Emerging Paradigms in Fetal Heart Rate Monitoring: Evaluating the Efficacy and Application of Innovative Textile-Based Wearables
Abstract
:1. Introduction
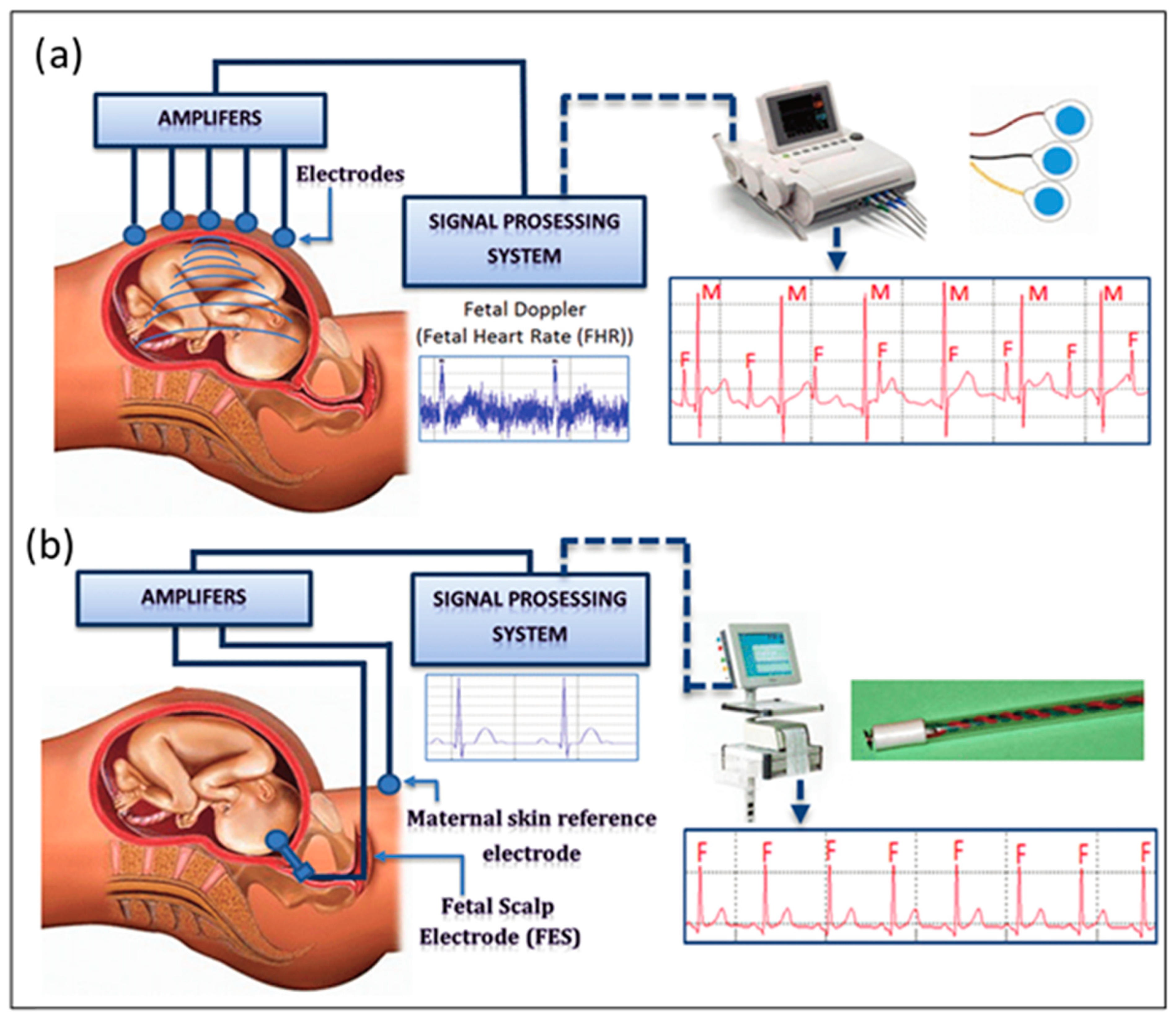
2. Measuring Techniques of fHR
2.1. Fetal Electrocardiography (fECG)
- Low-amplitude fECG results are caused by weak cardiac impulses and poor conductivity of the fetal layers on the surface of the mother’s body [26].
- Maternal ECG, uterine contractions, respiratory activity, and motion artefacts cause interference [27].
- Challenges include fetal movements and the need for a consistent fetal cardiac representation along the body axis [28].
- It is crucial to develop automated processes that can be applied to large datasets with little expert assistance.
- Key parts include creating criteria for predicted cardiac signals’ degree of confidence and setting theoretical boundaries for the data that can be gleaned from body surface recordings despite noise [29].

2.2. Cardiotocography (CTG)
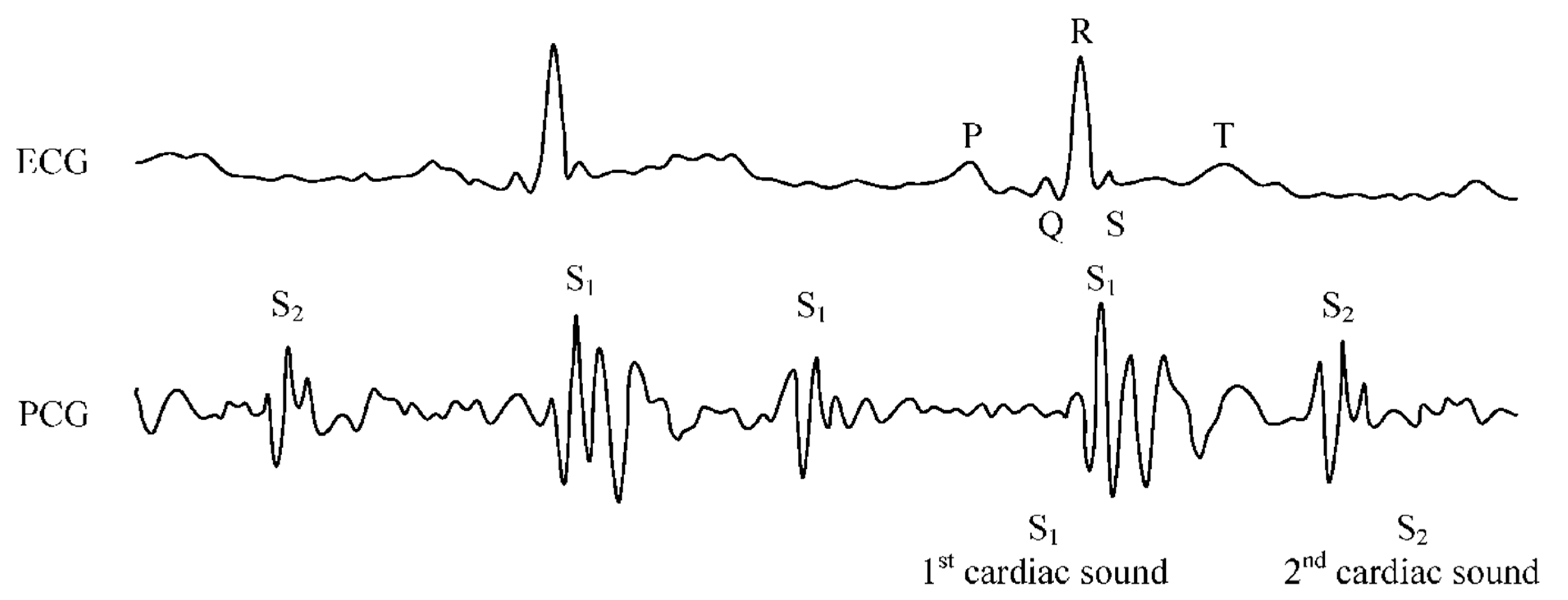
2.3. Phonocardiography (PCG)
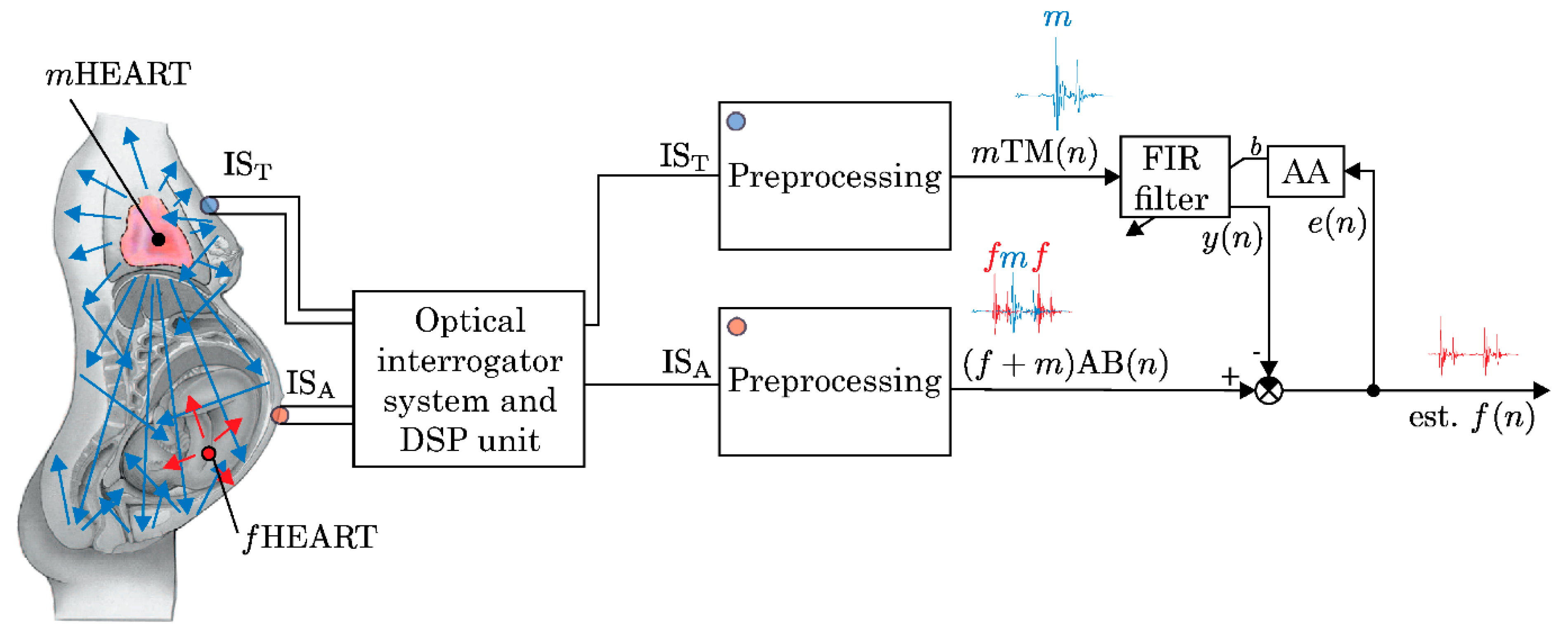
2.4. Ballistocardiography (BCG)
3. Commercially Available Fetal Monitoring Devices
4. Research in fHR Monitoring Systems
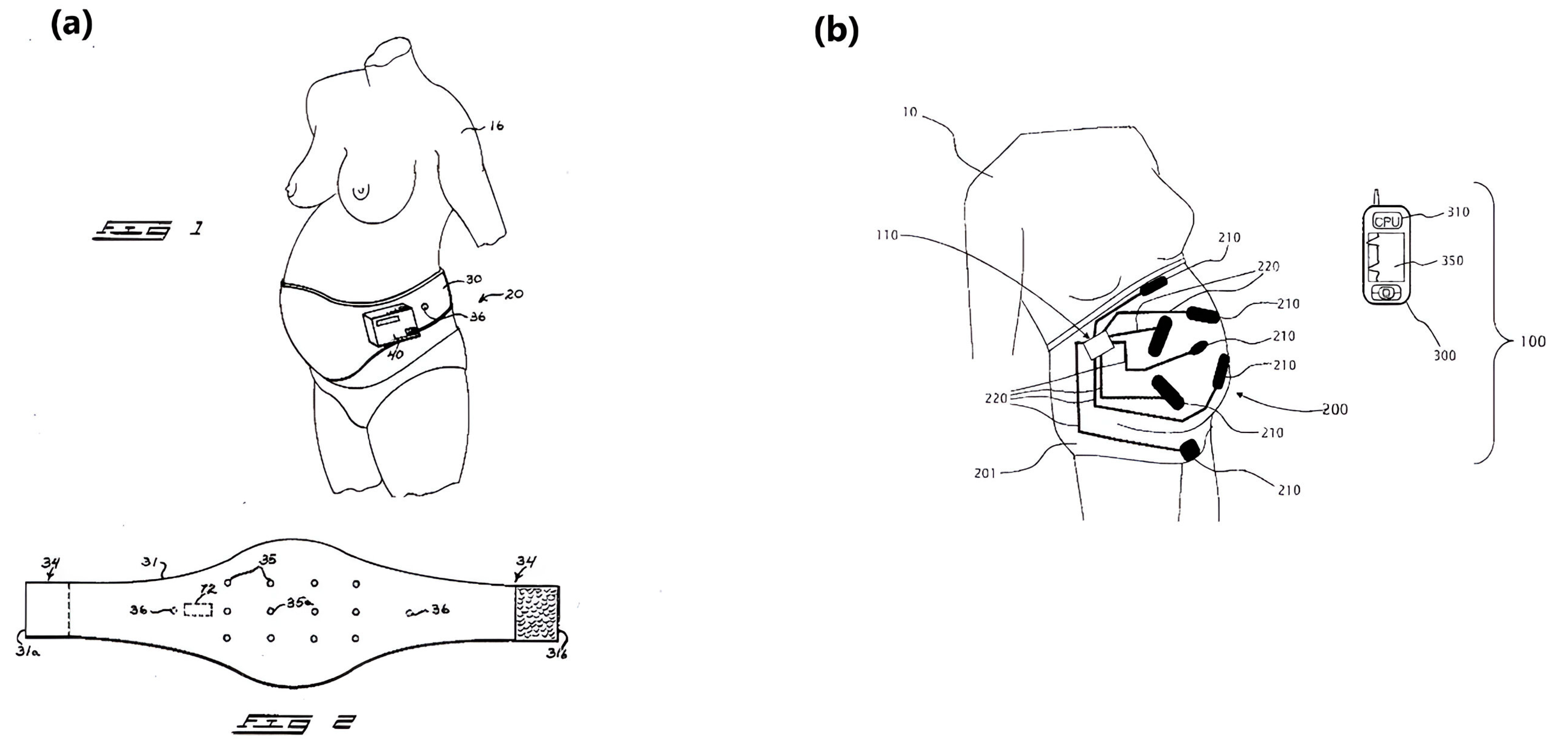
4.1. Nonambulatory fHR Monitoring Patents
4.2. Ambulatory fHR Monitoring Systems
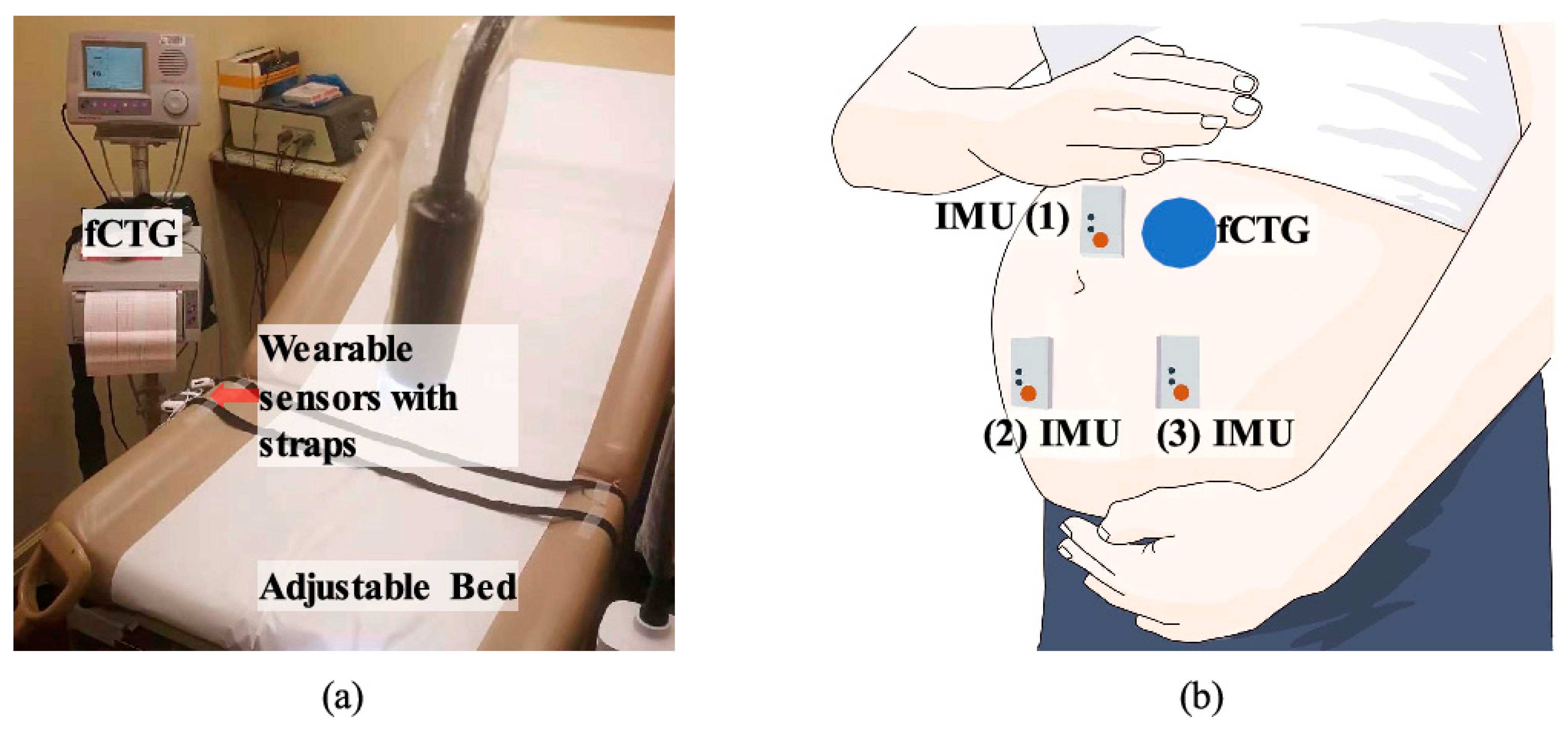
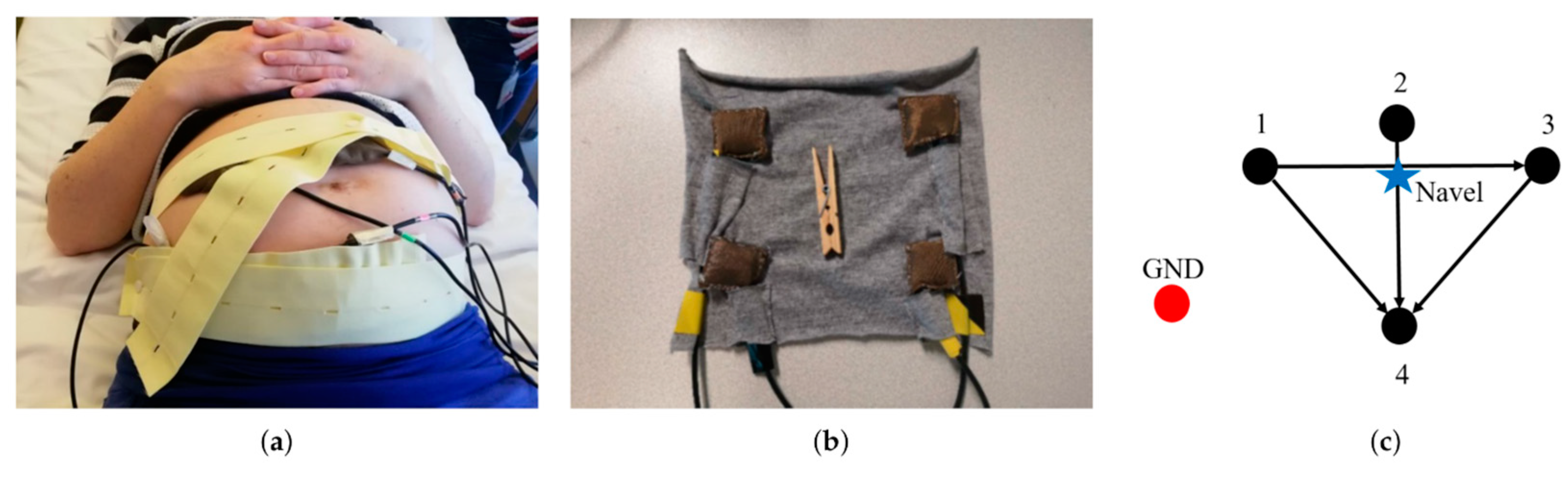
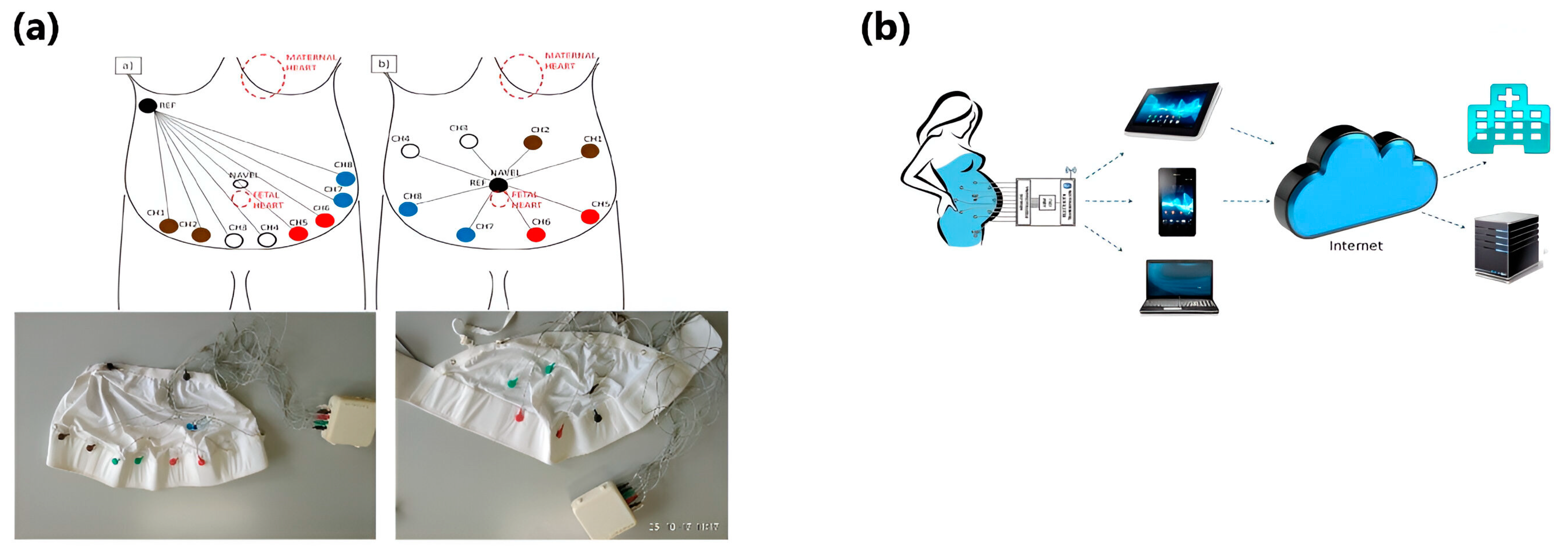
5. Textile-Based Fetal Heart Monitoring Systems
5.1. Fabric Structures in Textile-Based Fetal Monitoring Systems
5.1.1. Woven Fabrics
5.1.2. Knitted Fabrics
5.1.3. Non-Woven Fabrics
5.2. Sensor Materials in Textile-Based Fetal Monitoring
5.2.1. Metal-Coated Fibers (Silver, Stainless Steel)
5.2.2. Conductive Polymers
5.2.3. Hybrid Materials
5.3. Fabrication Methods for Textile-Based Fetal Monitoring Sensors
5.3.1. Embroidery and Sewing
5.3.2. Screen and Inkjet Printing
5.3.3. Weaving and Knitting Conductive Fibers
5.4. Recent Progress in Textile-Based Fetal Heart Monitoring Devices
6. Conclusions
7. Future Perspectives
Funding
Conflicts of Interest
References
- Vetter, V.L.; Elia, J.; Erickson, C.; Berger, S.; Blum, N.; Uzark, K.; Webb, C.L. Correction: Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation 2008, 117, 2407–2423. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, R.; Wells, J.; Castellsague, X. A second-generation study of 427 probands with congenital heart defects and their 837 children. J. Am. Coll. Cardiol. 1994, 23, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Canobbio, M.M.; Warnes, C.A.; Aboulhosn, J.; Connolly, H.M.; Khanna, A.; Koos, B.J.; Mital, S.; Rose, C.; Silversides, C.; Stout, K. Management of Pregnancy in Patients with Complex Congenital Heart Disease: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2017, 135, e50–e87. [Google Scholar] [CrossRef] [PubMed]
- Jawad, H.; Ali, N.N.; Lyon, A.R.; Chen, Q.Z.; Harding, S.E.; Boccaccini, A.R. Myocardial tissue engineering: A review. Tissue Eng. Regen. Med. 2007, 1, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Pajkrt, E.; Weisz, B.; Firth, H.V.; Chitty, L.S. Fetal cardiac anomalies and genetic syndromes. Prenat. Diagn. 2004, 24, 1104–1115. [Google Scholar] [CrossRef]
- Zuckerwar, A.J.; Pretlow, R.A.; Stoughton, J.W.; Baker, D.A. Development of a piezopolymer pressure sensor for a portable fetal heart rate monitor. IEEE Trans. Biomed. Eng. 1993, 40, 963–969. [Google Scholar] [CrossRef]
- Knox, G.E.; Simpson, K.R.; Garite, T.J. High reliability perinatal units: An approach to the prevention of patient injury and medical malpractice claims. J. Healthcare Risk Manag. 1999, 19, 24–32. [Google Scholar] [CrossRef]
- Paladini, D.; Volpe, P. Ultrasound of Congenital Fetal Anomalies: Differential Diagnosis and Prognostic Indicators, 2nd ed.; CRC Press: London, UK, 2014. [Google Scholar] [CrossRef]
- Mohebbian, M.R.; Vedaei, S.S.; Wahid, K.A.; Dinh, A.; Marateb, H.R.; Tavakolian, K. Fetal ECG Extraction from Maternal ECG Using Attention-Based CycleGAN. IEEE J. Biomed. Health. Inform. 2022, 26, 515–526. [Google Scholar] [CrossRef]
