Gas Sensing Properties of Indium–Oxide–Based Field–Effect Transistor: A Review
Abstract
:1. Introduction
2. Typical Transducers for In2O3 FET Gas Sensors
2.1. MOSFET
2.2. TFT
3. Properties of In2O3 FET Gas Sensors
3.1. Responsivity, Sensitivity, and Limit of Detection
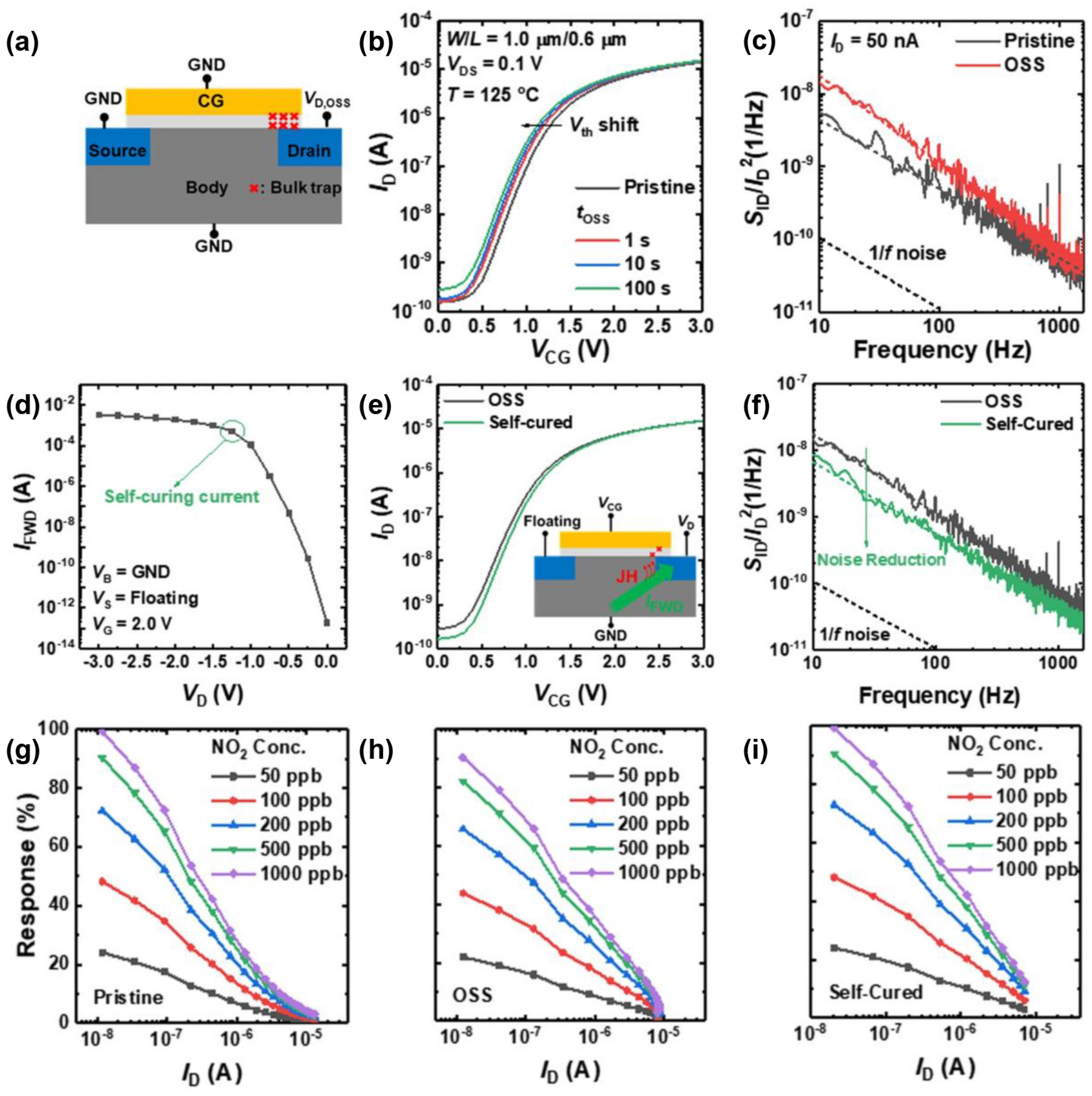
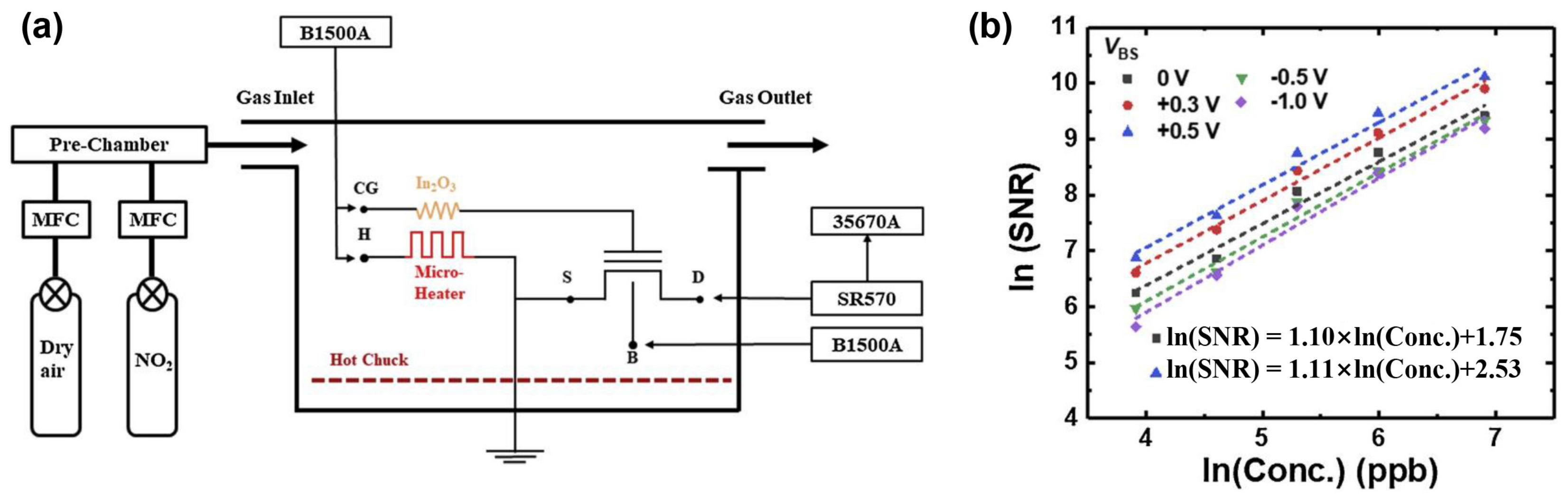
3.2. Signal–to–Noise Ratio
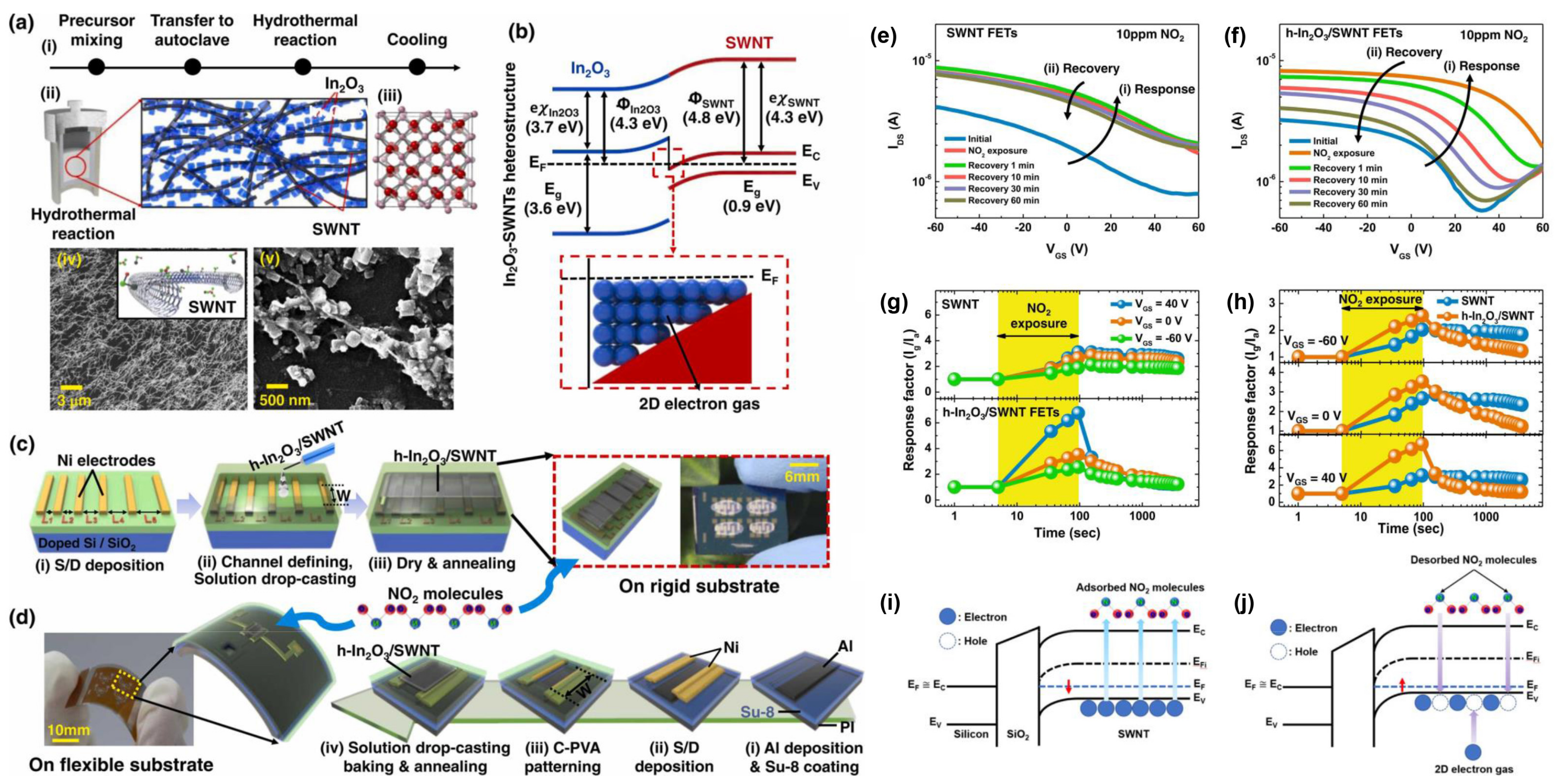
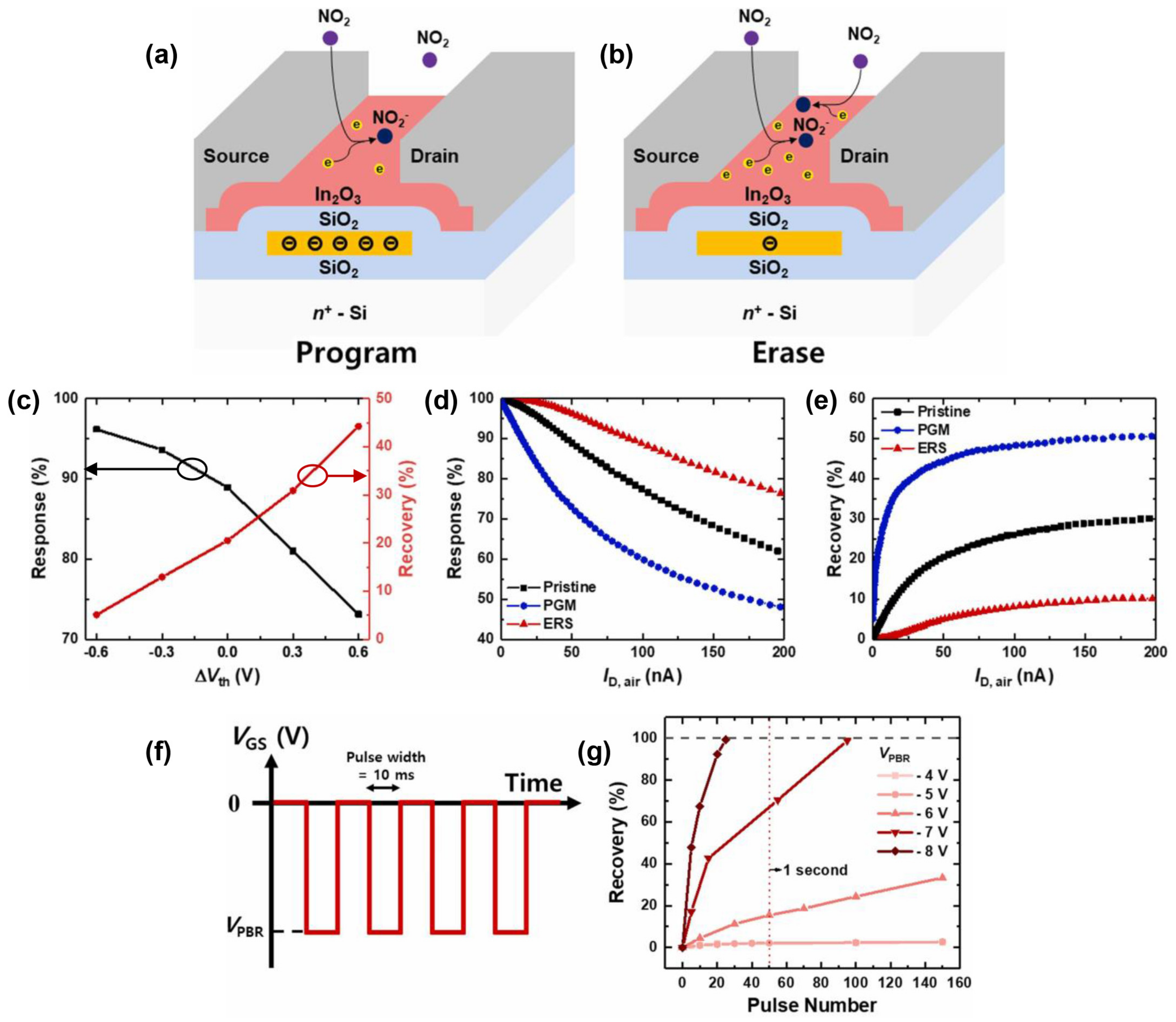
3.3. Response and Recovery
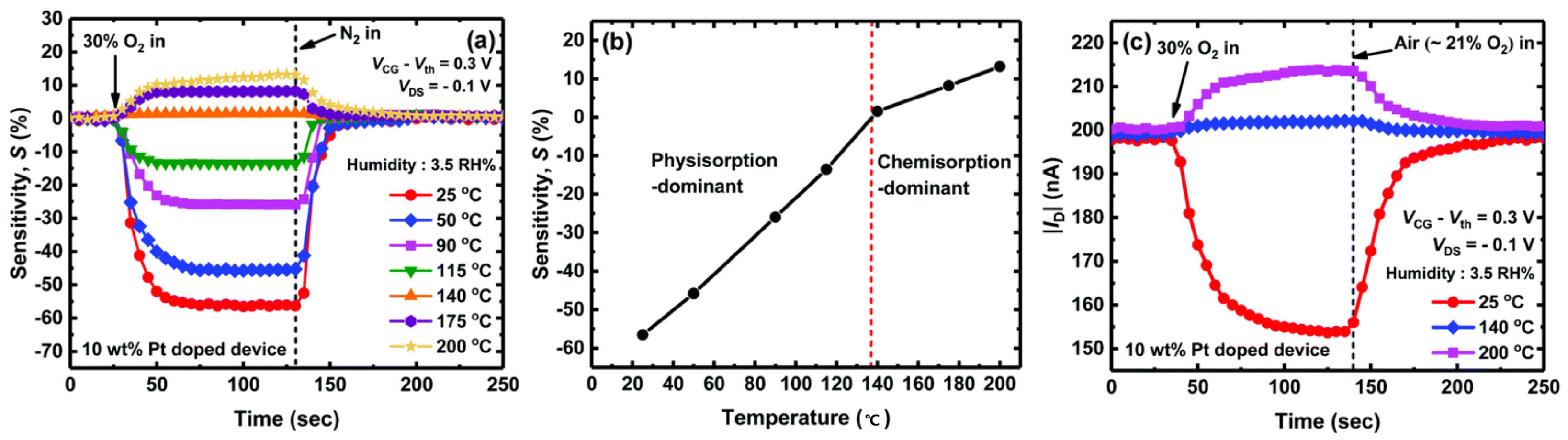
3.4. Low–Temperature Detection Capability
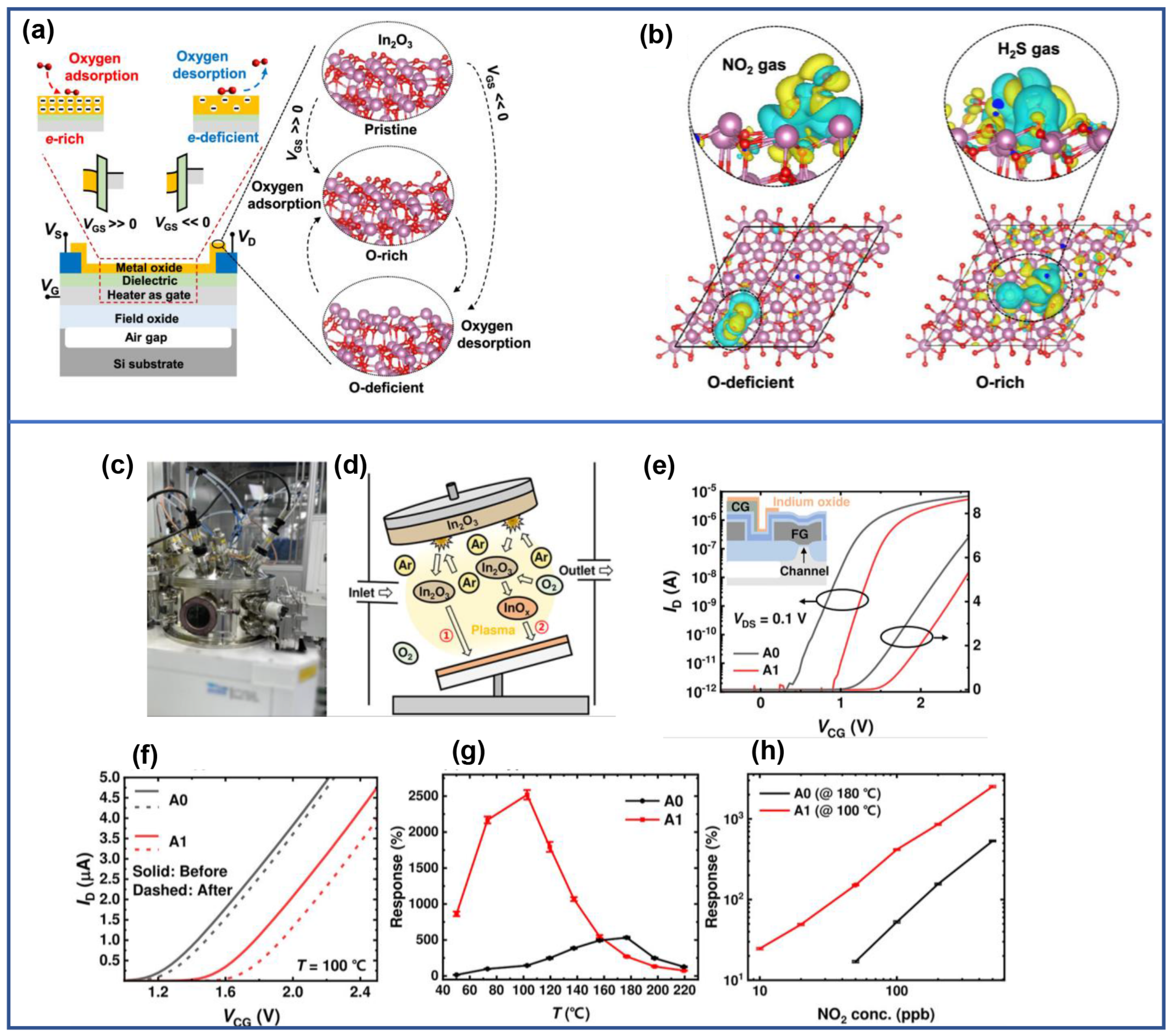
3.5. Selectivity
4. Performance Optimization of In2O3 FET Gas Sensors
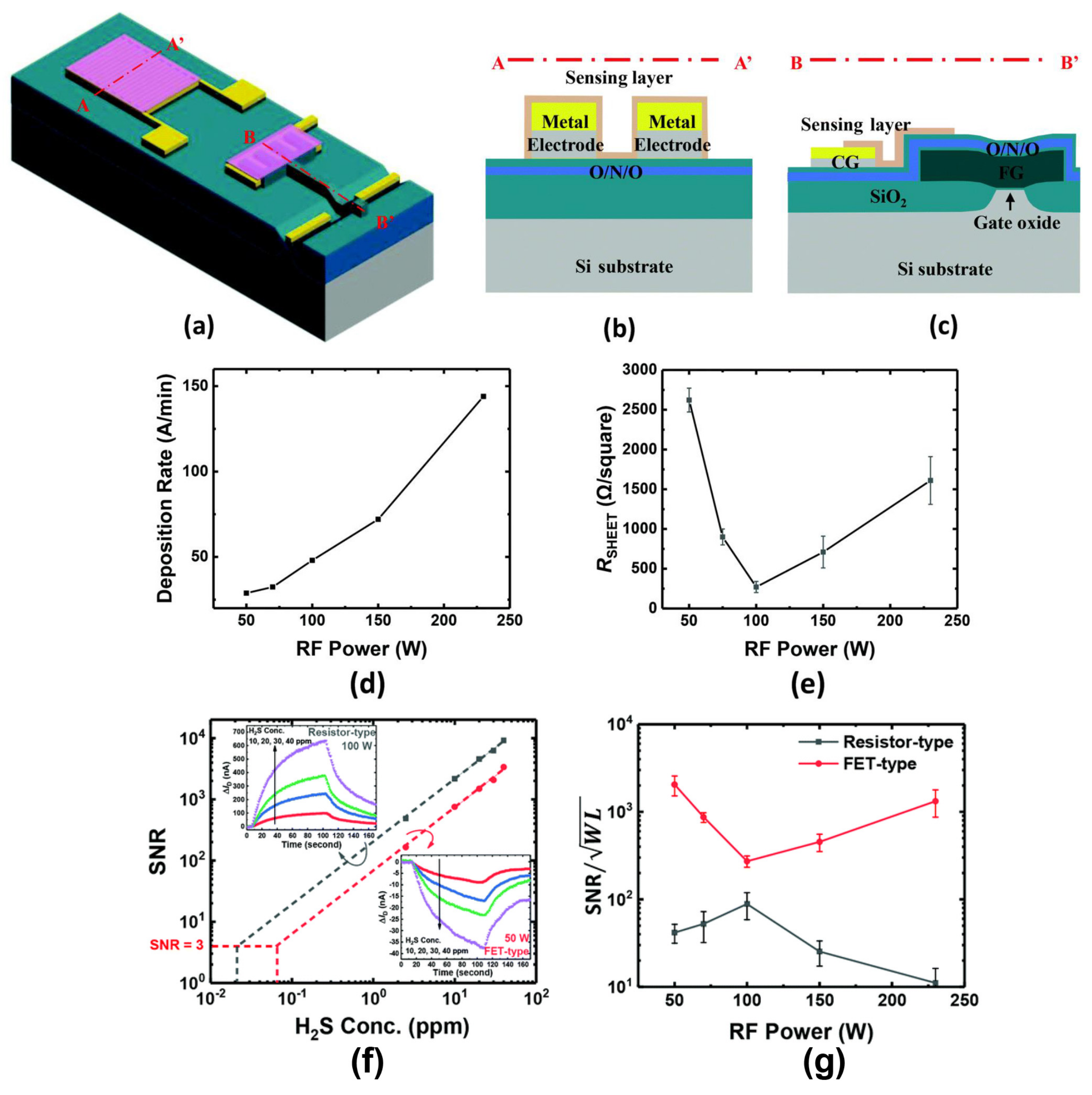
4.1. Optimization of FET Struture
4.2. Optimization of Operating Conditions
4.3. Algorithm
5. Challenges and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nadargi, D.Y.; Umar, A.; Nadargi, J.D.; Lokare, S.A.; Akbar, S.; Mulla, I.S.; Suryavanshi, S.S.; Bhandari, N.L.; Chaskar, M.G. Gas sensors and factors influencing sensing mechanism with a special focus on MOS sensors. J. Mater. Sci. 2023, 58, 559–582. [Google Scholar] [CrossRef]
- Verma, A.; Gupta, R.; Verma, A.S.; Kumar, T. Review–Recent Advances and Challenges of Conducting Polymer–Metal Nanocomposites for the Detection of Industrial Waste Gases. ECS J. Solid State Sci. Technol. 2023, 12, 20. [Google Scholar] [CrossRef]
- Ryabtsev, S.V.; Shaposhnick, A.V.; Lukin, A.N.; Domashevskaya, E.P. Application of semiconductor gas sensors for medical diagnostics. Sens. Actuator B–Chem. 1999, 59, 26–29. [Google Scholar] [CrossRef]
- Joshi, N.; Pransu, G.; Conte, C.A. Critical review and recent advances of 2D materials–Based gas sensors for food spoilage detection. Crit. Rev. Food Sci. Nutr. 2023, 63, 10536–10559. [Google Scholar] [CrossRef] [PubMed]
- Preethichandra, D.M.G.; Gholami, M.D.; Izake, E.L.; O’Mullane, A.P.; Sonar, P. Conducting Polymer Based Ammonia and Hydrogen Sulfide Chemical Sensors and Their Suitability for Detecting Food Spoilage. Adv. Mater. Technol. 2023, 8, 34. [Google Scholar] [CrossRef]
- Balan, T.; Dumitru, C.; Dudnik, G.; Alessi, E.; Lesecq, S.; Correvon, M.; Passaniti, F.; Licciardello, A. Smart Multi–Sensor Platform for Analytics and Social Decision Support in Agriculture. Sensors 2020, 20, 29. [Google Scholar] [CrossRef]
- Man, G.; Stoeber, B.; Walus, K. An assessment of sensing technologies for the detection of clandestine methamphetamine drug laboratories. Forensic Sci. Int. 2009, 189, 1–13. [Google Scholar] [CrossRef]
- Sun, Z.G.; Huang, L.X.; Zhang, Y.; Wu, X.F.; Zhang, M.H.; Liang, J.H.; Bao, Y.W.; Xia, X.H.; Gu, H.S.; Homewood, K.; et al. Homojunction TiO2 thin film–based room–temperature working H2 sensors with non–noble metal electrodes. Sens. Actuator B–Chem. 2024, 398, 11. [Google Scholar] [CrossRef]
