Multimodal Ultrasound Radiomic Technology for Diagnosing Benign and Malignant Thyroid Nodules of Ti-Rads 4-5: A Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
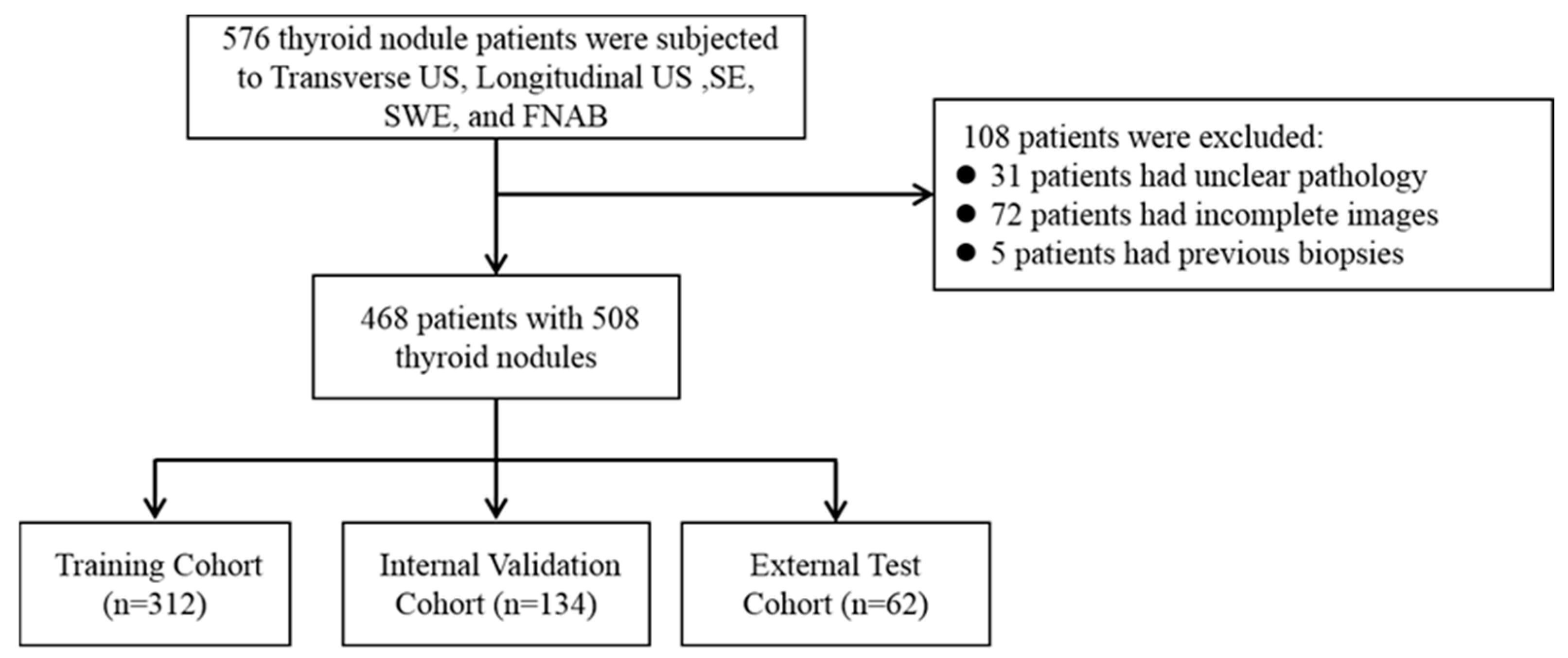
2.1. Study Population
2.2. Image Acquisition and Segmentation
2.3. Radiomic Feature Extraction and Selection
2.4. Model Building and Explanation
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
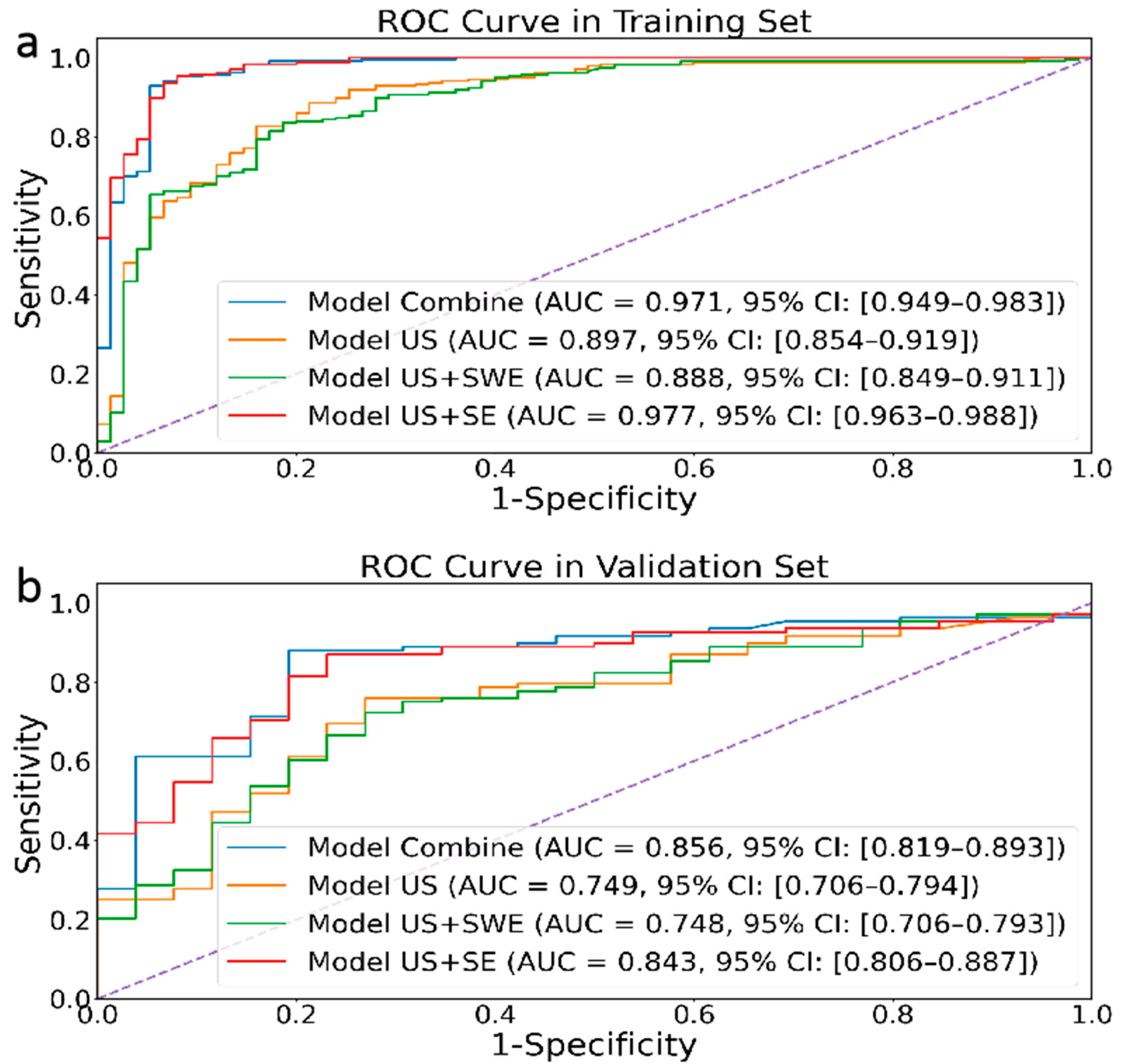
3.2. Model Evaluation
3.3. External Validation of Model Accuracy
3.4. The Interpretability of the Model
- (1)
- Feature 1 (Wavelet_HL_firstorder-RootMeanSquared2): By extracting horizontal edge information from longitudinal ultrasound images and describing the pixel intensity features of nodular edges, it can reflect the horizontal edge morphology and internal echo situation of nodules in clinical practice. This feature mainly corresponds to the presence of irregularity and lobulation at the edge of the nodule in clinical practice, as well as the high and low echogenicities inside the nodule;
- (2)
- Feature 2 (Wavelet_LH_firstorder-Median): By extracting vertical edge information from transverse ultrasound images and describing the average pixel intensity characteristics of nodular edge images, it can reflect the changes in the vertical edge morphology and internal tissue density of nodules in clinical practice. This feature mainly corresponds to whether the aspect ratio of the nodule is ≥1 in clinical practice, as well as the composition of the nodule;
- (3)
- Feature 3 (Wavelet-LL_firster_10Percentile4G): By extracting the overall structure and low-frequency features of SE ultrasound images, the basal grayscale level of the nodule is described, reflecting the overall shape and basal hardness of the nodule. This feature mainly analyzes the distribution of soft tissue areas in elastic ultrasound images to understand the characteristics of nodules more accurately;
- (4)
- Feature 4 (Wavelet-LH_glszm-SmallArenEmphasis): By extracting the vertical edge textural features of transverse ultrasound images and describing the distribution of high-intensity grayscale areas in nodules, it can reflect the textural complexity and non-uniformity of nodular edges. This feature mainly corresponds to nodular margin lobulation and calcification in clinical practice;
- (5)
