Statistical Analysis of the Influence of Various Types of Graphite Precursors and Oxidation Methods on the Gas Sensor Properties of Reduced Graphene Oxide
Abstract
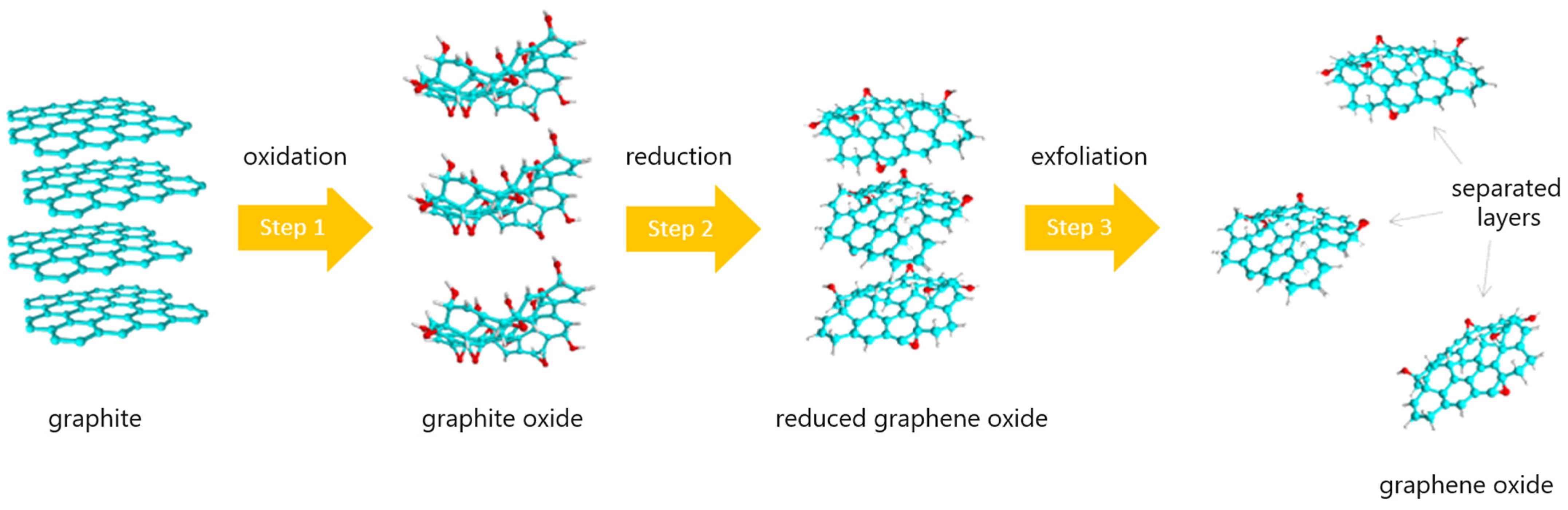
:1. Introduction
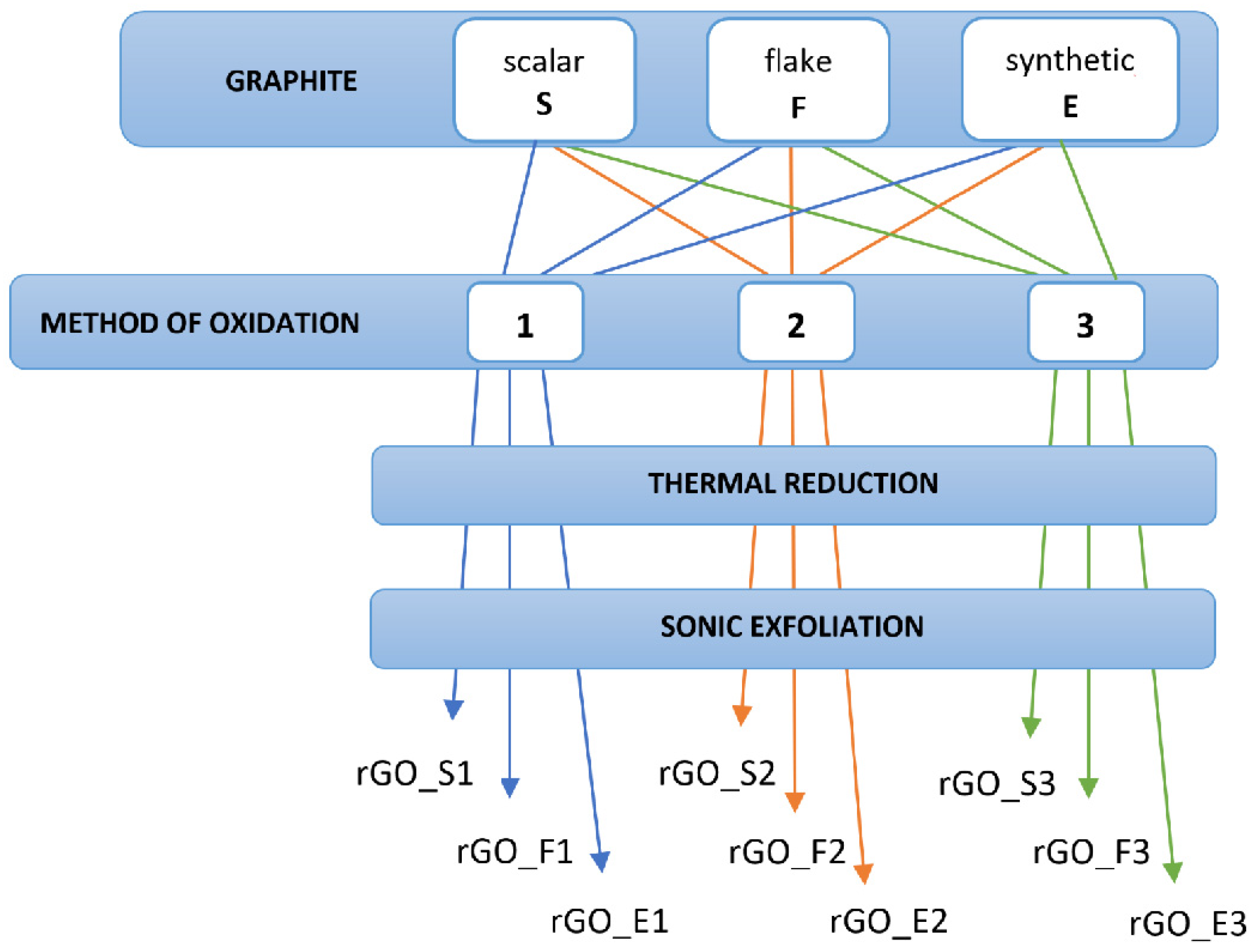
2. Materials and Methods
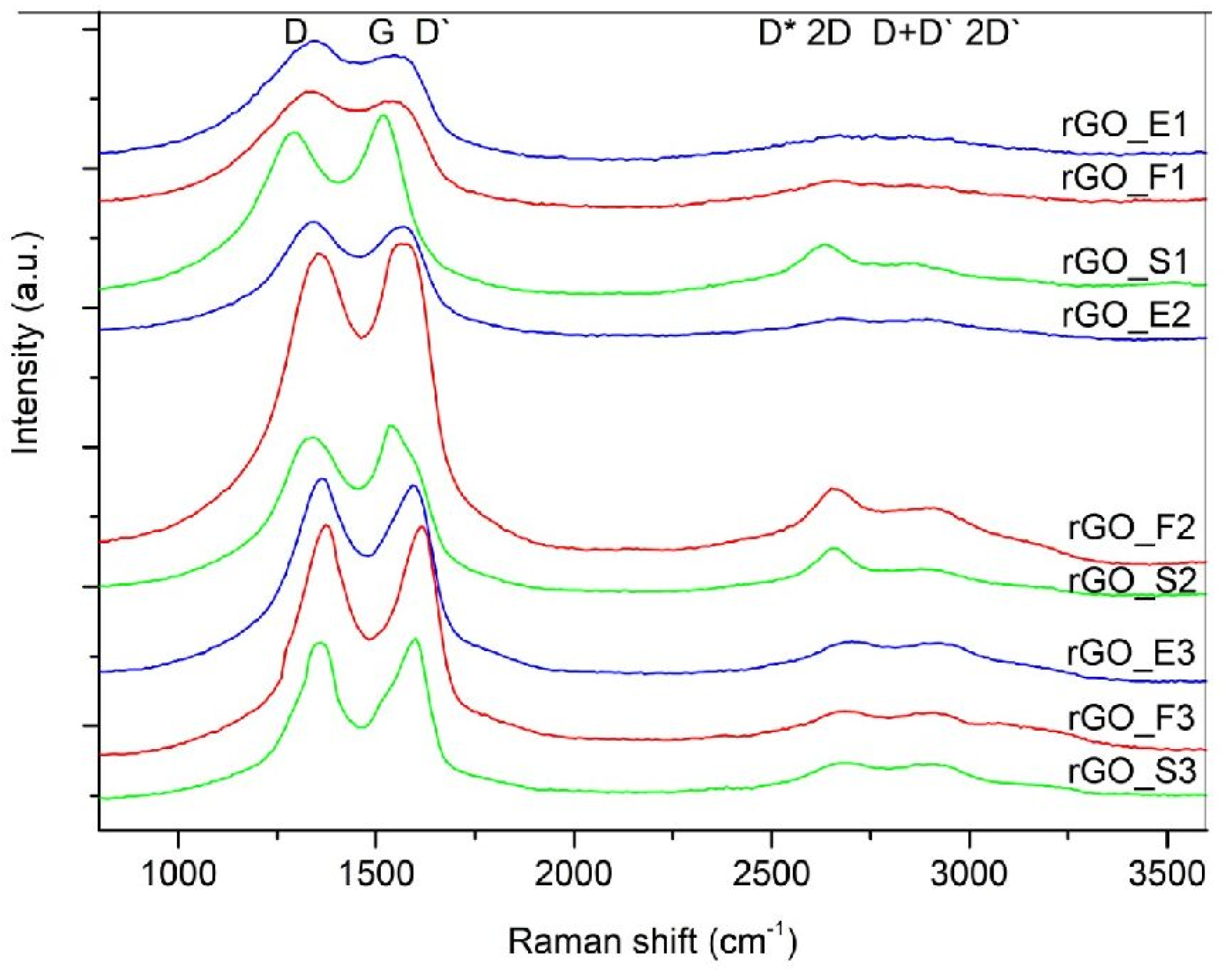
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Reduced graphene oxide (Rgo)-loaded metal-oxide nanofiber gas sensors: An overview. Sensors 2021, 21, 1352. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced graphene oxide molecular sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Renteria, J.A.; Ania, C.O.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R. Influence of protons on reduction degree and defect formation in electrochemically reduced graphene oxide. Carbon 2019, 149, 722–732. [Google Scholar] [CrossRef]