- Epping, R.; Koch, M. On–Site Detection of Volatile Organic Compounds (VOCs). Molecules 2023, 28, 19. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy–saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 25. [Google Scholar] [CrossRef]
- Li, Z.J.; Li, H.; Wu, Z.L.; Wang, M.K.; Luo, J.T.; Torun, H.D.; Hu, P.A.; Yang, C.; Grundmann, M.; Liu, X.T.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high–precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 15. [Google Scholar] [CrossRef]
- Chu, J.F.; Li, W.J.; Yang, X.; Wu, Y.; Wang, D.W.; Yang, A.J.; Yuan, H.; Wang, X.H.; Li, Y.J.; Rong, M.Z. Identification of gas mixtures via sensor array combining with neural networks. Sens. Actuator B–Chem. 2021, 329, 10. [Google Scholar] [CrossRef]
- Song, X.Y.; Liu, T.; Gu, K.K.; Luo, Z.B.; Zhang, M.Z. Highly selective and ultra–sensitive gas sensor based on Fe2O3/Ti3C2Tx MXene heterostructure for ppb–level n–butanol detection. J. Alloy Compd. 2024, 976, 10. [Google Scholar] [CrossRef]
- Lee, J.; Gam, D.; Nam, K.E.; Cho, S.J.; Kim, H. Feasibility study of a resistive–type sodium aerosol detector with ZnO nanowires for sodium–cooled fast reactors. Nucl. Eng. Technol. 2023, 55, 2373–2379. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable Sensors for Ubiquitous Monitoring of Gases in the Era of Internet of Things and Industrial Internet. Chem. Rev. 2016, 116, 14918. [Google Scholar] [CrossRef]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible Graphene–Based Wearable Gas and Chemical Sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, X.H.; Zhang, J.; Kumar, M. Room–Temperature Gas Sensors Under Photoactivation: From Metal Oxides to 2D Materials. Nano–Micro Lett. 2020, 12, 37. [Google Scholar] [CrossRef]
- Lu, H.; Seabaugh, A. Tunnel Field–Effect Transistors: State–of–the–Art. IEEE J. Electron Devices Soc. 2014, 2, 44–49. [Google Scholar] [CrossRef]
- Qiu, C.G.; Zhang, Z.Y.; Xiao, M.M.; Yang, Y.J.; Zhong, D.L.; Peng, L.M. Scaling carbon nanotube complementary transistors to 5–nm gate lengths. Science 2017, 355, 271–276. [Google Scholar] [CrossRef]
- Xu, K.K. Silicon MOS Optoelectronic Micro–Nano Structure Based on Reverse–Biased PN Junction. Phys. Status Solidi A–Appl. Mat. 2019, 216, 9. [Google Scholar] [CrossRef]
- Paghi, A.; Mariani, S.; Barillaro, G. 1D and 2D Field Effect Transistors in Gas Sensing: A Comprehensive Review. Small 2023, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Wu, M.; Hong, Y.; Jeong, Y.; Jung, G.; Shin, W.; Park, J.; Kim, D.; Jang, D.; Lee, J.H. FET–type gas sensors: A review. Sens. Actuator B–Chem. 2021, 330, 24. [Google Scholar] [CrossRef]
- Zeng, S.M.; Zhang, Y.; Zhang, Y.; Li, Y.N.; Tang, C.G.; Li, K.; Sun, J.Y.; Deng, T. A novel room temperature SO2 gas sensor based on TiO2/rGO buried–gate FET. Microelectron. Eng. 2022, 263, 7. [Google Scholar] [CrossRef]