- Feature 5 (Wavelet_LL_glcm_lmcl4R): By extracting the overall structure and low-frequency features of strain elastic ultrasound images and describing the frequency of low grayscale pixels appearing in the nodular area, it can reflect the presence of soft tissue or low-hardness areas in the nodule. This feature mainly corresponds to the distribution of the nodular hardness and the contrast between soft and hard areas.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Franceschi, S.; Bray, F.; Wild, C.P.; Plummer, M.; Dal Maso, L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N. Engl. J. Med. 2016, 375, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.; Lang, B.H.H.; McLeod, D.S.A.; Newbold, K.; Haymart, M.R. Thyroid cancer. Lancet 2023, 401, 1531–1544. [Google Scholar] [CrossRef]
- Ma, Y.; Yue, T.; He, Q. Tracheal injury following robotic thyroidectomy: A literature review of epidemiology, etiology, diagnosis, and treatment and 3 case reports. Asian J. Surg. 2024, 47, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Haddou, N.; Idrissi, N.; Ben Jebara, S. Analysis of Voice Quality after Thyroid Surgery. J. Voice Off. J. Voice Found. 2023. [Google Scholar] [CrossRef]
- AlSaedi, A.H.; Almalki, D.S.; ElKady, R.M. Approach to Thyroid Nodules: Diagnosis and Treatment. Cureus 2024, 16, e52232. [Google Scholar] [CrossRef] [PubMed]
- Monpeyssen, H.; Alamri, A.; Ben Hamou, A. Long-Term Results of Ultrasound-Guided Radiofrequency Ablation of Benign Thyroid Nodules: State of the Art and Future Perspectives—A Systematic Review. Front. Endocrinol. 2021, 12, 622996. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedüs, L.; Paschke, R.; Valcavi, R.; Vitti, P. American association of clinical endocrinologists, american college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 update. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2016, 22, 622–639. [Google Scholar] [CrossRef]
- Modi, L.; Sun, W.; Shafizadeh, N.; Negron, R.; Yee-Chang, M.; Zhou, F.; Simsir, A.; Sheth, S.; Brandler, T.C. Does a higher American College of Radiology Thyroid Imaging Reporting and Data System (ACR TI-RADS) score forecast an increased risk of malignancy? A correlation study of ACR TI-RADS with FNA cytology in the evaluation of thyroid nodules. Cancer Cytopathol. 2020, 128, 470–481. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Zhang, D.; Zhang, Y.Z.; Guo, L.; Jiang, Y.; Du, S.; Zhou, Q. An integrated AI model to improve diagnostic accuracy of ultrasound and output known risk features in suspicious thyroid nodules. Eur. Radiol. 2022, 32, 2120–2129. [Google Scholar] [CrossRef]
