Development of a Gait Analysis Application for Assessing Upper and Lower Limb Movements to Detect Pathological Gait
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approvals
2.2. Study Population
2.3. Three-Dimensional Pose Tracker for Gait Test (TDPT-GT) App for 3D Human Motion Estimation
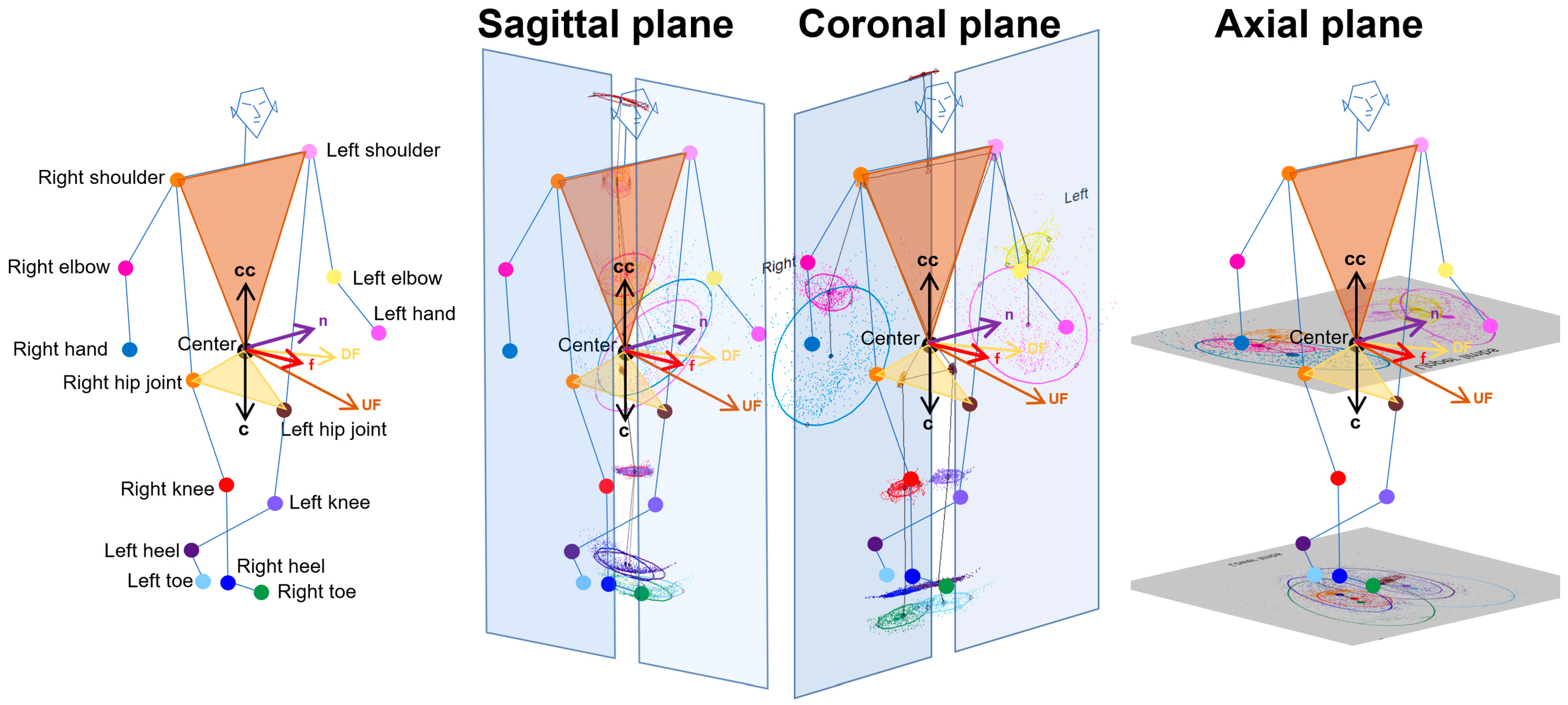
2.4. Projection of Relative Coordinates on the Body Axis Planes
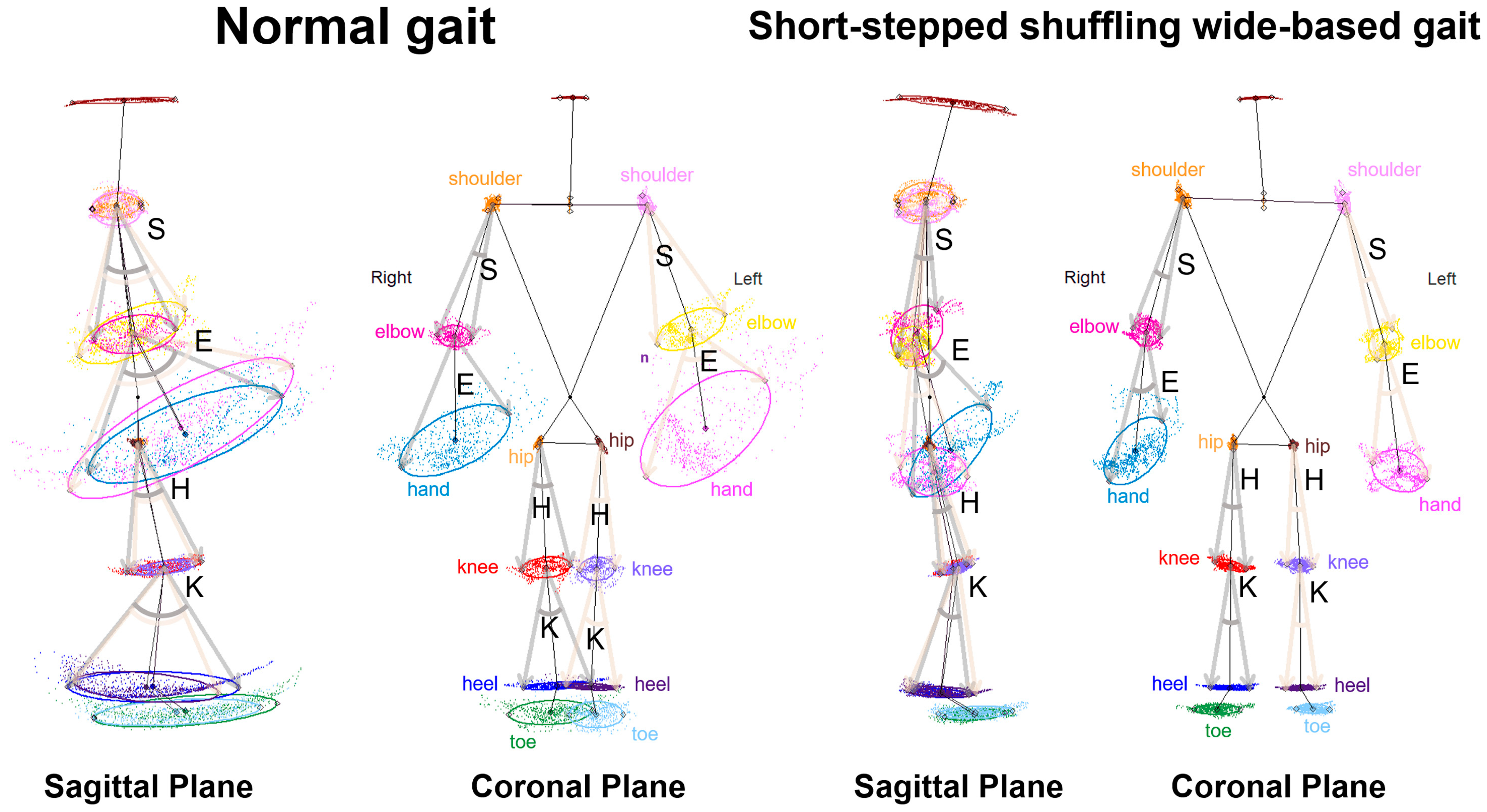
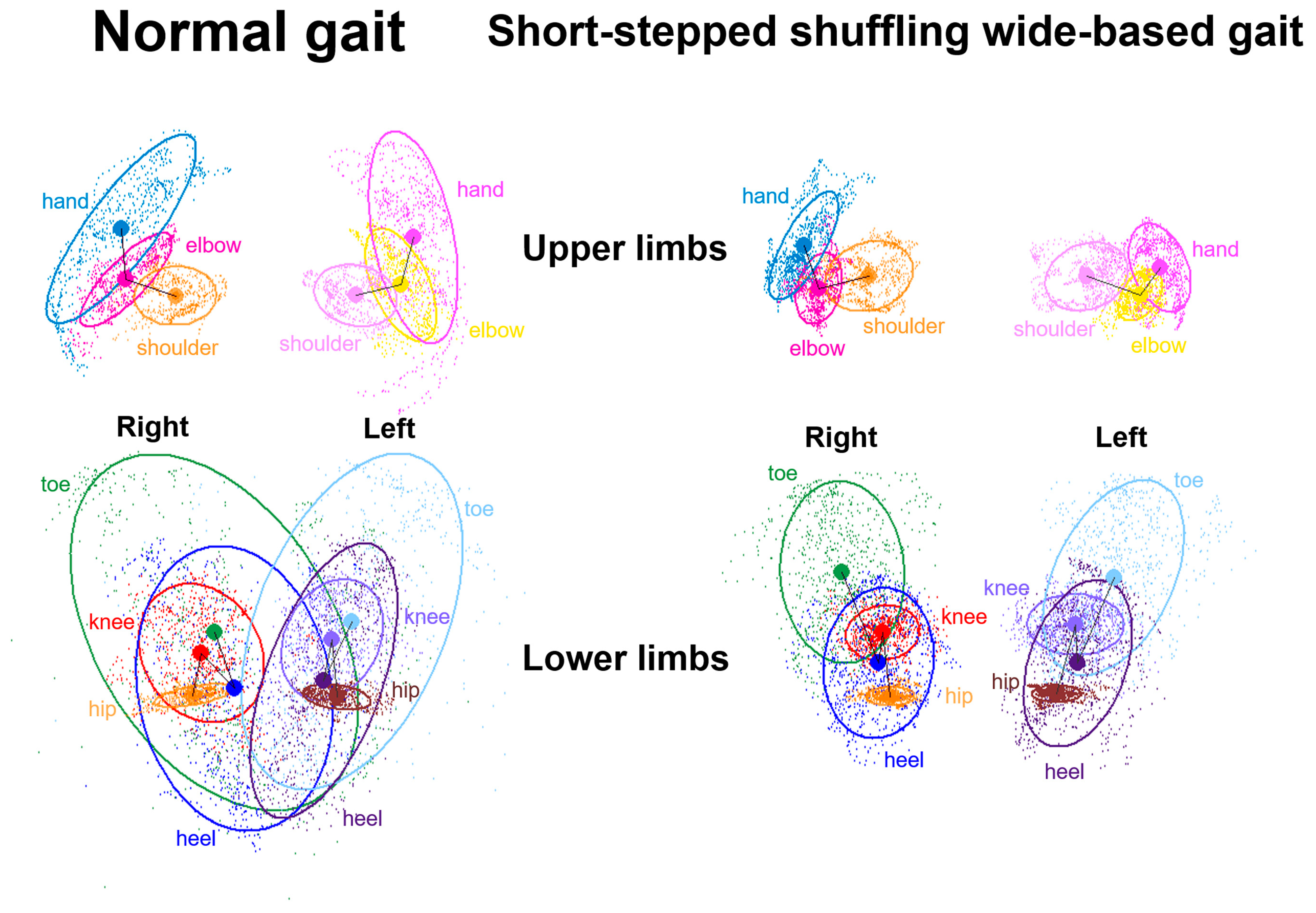
2.5. Data Processing for Gait Analysis
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
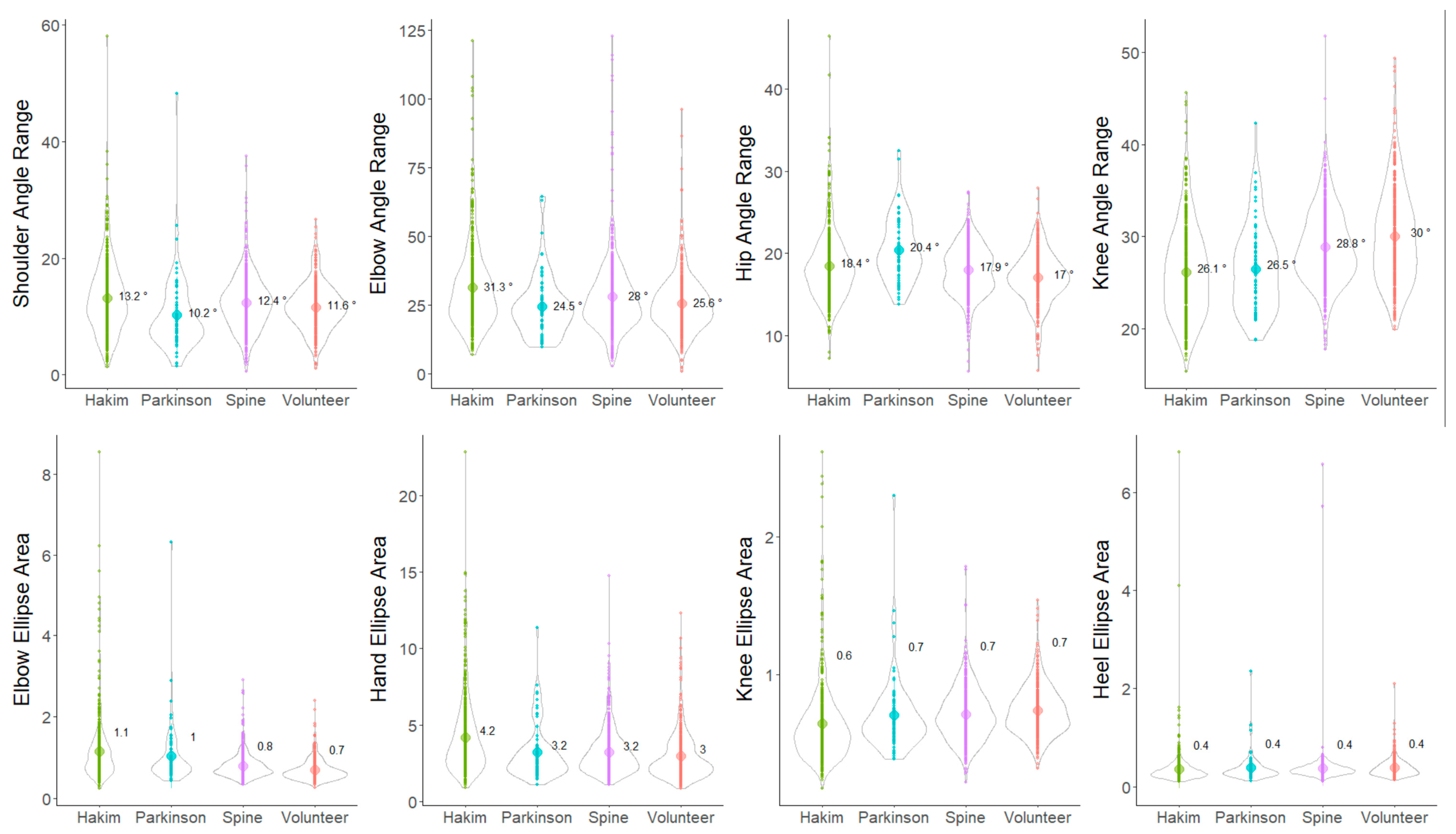
3.2. Indices of 2D Relative Coordinates on the Tri-Axial Projection Planes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gor-Garcia-Fogeda, M.D.; Cano de la Cuerda, R.; Carratala Tejada, M.; Alguacil-Diego, I.M.; Molina-Rueda, F. Observational Gait Assessments in People With Neurological Disorders: A Systematic Review. Arch. Phys. Med. Rehabil. 2016, 97, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Yamada, S.; Yamamoto, K. Agreement study on gait assessment using a video-assisted rating method in patients with idiopathic normal-pressure hydrocephalus. PLoS ONE 2019, 14, e0224202. [Google Scholar] [CrossRef] [PubMed]
- Marmarou, A.; Bergsneider, M.; Relkin, N.; Klinge, P.; Black, P.M. Development of guidelines for idiopathic normal-pressure hydrocephalus: Introduction. Neurosurgery 2005, 57 (Suppl. S3), S2-1–S2-3. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Yamada, S.; Miyajima, M.; Ishii, K.; Kuriyama, N.; Kazui, H.; Kanemoto, H.; Suehiro, T.; Yoshiyama, K.; Kameda, M.; et al. Guidelines for management of idiopathic normal pressure hydrocephalus (Third edition): Endorsed by the Japanese society of normal pressure hydrocephalus. Neurol. Med. Chir. 2021, 61, 63–97. [Google Scholar] [CrossRef] [PubMed]