- Dessì, A. Algorithms and Systems for Home Telemonitoring in Biomedical Applications; University of Cagliari: Cagliari, Italy, 2015. [Google Scholar]
- Peters, M.; Crowe, J.; Piéri, J.F.; Quartero, H.; Hayes-Gill, B.; James, D.; Stinstra, J.; Shakespeare, S. Monitoring the fetal heart non-invasively: A review of methods. J. Perinat. Med. 2001, 29, 408–416. [Google Scholar] [CrossRef]
- Dobkin, B.H.; Dorsch, A. The Promise of mHealth: Daily Activity Monitoring and Outcome Assessments by Wearable Sensors. Sage J. 2011, 25, 785–880. [Google Scholar] [CrossRef]
- Chan, M.; Estève, D.; Fourniols, J.-Y.; Escriba, C.; Campo, E. Smart wearable systems: Current status and future challenges. Artif. Intell. Med. 2012, 56, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Anikwe, C.V.; Nweke, H.F.; Ikegwu, A.C.; Egwuonwu, C.A.; Onu, F.U.; Alo, U.R.; Teh, Y.W. Mobile and wearable sensors for data-driven health monitoring system: State-of-the-art and future prospect. Expert Syst. Appl. 2022, 202, 117362. [Google Scholar] [CrossRef]
- Castano, L.M.; Flatau, A.B. Smart fabric sensors and e-textile technologies: A review. Smart Mater. Struct. 2014, 23, 053001. [Google Scholar] [CrossRef]
- Vullings, R.; Peters, C.; Mischi, M.; Sluijter, R.; Oei, G.; Bergmans, J. Artifact reduction in maternal abdominal ECG recordings for fetal ECG estimation. In Proceedings of the 2007 29th Annual International Conference IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 43–46. [Google Scholar]
- Schauss, G. Wearable Textile Electrocardiogram Sport Bra for Real Time Health Monitoring. Master’s Thesis, University of Colorado, Boulder, CO, USA, 2022. [Google Scholar]
- Cho, S.; Chang, T.; Yu, T.; Lee, C.H. Smart Electronic Textiles for Wearable Sensing and Display. Biosensors 2022, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, G.; Wei, Y. Non-Invasive Fetal Electrocardiogram Monitoring Techniques: Potential and Future Research Opportunities in Smart Textiles. Signals 2021, 2, 392–412. [Google Scholar] [CrossRef]
- Haws, R.A.; Yakoob, M.Y.; Soomro, T.; Menezes, E.V.; Darmstadt, G.L.; Bhutta, Z.A. Reducing stillbirths: Screening and monitoring during pregnancy and labour. BMC Pregnancy Childbirth 2009, 9, S5. [Google Scholar] [CrossRef]
- Ekblom, A.; Målqvist, M.; Gurung, R.; Rossley, A.; Basnet, O.; Bhattarai, P.; Ashish K., C. Factors associated with poor adherence to intrapartum fetal heart monitoring in relationship to intrapartum related death: A prospective cohort study. PLoS Glob. Public Health 2022, 2, e0000289. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, Y.; Zhou, Z.; Bai, Y.; Wu, S. A Fetal ECG Monitoring System Based on the Android Smartphone. Sensors 2019, 19, 446. [Google Scholar] [CrossRef]
- Cajavilca, C.; Varon, J. Willem Einthoven: The development of the human electrocardiogram. Resuscitation 2008, 76, 325–328. [Google Scholar] [CrossRef]
- Martens, S.M.M.; Rabotti, C.; Mischi, M.; Sluijter, R.J. A robust fetal ECG detection method for abdominal recordings. Physiol. Meas. 2007, 28, 373. [Google Scholar] [CrossRef]
- Hulsenboom, A.D.J.; Warmerdam, G.J.J.; Weijers, J.; Blijham, P.J.; Oei, S.G.; Laar, J.O.E.H.v.; Vullings, R.; Delhaas, T. Head orientation and electrode placement potentially influence fetal scalp ECG waveform. PLoS ONE 2019, 14, e0223282. [Google Scholar] [CrossRef] [PubMed]
- Warmerdam, G. Robust Spectral Analysis of Heart Rate Variability in Non-Invasive Fetal ECG Recordings. Master’s Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2012. [Google Scholar]
- Queyam, A.B.; Pahuja, S.K.; Singh, D. Non-invasive feto-maternal well-being monitoring: A review of methods. J. Eng. Sci. Technol. Rev. 2017, 6, 7–14. [Google Scholar]
- Vullings, R. Non-invasive fetal electrocardiogram: Analysis and interpretation. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2010. [Google Scholar]
- Clifford, G.D.; Azuaje, F.; Mcsharry, P. ECG statistics, noise, artifacts, and missing data. In Advanced Methods and Tools for ECG Data Analysis; Artech House: Boston, MA, USA, 2006; Volume 6, p. 18. [Google Scholar]