- Chua, C.K.; Sofer, Z.; Pumera, M. Graphite oxides: Effects of permanganate and chlorate oxidants on the oxygen composition. Chem. Eur. J. 2012, 18, 13453–13459. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Drewniak, S.; Pustelny, T.; Muzyka, R.; Plis, A. Studies of physicochemical properties of graphite oxide and thermally. Pol. J. Chem. Technol. 2015, 17, 109–114. [Google Scholar] [CrossRef]
- Wei, M.; Qiao, L.; Zhang, H.; Karakalos, S.; Ma, K.; Fu, Z.; Swihart, M.T.; Wu, G. Engineering reduced graphene oxides with enhanced electrochemical properties through multiple-step reductions. Electrochim. Acta 2017, 258, 735–743. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Abouei, E.; Hatamy, S.; Ghasemi, E. Accelerated differentiation of neural stem cells into neurons on ginseng-reduced graphene oxide sheets. Carbon 2014, 66, 395–406. [Google Scholar] [CrossRef]
- Diez, N.; Ś͆liwak, A.; Gryglewicz, S.; Grzyb, B.; Gryglewicz, G. Enhanced reduction of graphene oxide by high-pressure hydrothermal treatment. RSC Adv. 2015, 5, 81831–81837. [Google Scholar] [CrossRef]