- Shaji, M.; Saji, K.J.; Jayaraj, M.K. Low temperature operated ZTO thin film transistor based gas sensor for selective detection of H2S. Mater. Sci. Semicond. Process 2022, 150, 7. [Google Scholar] [CrossRef]
- Liao, F.; Chen, C.; Subramanian, V. Organic TFTs as gas sensors for electronic nose applications. Sens. Actuator B Chem. 2005, 107, 849–855. [Google Scholar] [CrossRef]
- Han, J.K.; Kang, M.; Jeong, J.; Cho, I.; Yu, J.M.; Yoon, K.J.; Park, I.; Choi, Y.K. Artificial Olfactory Neuron for an In–Sensor Neuromorphic Nose. Adv. Sci. 2022, 9, 11. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Goda, T.; Yatabe, R.; Oki, A.; Matsumoto, A.; Oka, H.; Washio, T.; Toko, K.; Miyahara, Y. Field–effect transistor array modified by a stationary phase to generate informative signal patterns for machine learning–assisted recognition of gas–phase chemicals. Mol. Syst. Des. Eng. 2019, 4, 386–389. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; Sangwan, V.K.; Wang, B.H.; Zeng, L.; Wang, G.; Huang, Y.; Lu, Z.Y.; Bedzyk, M.J.; Hersam, M.C.; et al. Polymer Doping Enables a Two–Dimensional Electron Gas for High–Performance Homojunction Oxide Thin–Film Transistors. Adv. Mater. 2019, 31, 8. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Meng, F.Y.; Shi, J.H.; Huang, W.; Liu, W.Z.; Liu, Z.X. High mobility Ti, Zr and Ga–codoping In2O3 transparent conductive oxide films prepared at low temperatures. J. Mater. Sci.–Mater. Electron. 2021, 32, 3201–3210. [Google Scholar] [CrossRef]
- Huang, W.; Yu, X.G.; Zeng, L.; Wang, B.H.; Takai, A.; Di Carlo, G.; Bedzyk, M.J.; Marks, T.J.; Facchetti, A. Ultraviolet Light–Densified Oxide–Organic Self–Assembled Dielectrics: Processing Thin–Film Transistors at Room Temperature. ACS Appl. Mater. Interfaces 2021, 13, 3445–3453. [Google Scholar] [CrossRef]
- Zhang, X.N.; Wang, B.H.; Huang, W.; Chen, Y.; Wang, G.; Zeng, L.; Zhu, W.G.; Bedzyk, M.J.; Zhang, W.F.; Medvedeva, J.E.; et al. Synergistic Boron Doping of Semiconductor and Dielectric Layers for High–Performance Metal Oxide Transistors: Interplay of Experiment and Theory. J. Am. Chem. Soc. 2018, 140, 12501–12510. [Google Scholar] [CrossRef]
- Pham, A.T.; Luu, O.A.M.; Nguyen, A.D.; Chu, M.H. Porous In2O3 nanorods fabricated by hydrothermal method for an effective CO gas sensor. Mater. Res. Bull. 2021, 137, 9. [Google Scholar]
- Son, D.N.; Hung, C.M.; Le, D.T.T.; Xuan, C.T.; Van Duy, N.; Dich, N.Q.; Nguyen, H.; Van Hieu, N.; Hoa, N.D. A novel design and fabrication of self–heated In2O3 nanowire gas sensor on for ethanol detection. Sens. Actuator A–Phys. 2022, 345, 11. [Google Scholar] [CrossRef]