- Zhang, Y.; Gu, A.; Xiao, Z.; Xing, Y.; Yang, C.; Li, J.; Liu, C. Wearable Fetal ECG Monitoring System from Abdominal Electrocardiography Recording. Biosensors 2022, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Ayres-de-Campos, D.; Bernardes, J. Twenty-five years after the FIGO guidelines for the use of fetal monitoring: Time for a simplified approach? Gynecol. Obstet. 2010, 110, 1–6. [Google Scholar] [CrossRef]
- Ruffo, M.; Cesarelli, M.; Romano, M.; Bifulco, P.; Fratini, A. An algorithm for FHR estimation from foetal phonocardiographic signals. Biomed. Signal Proc. Control 2010, 5, 131–141. [Google Scholar] [CrossRef]
- Behar, J.; Andreotti, F.; Zaunseder, S.; Oster, J.; Clifford, G.D. A practical guide to non-invasive foetal electrocardiogram extraction and analysis. Physiol. Meas. 2016, 37, R1. [Google Scholar] [CrossRef]
- Lele, P.P. Safety and potential hazards in the current applications of ultrasound in obstetrics and gynecology. Ultrasound Med. Biol. 1979, 5, 307–320. [Google Scholar] [CrossRef]
- Menihan, C.A. Ultrasound for Advanced Practitioners in Pregnancy and Women’s Health; Jones & Bartlett Learning: Burlington, MA, USA, 2018. [Google Scholar]
- Wretler, S. New Approaches on Fetal and Maternal Intrapartum Monitoring; Karolinska Institutet: Stockholm, Sweden, 2017. [Google Scholar]
- Jaros, R.; Kahankova, R.; Martinek, R.; Nedoma, J.; Fajkus, M.; Slanina, Z. Fetal phonocardiography signal processing from abdominal records by non-adaptive methods. In Proceedings of the Photonics Applications in Astronomy, Communications, Industry, and High-Energy Physics Experiments 2018, Wilga, Poland, 3–10 June 2018; p. 8. [Google Scholar]
- Martinek, R.; Nedoma, J.; Fajkus, M.; Kahankova, R.; Konecny, J.; Janku, P.; Kepak, S.; Bilik, P.; Nazeran, H. A Phonocardiographic-Based Fiber-Optic Sensor and Adaptive Filtering System for Noninvasive Continuous Fetal Heart Rate Monitoring. Sensors 2017, 17, 890. [Google Scholar] [CrossRef]
- Khandoker, A.; Ibrahim, E.; Oshio, S.; Kimura, Y. Validation of beat by beat fetal heart signals acquired from four-channel fetal phonocardiogram with fetal electrocardiogram in healthy late pregnancy. Sci. Rep. 2018, 8, 13635. [Google Scholar] [CrossRef]
- Mubarak, Q.-U.-A.; Akram, M.U.; Shaukat, A.; Hussain, F.; Khawaja, S.G.; Butt, W.H. Analysis of PCG signals using quality assessment and homomorphic filters for localization and classification of heart sounds. Comput. Methods Progr. Biomed. 2018, 164, 143–157. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, E215–E220. [Google Scholar] [CrossRef] [PubMed]
- Phanphaisarn, W.; Roeksabutr, A.; Wardkein, P.; Koseeyaporn, J.; Yupapin, P. Heart detection and diagnosis based on ECG and EPCG relationships. Med. Devices Evid. Res. 2011, 2011, 133–144. [Google Scholar] [CrossRef]
- Southern, E.M. Electrocardiography and phonocardiography of the foetal heart. J. Obstet. Gynaecol. Br. Emp. 1954, 61, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Adithya, P.C.; Sankar, R.; Moreno, W.A.; Hart, S. Trends in fetal monitoring through phonocardiography: Challenges and future directions. Biomed. Signal Process. Control 2017, 33, 289–305. [Google Scholar] [CrossRef]
- Ruffo, M.; Cesarelli, M.; Jin, C.; Gargiulo, G.; McEwan, A.; Sullivan, C.; Bifulco, P.; Romano, M.; Shephard, R.W.; Van Schaik, A. Non-invasive foetal monitoring with combined ECG-PCG system. In Biomedical Engineering, Trends in Electronics, Communications and Software; IntechOpen: London, UK, 2011; pp. 347–366. [Google Scholar]
- Strazza, A.; Sbrollini, A.; Di Battista, V.; Ricci, R.; Trillini, L.; Marcantoni, I.; Morettini, M.; Fioretti, S.; Burattini, L. PCG-Delineator: An efficient algorithm for automatic heart sounds detection in fetal phonocardiography. In Proceedings of the 2018 Computing in Cardiology Conference (CinC), Maastricht, The Netherlands, 23–26 September 2018; pp. 1–4. [Google Scholar]
- Gobillot, S.; Fontecave-Jallon, J.; Equy, V.; Rivet, B.; Gumery, P.; Hoffmann, P. Non-invasive fetal monitoring using electrocardiography and phonocardiography: A preliminary study. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 455–459. [Google Scholar] [CrossRef]
- Abbas, A.K.; Bassam, R. Phonocardiography Signal Processing; Morgan & Claypool Publishers: San Rafael, CA, USA, 2009; Volume 31. [Google Scholar]