- Dong, L.; Yang, J.; Chhowalla, M.; Loh, K.P. Synthesis and reduction of large sized graphene oxide sheets. Chem. Soc. Rev. 2017, 46, 7306–7316. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Z.; Li, H.; Song, S.; Lopez-Valdivieso, A. Synthesis of graphene oxide using mildly oxidized graphite through ultrasonic exfoliation. Surf. Rev. Lett. 2017, 24, 1750087. [Google Scholar] [CrossRef]
- Ferrari, I.; Motta, A.; Zanoni, R.; Scaramuzzo, F.A.; Amato, F.; Dalchiele, E.A.; Marrani, A.G. Understanding the nature of graphene oxide functional groups by modulation of the electrochemical reduction: A combined experimental and theoretical approach. Carbon 2022, 203, 29–38. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural characterization of graphene oxide: Surface functional groups and fractionated oxidative debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef]
- Sali, S.; Mackey, H.R.; Abdala, A.A. Effect of graphene oxide synthesis method on properties and performance of polysulfone-graphene oxide mixed matrix membranes. Nanomaterials 2019, 9, 769. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef]
- Bonavolontà, C.; Vettoliere, A.; Falco, G.; Aramo, C.; Rendina, I.; Ruggiero, B.; Silvestrini, P.; Valentino, M. Reduced graphene oxide on silicon-based structure as novel broadband photodetector. Sci. Rep. 2021, 11, 13015. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, C.; Santamaría, R.; Granda, M.; Gutiérrez, M.D.; Rodríguez-Reinoso, F.; Menéndez, R. Critical temperatures in the synthesis of graphene-like materials by thermal exfoliation-reduction of graphite oxide. Carbon 2013, 52, 476–485. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Totepvimarn, K.; Eimburanapravat, P.; Boonchompoo, W.; Buasri, A. Preparation and characterization