- Cheng, P.F.; Wang, Y.L.; Wang, C.; Ma, J.; Xu, L.P.; Lv, C.; Sun, Y.F. Investigation of doping effects of different noble metals for ethanol gas sensors based on mesoporous In2O3. Nanotechnology 2021, 32, 15. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.B.; An, B.X.; Bai, J.L.; Wang, Y.R.; Cheng, X.; Wang, Q.; Li, J.P.; Yang, Y.F.; Wu, Z.K.; Xie, E.Q. Ultrahigh–response hydrogen sensor based on PdO/NiO co–doped In2O3nanotubes. J. Colloid Interface Sci. 2021, 599, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Li, S.; Xiao, S.; Du, K. Down to ppb level NO2 detection by vertically MoS2 nanoflakes grown on In2O3 microtubes at room temperature. Colloid Surf. A–Physicochem. Eng. Asp. 2022, 648, 12. [Google Scholar] [CrossRef]
- Liu, M.; Song, P.; Yang, Z.X.; Wang, Q. MXene/In2O3nanocomposites for formaldehyde detection at low temperature. Inorg. Chem. Commun. 2023, 148, 8. [Google Scholar] [CrossRef]
- Du, N.; Zhang, H.; Chen, B.D.; Ma, X.Y.; Liu, Z.H.; Wu, J.B.; Yang, D.R. Porous indium oxide nanotubes: Layer–by–layer assembly on carbon–nanotube templates and application for room–temperature NH3 gas sensors. Adv. Mater. 2007, 19, 1641–1645. [Google Scholar] [CrossRef]
- Yi, S.; Tian, S.Q.; Zeng, D.W.; Xu, K.; Zhang, S.P.; Xie, C.S. An In2O3 nanowire–like network fabricated on coplanar sensor surface by sacrificial CNTs for enhanced gas sensing performance. Sens. Actuator B–Chem. 2013, 185, 345–353. [Google Scholar] [CrossRef]
- Hong, S.Z.; Huang, Q.Y.; Wu, T.M. Facile Synthesis of Polyaniline/Carbon–Coated Hollow Indium Oxide Nanofiber Composite with Highly Sensitive Ammonia Gas Sensor at the Room Temperature. Sensors 2022, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Fatema, K.N.; Sagadevan, S.; Liu, Y.; Cho, K.Y.; Jung, C.H.; Oh, W.C. New design of mesoporous SiO2 combined In2O3–graphene semiconductor nanocomposite for highly effective and selective gas detection. J. Mater. Sci. 2020, 55, 13085–13101. [Google Scholar] [CrossRef]
- Liu, X.J.; Jiang, L.; Jiang, X.M.; Tian, X.Y.; Huang, Y.; Hou, P.Y.; Zhang, S.W.; Xu, X.J. Design of superior ethanol gas sensor based on indium oxide/molybdenum disulfide nanocomposite via hydrothermal route. Appl. Surf. Sci. 2018, 447, 49–56. [Google Scholar] [CrossRef]
- Mansha, M.; Qurashi, A.; Ullah, N.; Bakare, F.O.; Khan, I.; Yamani, Z.H. Synthesis of In2O3/graphene heterostructure and their hydrogen gas sensing properties. Ceram. Int. 2016, 42, 11490–11495. [Google Scholar] [CrossRef]
- Hong, S.; Shin, J.; Hong, Y.; Wu, M.; Jang, D.; Jeong, Y.; Jung, G.; Bae, J.H.; Jang, H.W.; Lee, J.H. Observation of physisorption in a high–performance FET–type oxygen gas sensor operating at room temperature. Nanoscale 2018, 10, 18019–18027. [Google Scholar] [CrossRef]
- Hong, S.; Hong, Y.; Jeong, Y.; Jung, G.; Shin, W.; Park, J.; Lee, J.K.; Jang, D.; Bae, J.H.; Le, J.H. Improved CO gas detection of Si MOSFET gas sensor with catalytic Pt decoration and pre–bias effect. Sens. Actuator B–Chem. 2019, 300, 8. [Google Scholar] [CrossRef]