- Penders, J.; Altini, M.; Van Hoof, C.; Dy, E. Wearable sensors for healthier pregnancies. Proc. IEEE 2015, 103, 179–191. [Google Scholar] [CrossRef]
- Rai, D.; Thakkar, H.K.; Rajput, S.S.; Santamaria, J.; Bhatt, C.; Roca, F. A Comprehensive Review on Seismocardiogram: Current Advancements on Acquisition, Annotation, and Applications. Mathematics 2021, 9, 2243. [Google Scholar] [CrossRef]
- Wacker-Gussmann, A.; Strasburger, J.F.; Wakai, R.T. Contribution of Fetal Magnetocardiography to Diagnosis, Risk Assessment, and Treatment of Fetal Arrhythmia. J. Am. Heart Assoc. 2022, 11, e025224. [Google Scholar] [CrossRef]
- Murata, Y.; Martin, C.B., Jr. Systolic time intervals of the fetal cardiac cycle. Postgrad. Med. 1977, 61, 127–134. [Google Scholar] [CrossRef]
- Mahadevan, A. Real Time Ballistocardiogram Artifact Removal in EEG-fMRI Using Dilated Discrete Hermite Transform. Master’s Thesis, University of Akron, Akron, OH, USA, 2008. [Google Scholar]
- Sumali, B.; Mitsukura, Y.; Nishimura, T. Contactless continuous heart rate monitoring system using ballistocardiography. PLoS ONE 2022, 17, e0272072. [Google Scholar] [CrossRef]
- Gonzalez-Landaeta, R.; Ramirez, B.; Mejia, J. Estimation of systolic blood pressure by Random Forest using heart sounds and a ballistocardiogram. Sci. Rep. 2022, 12, 17196. [Google Scholar] [CrossRef] [PubMed]
- Kahankova, R.; Kolarik, J.; Brablik, J.; Barnova, K.; Simkova, I.; Martinek, R. Alternative measurement systems for recording cardiac activity in animals: A pilot study. Anim. Bio. 2022, 10, 15. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Q.; Li, B.; Yuan, C.; Wang, Y. Vital Sign Monitoring and Cardiac Triggering at 1.5 Tesla: A Practical Solution by an MR-Ballistocardiography Fiber-Optic Sensor. Sensors 2019, 19, 474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Padgett, S.; Cai, Z.; Conta, G.; Wu, Y.; He, Q.; Zhang, S.; Sun, C.; Liu, J.; Fan, E.; et al. Single-layered ultra-soft washable smart textiles for all-around ballistocardiograph, respiration, and posture monitoring during sleep. Biosens. Bioelectron. 2020, 155, 112064. [Google Scholar] [CrossRef] [PubMed]
- Ruffo, M. Foetal Heart Rate Recording: Analysis and Comparison of Different Methodologies. Master’s Thesis, Università di Bologna, Bologna, Italy, 2011. [Google Scholar]
- Rolfe, P. Sensors for Fetal and Neonatal Monitoring. In Sensors in Medicine and Health Care: Sensors Applications; Öberg, P.Å., Togawa, T., Spelman, F.A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2004; Volume 3, pp. 187–242. [Google Scholar]
- Adam, J. The Future of Fetal Monitoring. Obstet. Gynecol. 2012, 5, e132–e136. [Google Scholar]
- Kotas, M.; Jezewski, J.; Horoba, K.; Matonia, A. Application of spatio-temporal filtering to fetal electrocardiogram enhancement. Comput. Methods Progr. Biomed. 2011, 104, 1–9. [Google Scholar] [CrossRef]
- Sameni, R.; Clifford, G.D. A Review of Fetal ECG Signal Processing; Issues and Promising Directions. Open Pacing Electrophysiol. Ther. J. 2011, 3, 4–20. [Google Scholar] [CrossRef]
- Rosén, K.G.; Lindecrantz, K. STAN—The Gothenburg model for fetal surveillance during labour by ST analysis of the fetal electrocardiogram. Clin. Phys. Physiol. Meas. 1989, 10 (Suppl. SB), 51–56. [Google Scholar] [CrossRef]
- Reis-de-Carvalho, C.; Nogueira, P.; Ayres-de-Campos, D. Quality of fetal heart rate monitoring with transabdominal fetal ECG during maternal movement in labor: A prospective study. AOGS 2022, 101, 1269–1275. [Google Scholar] [CrossRef]
- NICE. Novii Wireless Patch System for Maternal and Fetal Monitoring. September 2020; pp 1–16. Available online: https://www.gehealthcare.com/products/maternal-infant-care/fetal-monitors/novii-wireless-patch-system (accessed on 10 June 2023).
- Healthcare, P. Philips Mother and Child Care: Avalon; Philips Medizin Systeme Böblingen GmbH: Böblingen, Germany, 2021; pp. 1–7. [Google Scholar]
- Tamirisa, K.P.; Elkayam, U.; Briller, J.E.; Mason, P.K.; Pillarisetti, J.; Merchant, F.M.; Patel, H.; Lakkireddy, D.R.; Russo, A.M.; Volgman, A.S.; et al. Arrhythmias in Pregnancy. J. Am. Coll. Cardiol. 2022, 8, 120–135. [Google Scholar] [CrossRef]
