Magnetometers | Sensitivity to light and temperature fluctuations, requires optical components, potentially bulky. |
| Induction Coil Magnetometers | Sensitivity to external noise, lower spatial resolution, bulky and less portable. |
| Atomic Magnetometers | Requires magnetic shielding, sensitivity to environmental noise, complex operation and maintenance. |
| Search Coil Magnetometers | Susceptibility to electromagnetic noise, requires specialized environments or shielding, temperature sensitivity. |
| Proton Precision Magnetometers | Sensitivity to external factors, lower sampling rate, complex calibration requirements. |
| Magnetoelectric Sensors | Sensitivity to temperature changes. |
| Magnetoresistive Sensors | Sensitivity to external magnetic interference, sensitivity to temperature fluctuations, high cost. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elfouly, T.; Alouani, A. Harnessing the Heart’s Magnetic Field for Advanced Diagnostic Techniques. Sensors 2024, 24, 6017. https://doi.org/10.3390/s24186017
Elfouly T, Alouani A. Harnessing the Heart’s Magnetic Field for Advanced Diagnostic Techniques. Sensors. 2024; 24(18):6017. https://doi.org/10.3390/s24186017
Chicago/Turabian StyleElfouly, Tarek, and Ali Alouani. 2024. "Harnessing the Heart’s Magnetic Field for Advanced Diagnostic Techniques" Sensors 24, no. 18: 6017. https://doi.org/10.3390/s24186017
APA StyleElfouly, T., & Alouani, A. (2024). Harnessing the Heart’s Magnetic Field for Advanced Diagnostic Techniques. Sensors, 24(18), 6017. https://doi.org/10.3390/s24186017