- Shin, W.; Jung, G.; Hong, S.; Jeong, Y.; Park, J.; Kim, D.; Jang, D.; Kwon, D.; Bae, J.H.; Park, B.G.; et al. Proposition of deposition and bias conditions for optimal signal–to–noise–ratio in resistor– and FET–type gas sensors. Nanoscale 2020, 12, 19768–19775. [Google Scholar] [CrossRef]
- Shin, W.; Jung, G.; Hong, S.; Jeong, Y.; Park, J.; Jang, D.; Park, B.G.; Lee, J.H. Low frequency noise characteristics of resistor– and Si MOSFET–type gas sensors fabricated on the same Si wafer with In2O3 sensing layer. Sens. Actuator B–Chem. 2020, 318, 11. [Google Scholar] [CrossRef]
- Shin, W.; Hong, S.; Jung, G.; Jeong, Y.; Park, J.; Kim, D.; Jang, D.; Park, B.G.; Lee, J.H. Improved signal–to–noise–ratio of FET–type gas sensors using body bias control and embedded micro–heater. Sens. Actuator B–Chem. 2021, 329, 9. [Google Scholar] [CrossRef]
- Jung, G.; Hong, S.; Jeong, Y.; Shin, W.; Park, J.; Kim, D.; Bae, J.H.; Park, B.G.; Lee, J.H. Response Comparison of Resistor– and Si FET–Type Gas Sensors on the Same Substrate. IEEE Trans. Electron Devices 2021, 68, 3552–3557. [Google Scholar] [CrossRef]
- Shin, W.; Jeong, Y.; Kim, M.; Lee, J.; Koo, R.H.; Hong, S.; Jung, G.; Kim, J.J.; Lee, J.H. Recovery of off–state stress–induced damage in FET–type gas sensor using self–curing method. Discov. Nano. 2023, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Ju, S.; Choi, K.; Kim, J.; Hong, S.; Park, J.; Shin, W.; Jeong, Y.; Han, S.; Choi, W.Y.; et al. Reconfigurable Manipulation of Oxygen Content on Metal Oxide Surfaces and Applications to Gas Sensing. ACS Nano 2023, 17, 17790–17798. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Shin, H.; Jeon, S.W.; Lim, Y.H.; Hong, S.; Kim, D.H.; Lee, J.H. Transducer–Aware Hydroxy–Rich–Surface Indium Oxide Gas Sensor for Low–Power and High–Sensitivity NO2 Gas Sensing. ACS Appl. Mater. Interfaces 2023, 15, 22651–22661. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.B.; Jeong, Y.; Jung, G.Y.W.; Shin, W.; Park, J.; Kim, D.; Lee, C.Y.; Lee, J.H. Macroscopic analysis and design of Si HFGFET gas sensor for sensitive gas detection. Solid–State Electron. 2023, 200, 6. [Google Scholar] [CrossRef]
- Park, J.; Hong, S.; Jeong, Y.; Jung, G.; Shin, W.; Kim, D.; Lee, C.; Lee, J.H. H2S gas sensing properties in polysilicon control–gate FET–type gas sensor. Solid–State Electron. 2023, 200, 7. [Google Scholar] [CrossRef]
- Seetha, M.; Mangalaraj, D. Nano–porous indium oxide transistor sensor for the detection of ethanol vapours at room temperature. Appl. Phys. A–Mater. Sci. Process. 2012, 106, 137–143. [Google Scholar] [CrossRef]
- Wang, L.P.; Xu, X.D. Semiconducting properties of In2O3 nanoparticle thin films in air and nitrogen. Ceram. Int. 2015, 41, 7687–7692. [Google Scholar] [CrossRef]
- Shariati, M.; Khosravinejad, F. The Laser–Assisted Field Effect Transistor Gas Sensor Based on Morphological Zinc–Excited Tin–Doped In2O3 Nanowires. Surf. Rev. Lett. 2017, 24, 11. [Google Scholar] [CrossRef]