- Jones, K.; Anness, A.; Siddiqui, F. Maternal Chronic Conditions and the Fetus. In Emerging Topics and Controversies in Neonatology; Boyle, E.M., Cusack, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 19–41. [Google Scholar] [CrossRef]
- Huhn, E.A.; Müller, M.I.; Meyer, A.H.; Manegold-Brauer, G.; Holzgreve, W.; Hoesli, I.; Wilhelm, F.H. Quality Predictors of Abdominal Fetal Electrocardiography Recording in Antenatal Ambulatory and Bedside Settings. Fetal Diagn. Ther. 2017, 41, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Sulas, E.; Urru, M.; Tumbarello, R.; Raffo, L.; Sameni, R.; Pani, D. A non-invasive multimodal foetal ECG–Doppler dataset for antenatal cardiology research. Sci. Data 2021, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Matonia, A.; Jezewski, J.; Kupka, T.; Jezewski, M.; Horoba, K.; Wrobel, J.; Czabanski, R.; Kahankowa, R. Fetal electrocardiograms, direct and abdominal with reference heartbeat annotations. Sci. Data 2020, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Jezewski, J.; Matonia, A.; Kupka, T.; Roj, D.; Czabanski, R. Determination of fetal heart rate from abdominal signals: Evaluation of beat-to-beat accuracy in relation to the direct fetal electrocardiogram. Biomed. Tech. 2012, 57, 383–394. [Google Scholar] [CrossRef]
- Barnova, K.; Martinek, R.; Jaros, R.; Kahankova, R.; Matonia, A.; Jezewski, M.; Czabanski, R.; Horoba, K.; Jezewski, J. A novel algorithm based on ensemble empirical mode decomposition for non-invasive fetal ECG extraction. PLoS ONE 2021, 16, e0256154. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, D.J.; Selvakumar, A. Issues and research on foetal electrocardiogram signal elicitation. Biomed. Signal Proc. Control 2014, 10, 224–244. [Google Scholar] [CrossRef]
- Hasan, M.; Reaz, M.; Ibrahimy, M.; Hussain, M.; Uddin, J. Detection and Processing Techniques of FECG Signal for Fetal Monitoring. Biol. Proceed. Online 2009, 11, 236–295. [Google Scholar] [CrossRef]
- Taylor, M.J.O.; Smith, M.J.; Thomas, M.; Green, A.R.; Cheng, F.; Oseku-Afful, S.; Wee, L.Y.; Fisk, N.M.; Gardiner, H.M. Non-invasive fetal electrocardiography in singleton and multiple pregnancies. BJOG 2003, 110, 668–678. [Google Scholar]
- Martinek, R.; Kahankova, R.; Jezewski, J.; Jaros, R.; Mohylova, J.; Fajkus, M.; Nedoma, J.; Janku, P.; Nazeran, H. Comparative Effectiveness of ICA and PCA in Extraction of Fetal ECG From Abdominal Signals: Toward Non-invasive Fetal Monitoring. Front. Physiol. 2018, 9, 648. [Google Scholar] [CrossRef]
- Sato, H.; Yoshimura, K.; Nakamoto, H.; Ishibashi, D.; Nakata, Y.; Yaginuma, Y.; Masui, S. 19.2 cm3 flexible fetal heart rate sensor for improved quality of pregnancy life. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS), Shanghai, China, 17–19 October 2016. [Google Scholar]
- Xu, J.; Hong, Z. Low Power Bio-Impedance Sensor Interfaces: Review and Electronics Design Methodology. IEEE Rev. Biomed. Eng. 2022, 15, 23–35. [Google Scholar] [CrossRef]
- Romano, M.; Iuppariello, L.; Ponsiglione, A.M.; Improta, G.; Bifulco, P.; Cesarelli, M. Frequency and Time Domain Analysis of Foetal Heart Rate Variability with Traditional Indexes: A Critical Survey. Comput. Math Method. Med. 2016, 2016, 9585431. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, E.J.; Orenstein, N.P. Fetal Heart Monitoring. USA7593765, 2 May 2006. [Google Scholar]
- Morris, S.; Bert, S. Foetal Monitor. US3409737A, 24 June 1965. [Google Scholar]
- Abbenante, T.J.; Gieler, V.H.; Weiss, R.E.M.G. Fetal Heart Rate and Intra-Uterine Pressure Monitor System. US3599628A, 17 August 1971. [Google Scholar]
- Epstein, P.; Ballas, J.S., Jr.; Mandler, J.J., Jr.; Van Horn, J.M. Fetal Heart Rate Monitor Apparatus and Method for Combining Electrically and Mechanically Derived Cardiographic Signals. US4519396A, 28 May 1985. [Google Scholar]
- Grumbeck, V.N. Sunburst Wall Plaque or the Like and Method of Making the Same. US4027057A, 31 May 1977. [Google Scholar]
- Epstein, P.; Ballas, J.S., Jr.; Mandler, J.J., Jr.; Van Horn, J.M. Fetal Heart Rate Monitor Apparatus and Method for Combining Electrically and Mechanically Derived Cardiographic Signals. CA1170312A, 3 July 1984. [Google Scholar]