- Scully, A.E.; Lim, E.C.W.; Teow, P.P.; Tan, D.M.L. A systematic review of the diagnostic utility of simple tests of change after trial removal of cerebrospinal fluid in adults with normal pressure hydrocephalus. Clin. Rehabil. 2018, 32, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Stolze, H.; Kuhtz-Buschbeck, J.P.; Drucke, H.; Johnk, K.; Illert, M.; Deuschl, G. Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2001, 70, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M. Gait dynamics, fractals and falls: Finding meaning in the stride-to-stride fluctuations of human walking. Hum. Mov. Sci. 2007, 26, 555–589. [Google Scholar] [CrossRef]
- Kitade, I.; Kitai, R.; Neishi, H.; Kikuta, K.I.; Shimada, S.; Matsumine, A. Relationship between gait parameters and MR imaging in idiopathic normal pressure hydrocephalus patients after shunt surgery. Gait Posture 2018, 61, 163–168. [Google Scholar] [CrossRef]
- Murray, M.P.; Sepic, S.B.; Gardner, G.M.; Downs, W.J. Walking patterns of men with parkinsonism. Am. J. Phys. Med. 1978, 57, 278–294. [Google Scholar]
- Panciani, P.P.; Migliorati, K.; Muratori, A.; Gelmini, M.; Padovani, A.; Fontanella, M. Computerized gait analysis with inertial sensor in the management of idiopathic normal pressure hydrocephalus. Eur. J. Phys. Rehabil. Med. 2018, 54, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; Thomas, G.; de Lateur, B.; Imteyaz, H.; Rose, J.G.; Shore, W.S.; Kharkar, S.; Rigamonti, D. Objective assessment of gait in normal-pressure hydrocephalus. Am. J. Phys. Med. Rehabil. 2008, 87, 39–45. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Xiong, F.; Zhang, W. Gait Recognition Using Optical Motion Capture: A Decision Fusion Based Method. Sensors 2021, 21, 3496. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Tang, P.F.; Jan, M.H. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch. Phys. Med. Rehabil. 2003, 84, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; McIlroy, W.E. Gait asymmetry in community-ambulating stroke survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Serrao, M.; Pierelli, F.; Ranavolo, A.; Draicchio, F.; Conte, C.; Don, R.; Di Fabio, R.; LeRose, M.; Padua, L.; Sandrini, G.; et al. Gait pattern in inherited cerebellar ataxias. Cerebellum 2012, 11, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Leardini, A.; Belvedere, C.; Nardini, F.; Sancisi, N.; Conconi, M.; Parenti-Castelli, V. Kinematic models of lower limb joints for musculo-skeletal modelling and optimization in gait analysis. J. Biomech. 