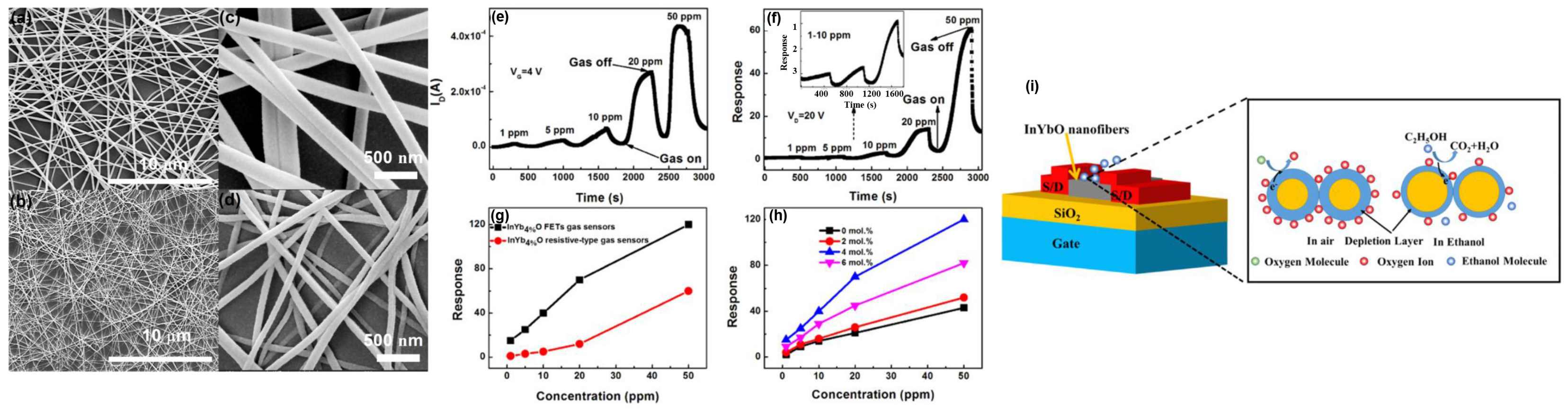
- Jun, L.; Chen, Q.; Fu, W.H.; Yang, Y.H.; Zhu, W.Q.; Zhang, J.H. Electrospun Yb–Doped In2O3 Nanofiber Field–Effect Transistors for Highly Sensitive Ethanol Sensors. ACS Appl. Mater. Interfaces 2020, 12, 38425–38434. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Fu, W.H.; Yang, Y.H.; Zhu, W.Q.; Zhang, J.H. Detection of N,N–dimethylformamide vapor down to ppb level using electrospun InYbO nanofibers field–effect transistor. Sens. Actuator B–Chem. 2020, 323, 10. [Google Scholar] [CrossRef]
- Li, J.; Li, L.K.; Chen, Q.; Zhu, W.Q.; Zhang, J.H. Ultrasensitive room–temperature acetone gas sensors employing green–solvent–processed aligned InNdO nanofiber field–effect transistors. J. Mater. Chem. C 2022, 10, 860–869. [Google Scholar] [CrossRef]
- Seo, S.G.; Baek, J.I.; Mishra, D.; Jo, H.; Kwon, H.I.; Jin, S.H. One–pot hydrothermal growth of indium oxide–CNT heterostructure via single walled carbon nanotube scaffolds and their application toward flexible NO2 gas sensors. J. Alloy. Compd. 2022, 922, 11. [Google Scholar] [CrossRef]
- Yeom, G.; Kwon, D.; Shin, W.; Park, M.K.; Kim, J.J.; Lee, J.H. Fast–response/recovery In2O3 thin–film transistor–type NO2 gas sensor with floating–gate at low temperature. Sens. Actuator B–Chem. 2023, 394, 9. [Google Scholar] [CrossRef]
- Lv, Q.J.; Li, R.F.; Qu, Y.H.; Yu, M.Y.; Zhang, L.; Li, H.H. CeO2 nanorods decorated In2O3nanoparticles for enhanced low temperature detection of hydrogen. Inorg. Chem. Commun. 2023, 158, 9. [Google Scholar] [CrossRef]
- Meng, D.; Qiao, T.T.; Ji, Y.; Wang, R.S.; Zhang, Y.; San, X.G.; Jin, Q.; Wang, X.L. Hydrangea–Like In2O3@In2S3 n–n Heterostructures for High–Efficiency TMA Measurement. IEEE Trans. Instrum. Meas. 2023, 72, 9. [Google Scholar] [CrossRef]
- An, D.M.; Wang, Y.Y.; Dai, J.L.; Guo, L.P.; Liu, N. Synthesis of Pr doped In2O3 nanoparticles and their enhanced ethanol gas–sensing performance. Ceram. Int. 2024, 50, 4945–4954. [Google Scholar] [CrossRef]
- Min, S.K.; Kim, H.S.; Chang, S.P. Highly sensitive toluene sensor based on porous core–shell–structured In2O3–ZnO nanofibers under UV irradiation at room temperature. Phys. E 2023, 154, 13. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.A.; Liu, J.W.; Wang, Z.; Sun, S.Y.; Li, M.T.; Yan, W. ZIF–L(Co) derived cobalt doped In2O3 hollow nanofibers with high surface activity for efficient formaldehyde gas sensing. Sens. Actuator B Chem. 2024, 403, 9. [Google Scholar] [CrossRef]