- Yang, C.; Antoine, C.; Young, B.K.; Tavassolian, N. A Pilot Study on Fetal Heart Rate Extraction from Wearable Abdominal Inertial Sensors. IEEE Sens. J. 2019, 19, 10773–10781. [Google Scholar] [CrossRef]
- Hu, K.; Xia, J.; Chen, B.; Tang, R.; Chen, Y.; Ai, J.; Yang, H. A Wireless and Wearable System for Fetal Heart Rate Monitoring. In Proceedings of the 3rd International Conference on Applied Machine Learning (ICAML), Changsha, China, 23–25 July 2021. [Google Scholar]
- Sarafan, S.; Le, T.; Ellington, F.; Zhang, Z.; Lau, M.P.H.; Ghirmai, T.; Hameed, A.; Cao, H. Development of a Home-based Fetal Electrocardiogram (ECG) Monitoring System. In Proceedings of the 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021. [Google Scholar]
- Braun, F.; Bonnier, G.; Rapin, M.; Yilmaz, G.; Proust, Y.-M.; Schneider, S.; Radan, A.-P.; Strahm, K.M.; Surbek, D.; Lemay, M.; et al. Evaluation of a Wearable System for Fetal ECG Monitoring Using Cooperative Sensors. In Proceedings of the 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022. [Google Scholar]
- Heo, J.S.; Eom, J.; Kim, Y.-H.; Park, S.K. Recent Progress of Textile-Based Wearable Electronics: A Comprehensive Review of Materials, Devices, and Applications. Small 2017, 14, 1703034. [Google Scholar] [CrossRef]
- Choudhry, N.A.; Arnold, L.; Rasheed, A.; Khan, I.A.; Wang, L. Textronics—A Review of Textile-Based Wearable Electronics. Adv. Eng. Mater. 2021, 23, 2100469. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Jeong, W. EMG Measurement with Textile-Based Electrodes in Different Electrode Sizes and Clothing Pressures for Smart Clothing Design Optimization. Polymers 2020, 12, 2406. [Google Scholar] [CrossRef]
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef]
- Webb, J.A.; Aggarwal, J.K. Visually Interpreting the Motion of Objects in Space. Computer 1981, 14, 40–46. [Google Scholar] [CrossRef]
- Vojtech, L.; Bortel, R.; Neruda, M.; Kozak, M. Wearable Textile Electrodes for ECG Measurement. Adv. Electr. Electron. Eng. 2013, 11, 410–414. [Google Scholar] [CrossRef]
- Kabakov, S.; Falk, S.M.; Fuchs, B.C. Fetal Heart Monitoring Range. CN103169497A, 12 April 2017. [Google Scholar]
- Galli, A.; Peri, E.; Zhang, Y.; Vullings, R.; Ven, M.v.d.; Giorgi, G.; Ouzounov, S.; Harpe, P.J.A.; Mischi, M. Dedicated Algorithm for Unobtrusive Fetal Heart Rate Monitoring Using Multiple Dry Electrodes. Sensors 2021, 21, 4298. [Google Scholar] [CrossRef]
- Fanelli, A. TeleFetal Care: Development of a Wearable System for Fetal Monitoring during Pregnancy. Ph.D. Thesis, Politecnico di Milano, Milano, Italy, 2013. [Google Scholar]
- Roham, M.; Saldivar, E.; Raghavan, S.; Mehregany, M.; Shah, M. Wireless Fetal Monitoring System. CA2816894C, 4 June 2019. [Google Scholar]
- Manna, S.; Sriraam, N.; Pandian, P. Prototype of Home Based Multi-Channel Wearable Wireless Fetal ECG Monitoring System. In Proceedings of the IEEE International Conference on Electronics, Computing and Communication Technologies (CONECCT), Bangalore, India, 12–14 July 2024. [Google Scholar]
- Manna, S.; Manjula, R. Wearable Fetal Ecg Home Monitoring System. Int. J. Recent Technol. Eng. 2020, 8, 1–5. [Google Scholar] [CrossRef]
- Signorini, M.G.; Lanzola, G.; Torti, E.; Fanelli, A.; Magenes, G. Antepartum Fetal Monitoring through a Wearable System and a Mobile Application. Technologies 2018, 6, 44. [Google Scholar] [CrossRef]
- Aghadavoda, R.; Zarrebini, M.; Shanbeh, M.; Shayegh, F.; Sabet, F. Investigation of fetal ECG signal using textile-based electrodes. J. Text. Inst. 2022, 115, 1–12. [Google Scholar] [CrossRef]
- Baker, D.A. Ambulatory Non-Invasive Automatic Fetal Monitoring System. US4781200A, 1 November 1988. [Google Scholar]
- Amir, U.; Malafriev, O.; Katz, I. Wearable Fetal Monitoring System Having Textile Electrodes. US20170150926A1, 9 December 2016. [Google Scholar]
- Begum, F.; Buckshee, K. Foetal compromise by spontaneous foetal heart rate deceleration in reactive non-stress test and decreased amniotic fluid index. Bangladesh Med. Res. Counc. Bull. 1998, 24, 60–66. [Google Scholar] [PubMed]
- Verma, U.; Garg, R.; Rani, R.; Jain, M.; Pathak, A. Comparative study of foetal colour doppler versus non-stress test as a predictor of perinatal outcome in high risk pregnancy. Obstet. Gynecol. Int. J. 2015, 2, 00065. [Google Scholar] [CrossRef]