2017, 62, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, Y.; Diao, Y.; Li, G.; Zhao, G. The reliability and validity of gait analysis system using 3D markerless pose estimation algorithms. Front. Bioeng. Biotechnol. 2022, 10, 857975. [Google Scholar] [CrossRef]
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef]
- Morris, M.; Iansek, R.; McGinley, J.; Matyas, T.; Huxham, F. Three-dimensional gait biomechanics in Parkinson’s disease: Evidence for a centrally mediated amplitude regulation disorder. Mov. Disord. 2005, 20, 40–50. [Google Scholar] [CrossRef]
- Baak, A.; M¨uller, M.; Bharaj, G.; Seidel, H.; Theobalt, C. A data-driven approach for real-time full body pose reconstruction from a depth camera. In IEEE 13th International Conference on Computer Vision; Springer: London, UK, 2011; p. 1092. [Google Scholar]
- Buker, L.C.; Zuber, F.; Hein, A.; Fudickar, S. HRDepthNet: Depth Image-Based Marker-Less Tracking of Body Joints. Sensors 2021, 21, 1356. [Google Scholar] [CrossRef]
- Das, K.; de Paula Oliveira, T.; Newell, J. Comparison of markerless and marker-based motion capture systems using 95% functional limits of agreement in a linear mixed-effects modelling framework. Sci. Rep. 2023, 13, 22880. [Google Scholar] [CrossRef] [PubMed]
- Gutta, V.; Fallavollita, P.; Baddour, N.; Lemaire, E.D. Development of a Smart Hallway for Marker-Less Human Foot Tracking and Stride Analysis. IEEE J. Transl. Eng. Health Med. 2021, 9, 2100412. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.W.T.; Tang, Y.M.; Fong, K.N.K. A systematic review of the applications of markerless motion capture (MMC) technology for clinical measurement in rehabilitation. J. Neuroeng. Rehabil. 2023, 20, 57. [Google Scholar] [CrossRef]