of reduced graphene oxide sheets via water-based exfoliation and reduction methods. Adv. Mater. Sci. Eng. 2013, 1, 923403. [Google Scholar] [CrossRef]
- Osmieri, L.; Monteverde Videla, A.H.A.; Specchia, S. The use of different types of reduced graphene oxide in the preparation of Fe-N-C electrocatalysts: Capacitive behavior and oxygen reduction reaction activity in alkaline medium. J. Solid. State Electrochem. 2016, 20, 3507–3523. [Google Scholar] [CrossRef]
- Sieradzka, M.; Ślusarczyk, C.; Biniaś, W.; Fryczkowski, R. The role of the oxidation and reduction parameters on the properties of the reduced graphene oxide. Coatings 2021, 11, 166. [Google Scholar] [CrossRef]
- Báez, D.F.; Pardo, H.; Laborda, I.; Marco, J.F.; Yáñez, C.; Bollo, S. Reduced graphene oxides: Influence of the reduction method on the electrocatalytic effect towards nucleic acid oxidation. Nanomaterials 2017, 7, 168. [Google Scholar] [CrossRef]
- Drewniak, S.; Procek, M.; Muzyka, R.; Pustelny, T. Comparison of gas sensing properties of reduced graphene oxide obtained by two different methods. Sensors 2020, 20, 3175. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaría, R.; Romasanta, L.J.; Verdejo, R.; López-Manchado, M.A.; Menéndez, R. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 2013, 65, 156–164. [Google Scholar] [CrossRef]
- González, Z.; Botas, C.; Álvarez, P.; Roldán, S.; Blanco, C.; Santamaría, R.; Granda, M.; Menéndez, R. Thermally reduced graphite oxide as positive electrode in vanadium redox flow batteries. Carbon 2012, 50, 828–834. [Google Scholar] [CrossRef]
- Sthle, L.; Wold, S. Analysis of variance (ANOVA). Chemom. Intell. Lab. Syst. 1989, 6, 259–272. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Y.; Xie, T.; Tai, H.; Xie, G. A novel sensing mechanism for resistive gas sensors based on layered reduced graphene oxide thin films at room temperature. Sens. Actuators B Chem. 2014, 203, 135–142. [Google Scholar] [CrossRef]