- Paliwal, S.; Shaheen, R.; Paliwal, S.; Parakh, P.; Yadav, K.; Chaudhary, G.; Jalandhara, J. Comparative role of non-stress test and colour doppler in high risk pregnancy predicted by placental histopathology and foetal outcome. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 6, 1444–1449. [Google Scholar] [CrossRef]
- Fanelli, A.; Ferrario, M.; Piccini, L.; Andreoni, G.; Matrone, G.; Magenes, G.; Signorini, M.G. Prototype of a wearable system for remote fetal monitoring during pregnancy. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5815–5818. [Google Scholar]
- Lanzola, G.; Secci, I.; Scarpellini, S.; Fanelli, A.; Magenes, G.; Signorini, M.G. A Mobile Remote Monitoring Service for Measuring Fetal Heart Rate. In Proceedings of the XIII Mediterranean Conference on Medical and Biological Engineering and Computing 2013, Seville, Spain, 25–28 September 2013; pp. 1435–1438. [Google Scholar]

| Heart Rate (HR) BPM | Expected HR BPM | QRS Spectral Energy (Hz) | Peak-to-Peak Amplitude (μV) | |
|---|---|---|---|---|
| Fetal | 60–240 | 140 | 20–60 | 3–25 |
| Maternal | 50–210 | 80 | 10–30 | 100 |
| Method | Advantages | Limitations | Gestational Age | Energy type | Accuracy Issues in FHR Monitoring | Installation Cost |
|---|---|---|---|---|---|---|
| fECG | Cost-effective, user-friendly, continuous long-term monitoring, enabling precise beat-to-beat variability tracking for comprehensive fetal heart assessment | Complex design requirements, dependency on fetal orientation in utero, signal quality variations due to maternal and fetal movement, potentially leading to data inaccuracies | Around the 20th week of gestation onwards | Electrical | Accuracy can be compromised by signal interference from maternal ECG and movement artifacts, leading to potential false readings. | Moderate to high, depending on the complexity of the system and the need for specialized electrodes |
| fCTG | Economic, low-power, safe, and versatile monitoring capabilities, making it easily manageable, suitable for extended recordings, adaptable to clinical environments, portable, and viable within MRI settings | May result in a higher rate of false positives and interventions due to its sensitivity to maternal factors and reduced specificity in predicting fetal distress | Around the 28th week onwards | Ultrasound | Potential for false positives due to sensitivity to external factors, leading to unnecessary interventions | Low to moderate, typically involves standard ultrasound equipment |
| fBCG | Non-invasive and continuous fetal heart activity monitoring provides a low-cost, radiation-free, and sensitive method for long-term pregnancy assessment | Accuracy can be affected by factors like fetal position and movement, potentially leading to inconsistent readings | Usually from the third trimester | Mechanical | Inconsistent readings due to fetal movements and sensitivity to maternal positioning, which can affect accuracy | Low to moderate, generally less expensive due to fewer required components |
| fPCG | Non-invasive and safe means of monitoring fetal heart sounds, facilitating early detection of anomalies and providing valuable insights into fetal well-being during pregnancy | Monitoring might be prone to external noise interference, which could impact the accuracy of detecting fetal heart sounds | Around the 20th week of gestation | Acoustic | Susceptible to external noise and maternal movement, which can cause inaccuracies in detecting fetal heart sounds | Low, as it primarily requires a stethoscope or microphone system |
| Device/Method | Advantages | Disadvantages | Applications/Use Case | Specific Devices |
|---|---|---|---|---|
| Ultrasound | Widely used in hospitals and public health facilities. Offers detailed fetal information, including heart rate. Portable versions for continuous monitoring still under development. | High cost limits the duration of monitoring. Non-ambulatory; requires clinical setting. Laborious alignment of transducer with fetal heart. Inaccurate readings due to patient movement. | Commonly used for prenatal diagnostics to monitor fetal heart rate and other organs. Not suitable for continuous, long-term monitoring. Limited by high-frequency ultrasound vibrations. High cost and specialized training required. | Standard ultrasound equipment. |