- Hoang, J.K.; Middleton, W.D.; Farjat, A.E.; Langer, J.E.; Reading, C.C.; Teefey, S.A.; Abinanti, N.; Boschini, F.J.; Bronner, A.J.; Dahiya, N.; et al. Reduction in Thyroid Nodule Biopsies and Improved Accuracy with American College of Radiology Thyroid Imaging Reporting and Data System. Radiology 2018, 287, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Koc, A.M.; Adıbelli, Z.H.; Erkul, Z.; Sahin, Y.; Dilek, I. Comparison of diagnostic accuracy of ACR-TIRADS, American Thyroid Association (ATA), and EU-TIRADS guidelines in detecting thyroid malignancy. Eur. J. Radiol. 2020, 133, 109390. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Liu, X.; Li, Z.; Zhang, X.; Chen, M.; Luo, Z. Real-time ultrasound elastography in the differential diagnosis of benign and malignant thyroid nodules. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2009, 28, 861–867. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [PubMed]
- Garra, B.S. Elastography: History, principles, and technique comparison. Abdom. Imaging 2015, 40, 680–697. [Google Scholar] [CrossRef]
- Gurun, E.; Akdulum, I.; Akyuz, M.; Tokgoz, N.; Ozhan Oktar, S. Shear Wave Elastography Evaluation of Meniscus Degeneration with Magnetic Resonance Imaging Correlation. Acad. Radiol. 2021, 28, 1383–1388. [Google Scholar] [CrossRef]
- Nam, K.; Peterson, S.M.; Wessner, C.E.; Machado, P.; Forsberg, F. Diagnosis of Carpal Tunnel Syndrome using Shear Wave Elastography and High-frequency Ultrasound Imaging. Acad. Radiol. 2021, 28, e278–e287. [Google Scholar] [CrossRef]
- Ma, H.J.; Yang, J.C.; Leng, Z.P.; Chang, Y.; Kang, H.; Teng, L.H. Preoperative prediction of papillary thyroid microcarcinoma via multiparameter ultrasound. Acta Radiol. 2017, 58, 1303–1311. [Google Scholar] [CrossRef]
- Duan, S.B.; Yu, J.; Li, X.; Han, Z.Y.; Zhai, H.Y.; Liang, P. Diagnostic value of two-dimensional shear wave elastography in papillary thyroid microcarcinoma. OncoTargets Ther. 2016, 9, 1311–1317. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Q.; Gu, J.; Jiang, L.; Bai, M.; Liu, L.; Wu, Y.; Du, L. Combined value of Virtual Touch tissue quantification and conventional sonographic features for differentiating benign and malignant thyroid nodules smaller than 10 mm. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2014, 33, 257–264. [Google Scholar] [CrossRef]
- Du, Y.R.; Ji, C.L.; Wu, Y.; Gu, X.G. Combination of ultrasound elastography with TI-RADS in the diagnosis of small thyroid nodules (≤10 mm): A new method to increase the diagnostic performance. Eur. J. Radiol. 2018, 109, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Lohmann, P.; Bousabarah, K.; Hoevels, M.; Treuer, H. Radiomics in radiation oncology-basics, methods, and limitations. Strahlenther. Onkol. 2020, 196, 848–855. [Google Scholar] [CrossRef]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef]