- Kong, D.L.; Niu, J.Y.; Hong, B.; Xu, J.C.; Han, Y.B.; Peng, X.L.; Ge, H.L.; Li, J.; Zeng, Y.X.; Wang, X.Q. Ag–nanoparticles–anchored mesoporous In2O3 nanowires for ultrahigh sensitive formaldehyde gas sensors. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2023, 291, 10. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Liu, Y.A.; Wang, C.; Xu, J.Q.; Bai, Y.L. In2O3 decorated Co32 cluster for high performance H2S MEMS sensors. Inorg. Chem. Commun. 2023, 157, 7. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, Y.W.; Jin, X.H.; Sun, G.; Cao, J.L.; Wang, Y. The effects of Co doping on the gas sensing performance of In2O3 porous nanospheres. Sens. Actuator B Chem. 2024, 403, 11. [Google Scholar] [CrossRef]
- He, T.; Liu, H.C.; Zhang, J.; Yang, Y.P.; Hu, Y.J.; Zhang, Y.; Hu, K.L. DFT study on the adsorption and sensing properties of dissolved gases (H2, CO and CH4) in transformer oil on PdO–doped In2O3 (110) surfaces. Chem. Phys. Lett. 2023, 832, 9. [Google Scholar] [CrossRef]
- Liu, Y.P.; Yang, Z.; Huang, L.; Zeng, W.; Zhou, Q. Anti–interference detection of mixed NOX via In2O3–based sensor array combining with neural network model at room temperature. J. Hazard. Mater. 2024, 463, 12. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.M.; Wang, J.L.; Liu, X.Q.; Wang, C.L.; Jiang, Y.; Wang, Y.; Xiao, X.H.; Ho, J.C.; Li, J.C.; Jiang, C.Z.; et al. Rational Design of Sub–Parts per Million Specific Gas Sensors Array Based on Metal Nanoparticles Decorated Nanowire Enhancement–Mode Transistors. Nano Lett. 2013, 13, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Takahashi, T.; Kanai, M.; Zhang, G.Z.; He, Y.; Nagashima, K.; Yanagida, T. Long–Term Stability of Oxide Nanowire Sensors via Heavily Doped Oxide Contact. ACS Sens. 2017, 2, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Lee, Y.; Xu, W.T.; Kim, Y.H.; Kim, M.; Min, S.Y.; Kim, T.H.; Jang, H.W.; Lee, T.W. Direct–printed nanoscale metal–oxide–wire electronics. Nano Energy 2019, 58, 437–446. [Google Scholar] [CrossRef]
- Kwon, D.; Jung, G.; Shin, W.; Jeong, Y.; Hong, S.; Oh, S.; Bae, J.H.; Park, B.G.; Lee, J.H. Low–power and reliable gas sensing system based on recurrent neural networks. Sens. Actuator B–Chem. 2021, 340, 11. [Google Scholar] [CrossRef]
- Kwon, D.; Jung, G.; Shin, W.; Jeong, Y.; Hong, S.; Oh, S.; Kim, J.; Bae, J.H.; Park, B.G.; Lee, J.H. Efficient fusion of spiking neural networks and FET–type gas sensors for a fast and reliable artificial olfactory system. Sens. Actuator B–Chem. 2021, 345, 9. [Google Scholar] [CrossRef]
- Wang, B.; Cancilla, J.C.; Torrecilla, J.S.; Haick, H. Artificial Sensing Intelligence with Silicon Nanowires for Ultraselective Detection in the Gas Phase. Nano Lett. 2014, 14, 933–938. [Google Scholar] [CrossRef]
- Royer, J.E.; Kappe, E.D.; Zhang, C.Y.; Martin, D.T.; Trogler, W.C.; Kummel, A.C. Organic Thin–Film Transistors for Selective Hydrogen Peroxide and Organic Peroxide Vapor Detection. J. Phys. Chem. C 2012, 116, 24566–24572. [Google Scholar] [CrossRef]