- Vafadar, S.; Skalli, W.; Bonnet-Lebrun, A.; Assi, A.; Gajny, L. Assessment of a novel deep learning-based marker-less motion capture system for gait study. Gait Posture 2022, 94, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Scataglini, S.; Abts, E.; Van Bocxlaer, C.; Van den Bussche, M.; Meletani, S.; Truijen, S. Accuracy, Validity, and Reliability of Markerless Camera-Based 3D Motion Capture Systems versus Marker-Based 3D Motion Capture Systems in Gait Analysis: A Systematic Review and Meta-Analysis. Sensors 2024, 24, 3686. [Google Scholar] [CrossRef]
- Wishaupt, K.; Schallig, W.; van Dorst, M.H.; Buizer, A.I.; van der Krogt, M.M. The applicability of markerless motion capture for clinical gait analysis in children with cerebral palsy. Sci. Rep. 2024, 14, 11910. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.; Needham, L.; McGuigan, P.; Bilzon, J. Applications and limitations of current markerless motion capture methods for clinical gait biomechanics. PeerJ 2022, 10, e12995. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, Y.; Yamada, S.; Ueda, S.; Iseki, C.; Kondo, T.; Mori, K.; Kobayashi, Y.; Fukami, T.; Hoshimaru, M.; Ishikawa, M.; et al. Development of Smartphone Application for Markerless Three-Dimensional Motion Capture Based on Deep Learning Model. Sensors 2022, 22, 5282. [Google Scholar] [CrossRef]
- Yamada, S.; Aoyagi, Y.; Iseki, C.; Kondo, T.; Kobayashi, Y.; Ueda, S.; Mori, K.; Fukami, T.; Tanikawa, M.; Mase, M.; et al. Quantitative Gait Feature Assessment on Two-Dimensional Body Axis Projection Planes Converted from Three-Dimensional Coordinates Estimated with a Deep Learning Smartphone App. Sensors 2023, 23, 617. [Google Scholar] [CrossRef] [PubMed]
- Duprey, S.; Naaim, A.; Moissenet, F.; Begon, M.; Cheze, L. Kinematic models of the upper limb joints for multibody kinematics optimisation: An overview. J. Biomech. 2017, 62, 87–94. [Google Scholar] [CrossRef]