- Gao, X.; Ran, X.; Ding, W. The progress of radiomics in thyroid nodules. Front. Oncol. 2023, 13, 1109319. [Google Scholar] [CrossRef]
- Dondi, F.; Gatta, R.; Treglia, G.; Piccardo, A.; Albano, D.; Camoni, L.; Gatta, E.; Cavadini, M.; Cappelli, C.; Bertagna, F. Application of radiomics and machine learning to thyroid diseases in nuclear medicine: A systematic review. Rev. Endocr. Metab. Disord. 2024, 25, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Song, F.; Zhao, Y.; Xie, Y.; Wen, Y.; Zhou, P. Ultrasonography-based radiomics and computer-aided diagnosis in thyroid nodule management: Performance comparison and clinical strategy optimization. Front. Endocrinol. 2023, 14, 1140816. [Google Scholar] [CrossRef]
- Chen, J.H.; Zhang, Y.Q.; Zhu, T.T.; Zhang, Q.; Zhao, A.X.; Huang, Y. Applying machine-learning models to differentiate benign and malignant thyroid nodules classified as C-TIRADS 4 based on 2D-ultrasound combined with five contrast-enhanced ultrasound key frames. Front. Endocrinol. 2024, 15, 1299686. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.R.; Zhu, P.S.; Li, J.; Chen, M.; Cao, C.L.; Shi, L.N.; Li, W.X. Study on diagnosing thyroid nodules of ACR TI-RADS 4-5 with multimodal ultrasound radiomics technology. J. Clin. Ultrasound JCU 2024, 52, 274–283. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, D.; Han, L.Z.; Pan, Y.S.; Wei, Q.; Lv, W.Z.; Dietrich, C.F.; Wang, Z.Y.; Cui, X.W. Predicting Malignancy of Thyroid Micronodules: Radiomics Analysis Based on Two Types of Ultrasound Elastography Images. Acad. Radiol. 2023, 30, 2156–2168. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Hearst, M.A.; Dumais, S.T.; Osman, E.; Platt, J.; Scholkopf, B. Support vector machines. IEEE Intell. Syst. Their Appl. 1998, 13, 18–28. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, R.; Bajorath, J. Interpretation of Compound Activity Predictions from Complex Machine Learning Models Using Local Approximations and Shapley Values. J. Med. Chem. 2020, 63, 8761–8777. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.E. The Shapley Value (Essays in Honor of Lloyd S. Shapley)||A Value for n-Person Games; Cambridge University Press: Cambridge, UK, 1988; pp. 31–40. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.-I. A unified approach to interpreting model predictions. arXiv 2017, arXiv:1705.07874. [Google Scholar]