| STAN Monitoring System | Provides ST-segment analysis using fetal scalp electrodes. Analyzes the relationship between T-wave and R-wave for clinical assessment. | Requires invasive procedure (fetal scalp electrode). Limited to the labor stage; not applicable earlier in pregnancy. Sensitivity limited by noise; difficult to detect activities affecting specific areas of the heart. | Used during labor to monitor fetal heart health. Particularly useful for high-risk pregnancies. | STAN Monitor (Neoventa Medical) |
| Transabdominal ECG (fECG) | Continuous signal transmission, even with maternal movement. Non-invasive; allows for monitoring at home when coupled with a mobile application. High accuracy for ECG and Doppler telemetry (88.5–89.4%). | Signal quality can be affected by maternal ECG and abdominal muscle activity. Low SNR due to interference from maternal ECG. Challenging to extract clear signals due to noise and interference. | High-risk pregnancies (e.g., fetal growth restriction, arrhythmias). Can be used from earlier stages of pregnancy up to labor. | Novii Wireless Patch System (GE Healthcare) Monica AN24 Monitor (Monica Healthcare) Philips Avalon FM30 (Philips Healthcare) |
| Fetal Phonocardiography (fPCG) | Non-invasive and safe; detects fetal heart sounds. | Highly susceptible to external noise interference. | Early detection of cardiac anomalies. | Standard phonocardiography devices. |
| MindChild Medical | Simple and low-cost setup. Continuous fetal monitoring with advanced technology. Portable and adaptable to different environments. | Limited by the acoustic properties of the maternal abdomen. Installation and operational costs might be high. May require specialized training for effective use. | Can be used from the 20th week of gestation. Used in clinical settings for detailed fetal monitoring. Suitable for long-term and high-risk pregnancy monitoring. | MindChild Medical Monitor |
| Chosen Yarn | Fabrication Method | Comparable to Gel fECG? |
|---|---|---|
| Silver Plated Nylon [102] | Electrodes sewn onto belt | Yes, but uncertain which electrode worked |
| Cotton/Silver/Spandex [102] | Electrodes sewn onto the belt | Yes, but uncertain which electrode worked |
| Silver Plated Nylon [103] | Electrodes sewn onto the belt | Yes, but uncertain which electrode worked |
| Cotton/Silver/Spandex [103] | Electrodes sewn onto the belt | Yes, but uncertain which electrode worked |
| 40% Stainless Steel/60% Polyester [99] | Knitted electrode that is sewn onto the belt | No |
| 80% Stainless Steel/20% Polyester [99] | Knitted electrode that is sewn onto the belt | No |
| 100% Stainless Steel [99] | Sewn electrode that is sewn onto the belt | Yes |
| Conductive Fabric [104] | Held in place by an elastic belt | Yes |
| Silver Yarn [105] | Yarn sewn directly on cotton/lycra bodysuit | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.R.; Newby, S.; Potluri, P.; Mirihanage, W.; Fernando, A. Emerging Paradigms in Fetal Heart Rate Monitoring: Evaluating the Efficacy and Application of Innovative Textile-Based Wearables. Sensors 2024, 24, 6066. https://doi.org/10.3390/s24186066
Ahmed MR, Newby S, Potluri P, Mirihanage W, Fernando A. Emerging Paradigms in Fetal Heart Rate Monitoring: Evaluating the Efficacy and Application of Innovative Textile-Based Wearables. Sensors. 2024; 24(18):6066. https://doi.org/10.3390/s24186066
Chicago/Turabian StyleAhmed, Md Raju, Samantha Newby, Prasad Potluri, Wajira Mirihanage, and Anura Fernando. 2024. "Emerging Paradigms in Fetal Heart Rate Monitoring: Evaluating the Efficacy and Application of Innovative Textile-Based Wearables" Sensors 24, no. 18: 6066. https://doi.org/10.3390/s24186066
APA StyleAhmed, M. R., Newby, S., Potluri, P., Mirihanage, W., & Fernando, A. (2024). Emerging Paradigms in Fetal Heart Rate Monitoring: Evaluating the Efficacy and Application of Innovative Textile-Based Wearables. Sensors, 24(18), 6066. https://doi.org/10.3390/s24186066