- Meyns, P.; Bruijn, S.M.; Duysens, J. The how and why of arm swing during human walking. Gait Posture 2013, 38, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.D.; Fehlman, L.A.; Farley, C.T. Effects of aging and arm swing on the metabolic cost of stability in human walking. J. Biomech. 2008, 41, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Liu, A.M.; Xiao, F.; Wang, Y.Z.; Hu, F.; Chen, J.L.; Dai, K.R.; Gu, D.Y. Effect of active arm swing to local dynamic stability during walking. Hum. Mov. Sci. 2016, 45, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Amprimo, G.; Masi, G.; Vismara, L.; Cremascoli, R.; Sinagra, S.; Pettiti, G.; Mauro, A.; Priano, L. Evaluation of Arm Swing Features and Asymmetry during Gait in Parkinson’s Disease Using the Azure Kinect Sensor. Sensors 2022, 22, 6282. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.B.; Park, Y.M.; Kim, M.J.; Kim, W.S. Influences of elbow, shoulder, trunk motion and temporospatial parameters on arm swing asymmetry of Parkinson’s disease during walking. Hum. Mov. Sci. 2019, 68, 102527. [Google Scholar] [CrossRef]
- Navarro-Lopez, V.; Fernandez-Vazquez, D.; Molina-Rueda, F.; Cuesta-Gomez, A.; Garcia-Prados, P.; Del-Valle-Gratacos, M.; Carratala-Tejada, M. Arm-swing kinematics in Parkinson’s disease: A systematic review and meta-analysis. Gait Posture 2022, 98, 85–95. [Google Scholar] [CrossRef]
- Relkin, N.; Marmarou, A.; Klinge, P.; Bergsneider, M.; Black, P.M. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005, 57 (Suppl. S3), S2-4–S2-16. [Google Scholar] [CrossRef]
- Tsakanikas, D.; Katzen, H.; Ravdin, L.D.; Relkin, N.R. Upper extremity motor measures of Tap Test response in Normal Pressure Hydrocephalus. Clin. Neurol. Neurosurg. 2009, 111, 752–757. [Google Scholar] [CrossRef]
- Lee, D.H.; Yoo, J.Y.; Cho, J.H.; Hwang, C.J.; Lee, C.S.; Kim, C.; Ha, J.K.; Park, K.B. Subclinical gait disturbance and postoperative gait improvement in patients with degenerative cervical myelopathy. Sci. Rep. 2021, 11, 11179. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Watanabe, K.; Mizouchi, T.; Urakawa, T.; Ohashi, M.; Tashi, H.; Minato, K.; Tanaka, Y.; Kawashima, H. Gait Analysis by the Severity of Gait Disturbance in Patients with Compressive Cervical Myelopathy. Spine Surg. Relat. Res. 2023, 7, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Iseki, C.; Hayasaka, T.; Yanagawa, H.; Komoriya, Y.; Kondo, T.; Hoshi, M.; Fukami, T.; Kobayashi, Y.; Ueda, S.; Kawamae, K.; et al. Artificial Intelligence Distinguishes Pathological Gait: The Analysis of Markerless Motion Capture Gait Data Acquired by an iOS Application (TDPT-GT). Sensors 2023, 23, 6217. [Google Scholar] [CrossRef] [PubMed]
- Iseki, C.; Takahashi, Y.; Adachi, M.; Igari, R.; Sato, H.; Koyama, S.; Ishizawa, K.; Ohta, Y.; Kato, T. Prevalence and development of idiopathic normal pressure hydrocephalus: A 16-year longitudinal study in Japan. Acta Neurol. Scand. 2022, 146, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Bugalho, P.; Alves, L.; Miguel, R. Gait dysfunction in Parkinson’s disease and normal pressure hydrocephalus: A comparative study. J. Neural Transm. 2013, 120, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Iseki, C.; Suzuki, S.; Fukami, T.; Yamada, S.; Hayasaka, T.; Kondo, T.; Hoshi, M.; Ueda, S.; Kobayashi, Y.; Ishikawa, M.; et al. Fluctuations in Upper and Lower Body Movement during Walking in Normal Pressure Hydrocephalus and Parkinson’s Disease Assessed by Motion Capture with a Smartphone Application, TDPT-GT. Sensors 2023, 23, 9263. [Google Scholar] [CrossRef]
- Gallagher, R.M.; Marquez, J.; Osmotherly, P. Cognitive and upper limb symptom changes from a tap test in Idiopathic Normal Pressure Hydrocephalus. Clin. Neurol. Neurosurg. 2018, 174, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Tanikawa, M.; Horiba, M.; Sahashi, K.; Kawashima, S.; Kandori, A.; Yamanaka, T.; Nishikawa, Y.; Matsukawa, N.; Ueki, Y.; et al. Clinical utility of paced finger tapping assessment in idiopathic normal pressure hydrocephalus. Front. Hum. Neurosci. 2023, 17, 1109670. [Google Scholar] [CrossRef] [PubMed]
- Sirkka, J.; Parviainen, M.; Jyrkkanen, H.K.; Koivisto, A.M.; Saisanen, L.; Rauramaa, T.; Leinonen, V.; Danner, N. Upper limb dysfunction and activities in daily living in idiopathic normal pressure hydrocephalus. Acta Neurochir. 2021, 163, 2675–2683. [Google Scholar] [CrossRef]