- Zhou, J.; Lu, J.; Gao, C.; Zeng, J.; Zhou, C.; Lai, X.; Cai, W.; Xu, M. Predicting the response to neoadjuvant chemotherapy for breast cancer: Wavelet transforming radiomics in MRI. BMC Cancer 2020, 20, 100. [Google Scholar] [CrossRef]
- Chaddad, A.; Daniel, P.; Niazi, T. Radiomics Evaluation of Histological Heterogeneity Using Multiscale Textures Derived from 3D Wavelet Transformation of Multispectral Images. Front. Oncol. 2018, 8, 96. [Google Scholar] [CrossRef]
- Han, Z.; Huang, Y.; Wang, H.; Chu, Z. Multimodal ultrasound imaging: A method to improve the accuracy of diagnosing thyroid TI-RADS 4 nodules. J. Clin. Ultrasound JCU 2022, 50, 1345–1352. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Frates, M.C.; Benson, C.B.; Charboneau, J.W.; Cibas, E.S.; Clark, O.H.; Coleman, B.G.; Cronan, J.J.; Doubilet, P.M.; Evans, D.B.; Goellner, J.R.; et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2005, 237, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.; Royer, B.; Bigorgne, C.; Rouxel, A.; Bienvenu-Perrard, M.; Leenhardt, L. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur. J. Endocrinol. 2013, 168, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, J.; Ai, Y.; Zhu, K.; Xiao, C.; Xie, C.; Jin, X. Computer Tomography Radiomics-Based Nomogram in the Survival Prediction for Brain Metastases from Non-Small Cell Lung Cancer Underwent Whole Brain Radiotherapy. Front. Oncol. 2020, 10, 610691. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Training Cohort (n = 252) | Testing Cohort | p-Value * | Validation Cohort | p-Value * |
|---|---|---|---|---|---|
| Final pathology | 0.80 | 0.02 | |||
| Benign | 78 (25%) | 32 (23.9%) | 7 (11.3%) | ||
| Malignant | 234 (75%) | 102 (76.1%) | 55 (88.7%) | ||
| Age (years) | 44.3 [17–77] | 45.4 [6–74] | 0.34 | 46 [22–73] | 0.29 |
| Sex | 0.33 | 0.98 | |||
| Male | 81 (25.9%) | 29 (21.6%) | 16 (25.8%) | ||
| Female | 231 (74.1%) | 105 (78.4%) | 46 (74.2%) | ||
| Nodular size (mm) Maximum diameter | 9.02 [2.8–40.4] | 8.5 [3–36] | 0.94 | 9.23 [3–31.4] | 0.46 |
| Location | 0.92 | 0.99 | |||
| Right | 166 (53.2%) | 72 (53.7%) | 33 (53.2%) | ||
| Left | 146 (46.8%) | 62 (46.3%) | 29 (46.8%) | ||
| Echogenicity | 0.52 | 0.83 | |||
| Hypoechoic | 279 (89.4%) | 117 (87.3%) | 56 (90.3%) | ||
| Isoechogenic or Hyperechoic | 33 (10.6%) | 17 (12.7%) | 6 (9.7%) | ||
| Microcalcification | 0.54 | 0.95 | |||
| Yes | 205 (65.7%) | 84 (62.7%) | 41 (66.1%) | ||