- Pearce, R.; Iakimov, T.; Andersson, M.; Hultman, L.; Spetz, A.L.; Yakimova, R. Epitaxially grown graphene based gas sensors for ultra sensitive NO2 detection. Sens. Actuators B Chem. 2011, 155, 451–455. [Google Scholar] [CrossRef]
- Chen, H.; Ding, L.; Zhang, K.; Chen, Z.; Lei, Y.; Zhou, Z.; Hou, R. Preparation of chemically reduced graphene using hydrazine hydrate as the reduction agent and its NO2 sensitivity at room Temperature. Int. J. Electrochem. Sci. 2020, 15, 10231–10242. [Google Scholar] [CrossRef]
- Su, P.-G.; Shieh, H.-C. Flexible NO2 sensors fabricated by layer-by-layer covalent anchoring and in situ reduction of graphene oxide. Sens. Actuators B Chem. 2014, 190, 865–872. [Google Scholar] [CrossRef]
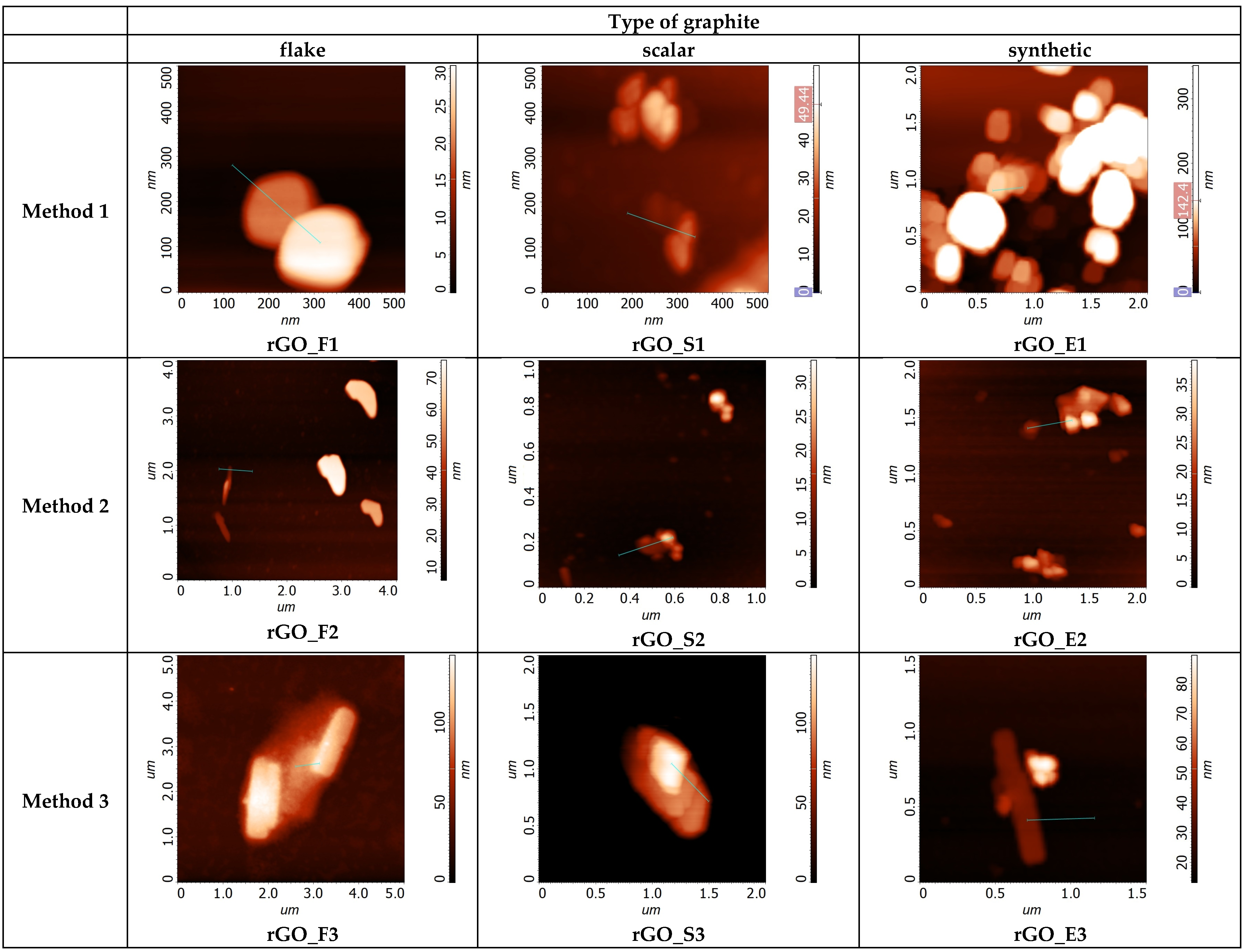
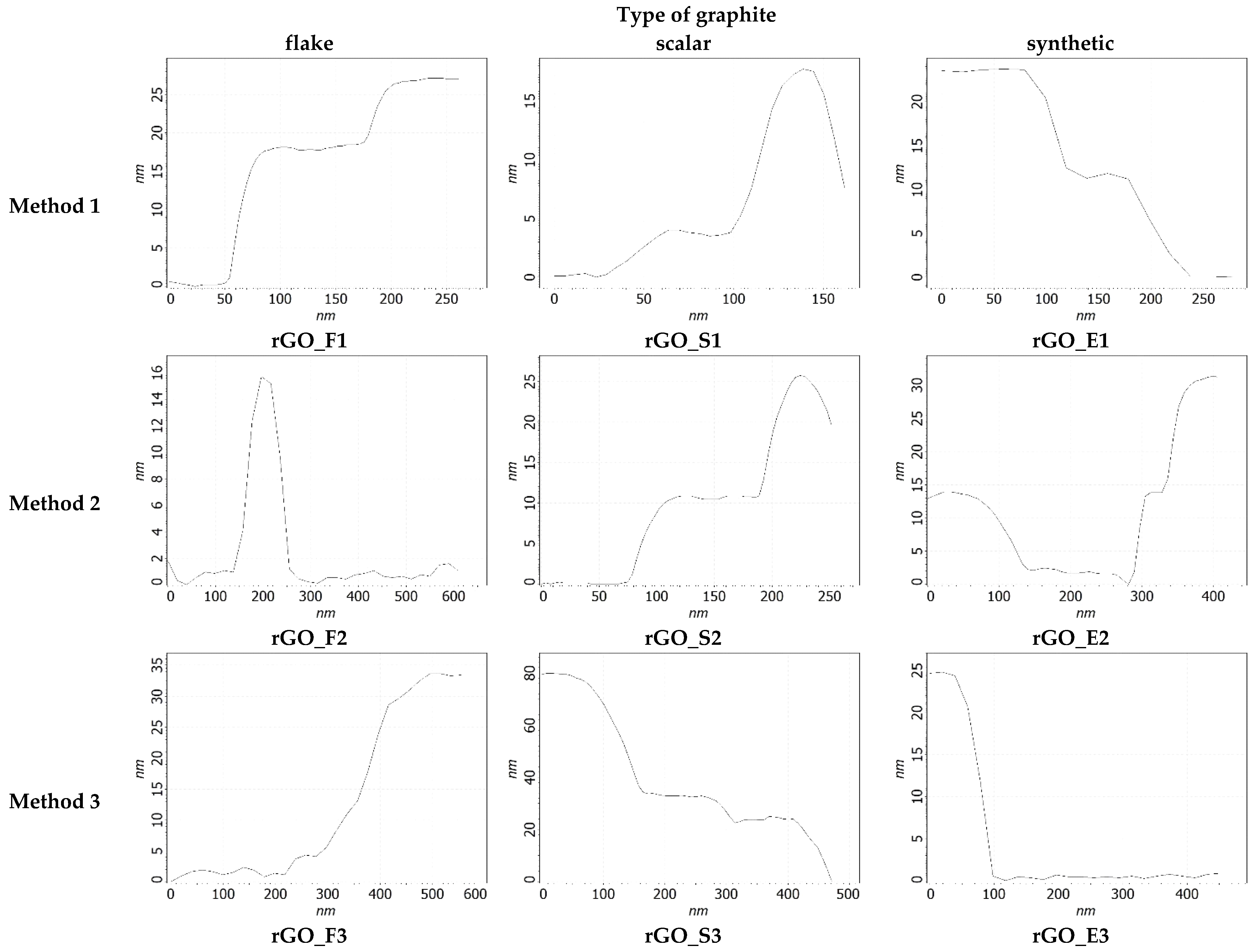



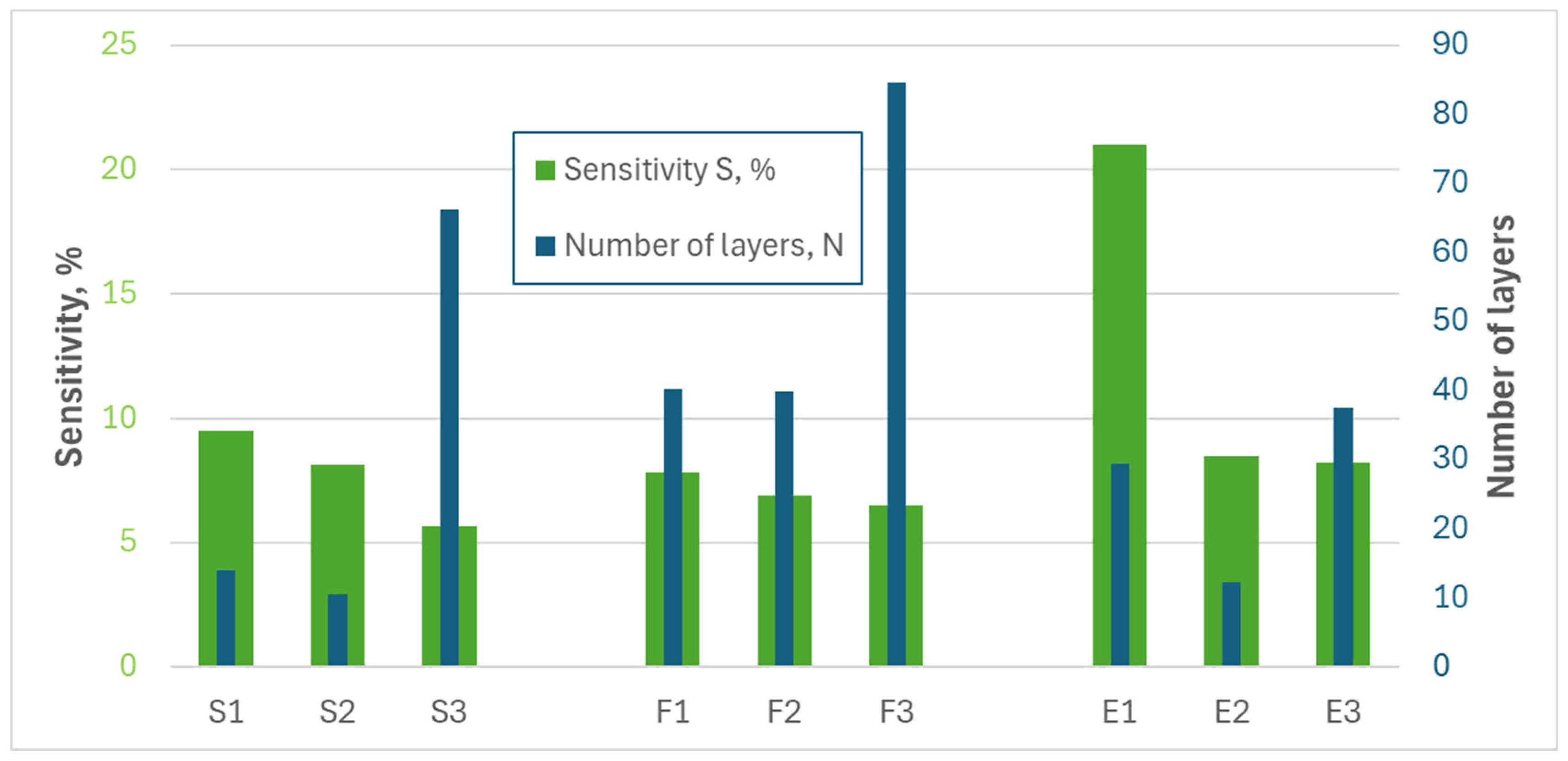