- Na, C.H.; Siebers, H.L.; Reim, J.; Eschweiler, J.; Hildebrand, F.; Clusmann, H.; Betsch, M. Kinematic movement and balance parameter analysis in neurological gait disorders. J. Biol. Eng. 2024, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Arvin, M.; Mazaheri, M.; Hoozemans, M.J.M.; Pijnappels, M.; Burger, B.J.; Verschueren, S.M.P.; van Dieen, J.H. Effects of narrow base gait on mediolateral balance control in young and older adults. J. Biomech. 2016, 49, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Silder, A.; Heiderscheit, B.C.; Thelen, D.G. Effect of age on center of mass motion during human walking. Gait Posture 2009, 30, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hurt, C.P.; Rosenblatt, N.; Crenshaw, J.R.; Grabiner, M.D. Variation in trunk kinematics influences variation in step width during treadmill walking by older and younger adults. Gait Posture 2010, 31, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Mourcou, Q.; Fleury, A.; Diot, B.; Franco, C.; Vuillerme, N. Mobile Phone-Based Joint Angle Measurement for Functional Assessment and Rehabilitation of Proprioception. Biomed. Res. Int. 2015, 2015, 328142. [Google Scholar] [CrossRef] [PubMed]
- Cronin, N.J.; Rantalainen, T.; Ahtiainen, J.P.; Hynynen, E.; Waller, B. Markerless 2D kinematic analysis of underwater running: A deep learning approach. J. Biomech. 2019, 87, 75–82. [Google Scholar] [CrossRef] [PubMed]



| Hakim’s | Parkinson’s | Cervical Myelopathy | Healthy Control | |
|---|---|---|---|---|
| Total number | 122 | 12 | 93 | 200 |
| Sex (Male/female/unknown) | 69:53:0 | 8:4:0 | 67:26:0 | 71:78:51 |
| Mean ± SD of age (years) | 75.9 ± 7.4 | 70.8 ± 7.9 | 66.1 ± 11.8 | 60.7 ± 20.2 |
| Range of age (years) | 60–93 | 56–83 | 42–88 | 20–91 |
| 1st Parameter | 2nd Parameter | Hakim’s | Parkinson’s | Cervical Myelopathy | Healthy Control |
|---|---|---|---|---|---|
| Shoulder angle | Hip angle | 0.23 | 0.38 | 0.37 | 0.33 |
| Shoulder angle | Knee angle | 0.16 | 0.27 | 0.36 | 0.28 |