| No | 107 (34.3%) | 50 (37.3%) | 21 (33.9%) | ||
| Aspect ratio | 0.44 | 0.01 | |||
| ≥1 | 132 (42.3%) | 62 (46.3%) | 37 (59.7%) | ||
| <1 | 180 (57.7%) | 72 (53.7%) | 25 (40.3%) | ||
| Composition | 0.22 | 0.41 | |||
| Solid cystic | 48 (15.4%) | 27 (20.1%) | 7 (11.3%) | ||
| Solid | 264 (84.6%) | 107 (79.9%) | 55 (88.7%) | ||
| Margin | 0.78 | 0.58 | |||
| Smooth or ill-defined | 187 (59.9%) | 85 (63.4%) | 33 (53.2%) | ||
| Lobulated or irregular | 105 (33.7%) | 41 (30.6%) | 25 (40.3%) | ||
| Extra-thyroidal extension | 20 (6.4%) | 8 (6.0%) | 4 (6.5%) | ||
| Blood flow | 0.50 | 0.52 | |||
| Yes | 224 (71.8%) | 92 (68.7%) | 47 (75.8%) | ||
| No | 88 (28.2%) | 42 (31.3%) | 15 (24.2%) | ||
| TI-RADS | 0.19 | 0.23 | |||
| 4a | 205 (65.7%) | 95 (70.9%) | 35 (56.5%) | ||
| 4b | 84 (26.9%) | 35 (26.1%) | 23 (37.1%) | ||
| 4c | 16 (5.1%) | 4 (3.0%) | 3 (4.8%) | ||
| 5 | 7 (2.3%) 7 | 0 (0.0%) | 1 (1.6%) |
| Training Set | Validation Set | |||||
|---|---|---|---|---|---|---|
| ACC | Sensitivity | Specificity | ACC | Sensitivity | Specificity | |
| Combined model | 94.6 | 95.4 | 92 | 85.8 | 87 | 80.8 |
| Model 1: US | 84.3 | 86.1 | 78.7 | 73.1 | 73.1 | 73.1 |
| Model 2: US + SWE | 83.7 | 87.3 | 72 | 73.1 | 75 | 65.4 |
| Model 3: US + SE | 94.2 | 94.9 | 92 | 84.3 | 86.1 | 76.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, C.; Deng, X.; Li, Y.; Zhou, W.; Huang, Y.; Chu, X.; Wang, T.; Li, H.; Chen, Y. Multimodal Ultrasound Radiomic Technology for Diagnosing Benign and Malignant Thyroid Nodules of Ti-Rads 4-5: A Multicenter Study. Sensors 2024, 24, 6203. https://doi.org/10.3390/s24196203
Wang L, Wang C, Deng X, Li Y, Zhou W, Huang Y, Chu X, Wang T, Li H, Chen Y. Multimodal Ultrasound Radiomic Technology for Diagnosing Benign and Malignant Thyroid Nodules of Ti-Rads 4-5: A Multicenter Study. Sensors. 2024; 24(19):6203. https://doi.org/10.3390/s24196203
Chicago/Turabian StyleWang, Luyao, Chengjie Wang, Xuefei Deng, Yan Li, Wang Zhou, Yilv Huang, Xuan Chu, Tengfei Wang, Hai Li, and Yongchao Chen. 2024. "Multimodal Ultrasound Radiomic Technology for Diagnosing Benign and Malignant Thyroid Nodules of Ti-Rads 4-5: A Multicenter Study" Sensors 24, no. 19: 6203. https://doi.org/10.3390/s24196203
APA StyleWang, L., Wang, C., Deng, X., Li, Y., Zhou, W., Huang, Y., Chu, X., Wang, T., Li, H., & Chen, Y. (2024). Multimodal Ultrasound Radiomic Technology for Diagnosing Benign and Malignant Thyroid Nodules of Ti-Rads 4-5: A Multicenter Study. Sensors, 24(19), 6203. https://doi.org/10.3390/s24196203