- Ghediya, P.R.; Magari, Y.; Sadahira, H.; Endo, T.; Furuta, M.; Zhang, Y.; Matsuo, Y.; Ohta, H. Reliable Operation in High–Mobility Indium Oxide Thin Film Transistors. Small Methods 2024, 2400578. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Liang, B.; Wang, X.; Chen, P.C.; Zhou, C. Indium oxide nanospirals made of kinked nanowires. ACS Nano. 2011, 5, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yan, C.; Lee, P.S. Room temperature CO gas sensing using Zn–doped In2O3 single nanowire field effect transistors. Sens. Actuators B Chem. 2010, 150, 19–24. [Google Scholar] [CrossRef]
- Huang, W.; Zeng, L.; Yu, X.; Guo, P.; Wang, B.; Ma, Q.; Chang, R.P.H.; Yu, J.; Bedzyk, M.J.; Marks, T.J.; et al. Metal Oxide Transistors via Polyethylenimine Doping of the Channel Layer: Interplay of Doping, Microstructure, and Charge Transport. Adv. Funct. Mater. 2016, 26, 6179–6187. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; She, X.; Chen, Y.; Wang, M.; Wang, Y.; Du, A.; Tang, C.; Zou, C.; Zhou, Y. Oxygen Vacancy–Rich Bimetallic Au@Pt Core–Shell Nanosphere–Functionalized Electrospun ZnFe2O4 Nanofibers for Chemiresistive Breath Acetone Detection. ACS Sens. 2024, 9, 2183–2193. [Google Scholar] [CrossRef]
- Yu, C.; Liu, J.; Zhao, H.; Wang, M.; Li, J.; She, X.; Chen, Y.; Wang, Y.; Liu, B.; Zou, C.; et al. Sensitive Breath Acetone Detection Based on α–Fe2O3 Nanoparticles Modified WO3 Nanoplate Heterojunctions. IEEE Trans. Instrum. Meas. 2024, 73, 1–8. [Google Scholar] [CrossRef]








| Type | Preparation Method | Mobility/μ cm2v−1s−1 | Refs. |
|---|---|---|---|
| Commercial IGZO thin film | PVD | 10 | / |
| Unpassivated In2O3 thin film | PVD | 85 | [81] |
| In2O3 nanowires | CVD | >200 | [82] |
| Zn–doped In2O3 nanowires | CVD | 139 | [83] |
| PVA–doped In2O3 thin film | Solution processed | 4 | [84] |
| PEI–doped In2O3 thin film | Solution processed | 9 | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Cao, Z.; Hao, J.; Zhao, S.; Yu, Y.; Dong, Y.; Liu, H.; Huang, C.; Gao, C.; Zhou, Y.; et al. Gas Sensing Properties of Indium–Oxide–Based Field–Effect Transistor: A Review. Sensors 2024, 24, 6150. https://doi.org/10.3390/s24186150
Liang C, Cao Z, Hao J, Zhao S, Yu Y, Dong Y, Liu H, Huang C, Gao C, Zhou Y, et al. Gas Sensing Properties of Indium–Oxide–Based Field–Effect Transistor: A Review. Sensors. 2024; 24(18):6150. https://doi.org/10.3390/s24186150
Chicago/Turabian StyleLiang, Chengyao, Zhongyu Cao, Jiongyue Hao, Shili Zhao, Yuanting Yu, Yingchun Dong, Hangyu Liu, Chun Huang, Chao Gao, Yong Zhou, and et al. 2024. "Gas Sensing Properties of Indium–Oxide–Based Field–Effect Transistor: A Review" Sensors 24, no. 18: 6150. https://doi.org/10.3390/s24186150
APA StyleLiang, C., Cao, Z., Hao, J., Zhao, S., Yu, Y., Dong, Y., Liu, H., Huang, C., Gao, C., Zhou, Y., & He, Y. (2024). Gas Sensing Properties of Indium–Oxide–Based Field–Effect Transistor: A Review. Sensors, 24(18), 6150. https://doi.org/10.3390/s24186150