| Elbow angle | Hip angle | 0.25 | 0.42 | 0.38 | 0.32 |
| Elbow angle | Knee angle | 0.36 | 0.47 | 0.41 | 0.39 |
| Shoulder ellipse | Hip ellipse | 0.40 | 0.03 | 0.17 | 0.32 |
| Shoulder ellipse | Knee ellipse | 0.15 | −0.07 | 0.06 | 0.14 |
| Shoulder ellipse | Heel ellipse | 0.13 | 0.00 | 0.27 | 0.14 |
| Elbow ellipse | Hip ellipse | 0.38 | 0.28 | 0.25 | 0.42 |
| Elbow ellipse | Knee ellipse | 0.13 | 0.35 | 0.20 | 0.31 |
| Elbow ellipse | Heel ellipse | 0.08 | 0.31 | 0.36 | 0.39 |
| Hand ellipse | Hip ellipse | 0.25 | 0.29 | 0.28 | 0.36 |
| Hand ellipse | Knee ellipse | 0.21 | 0.37 | 0.25 | 0.30 |
| Hand ellipse | Heel ellipse | 0.24 | 0.34 | 0.35 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taishaku, A.; Yamada, S.; Iseki, C.; Aoyagi, Y.; Ueda, S.; Kondo, T.; Kobayashi, Y.; Sahashi, K.; Shimizu, Y.; Yamanaka, T.; et al. Development of a Gait Analysis Application for Assessing Upper and Lower Limb Movements to Detect Pathological Gait. Sensors 2024, 24, 6329. https://doi.org/10.3390/s24196329
Taishaku A, Yamada S, Iseki C, Aoyagi Y, Ueda S, Kondo T, Kobayashi Y, Sahashi K, Shimizu Y, Yamanaka T, et al. Development of a Gait Analysis Application for Assessing Upper and Lower Limb Movements to Detect Pathological Gait. Sensors. 2024; 24(19):6329. https://doi.org/10.3390/s24196329
Chicago/Turabian StyleTaishaku, Atsuhito, Shigeki Yamada, Chifumi Iseki, Yukihiko Aoyagi, Shigeo Ueda, Toshiyuki Kondo, Yoshiyuki Kobayashi, Kento Sahashi, Yoko Shimizu, Tomoyasu Yamanaka, and et al. 2024. "Development of a Gait Analysis Application for Assessing Upper and Lower Limb Movements to Detect Pathological Gait" Sensors 24, no. 19: 6329. https://doi.org/10.3390/s24196329
APA StyleTaishaku, A., Yamada, S., Iseki, C., Aoyagi, Y., Ueda, S., Kondo, T., Kobayashi, Y., Sahashi, K., Shimizu, Y., Yamanaka, T., Tanikawa, M., Ohta, Y., & Mase, M. (2024). Development of a Gait Analysis Application for Assessing Upper and Lower Limb Movements to Detect Pathological Gait. Sensors, 24(19), 6329. https://doi.org/10.3390/s24196329