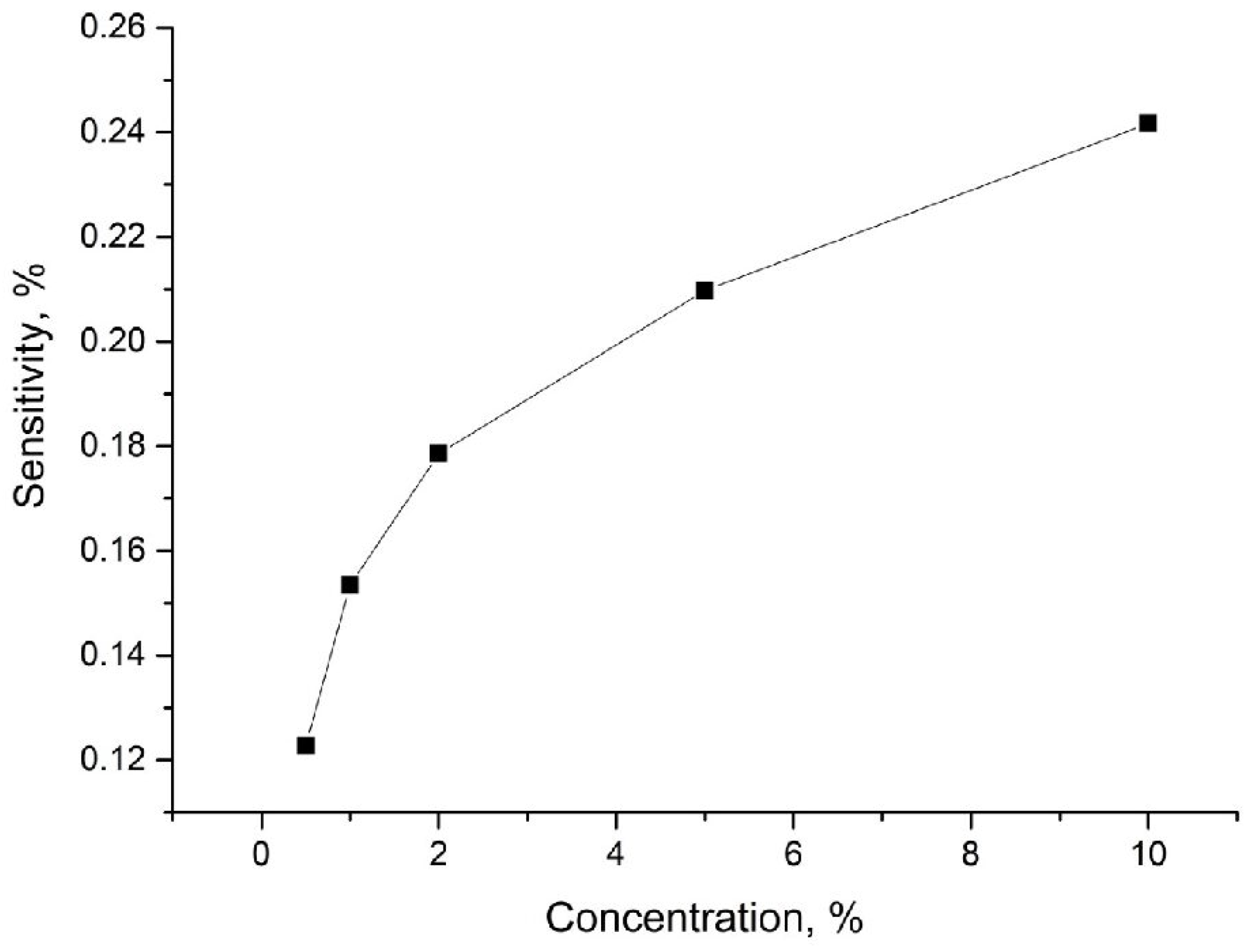
| The Null Hypothesis (H0) | Alternative Hypothesis (Ha) |
|---|---|
| Graphite precursor has no effect on the average number of graphene layers in rGO | Graphite precursor has an effect on the average number of graphene layers in rGO |
| Oxidation method has no effect on the average number of graphene layers in rGO | Oxidation method has an effect on the average number of graphene layers in rGO |
| There is no interaction effect between the type of graphite precursor and the oxidation method on the average number of graphene layers in rGO | There is an interaction effect between the type of graphite precursor and the oxidation method on the average number of graphene layers in rGO |
| rGO_E1 | rGO_F1 | rGO_S1 | rGO_E2 | rGO_F2 | rGO_S2 | rGO_E3 | rGO_F3 | rGO_S3 | |
|---|---|---|---|---|---|---|---|---|---|
| (nm) | 44.2 | 60.4 | 21 | 18.4 | 59.7 | 15.7 | 56.4 | 126.9 | 99.4 |
| σ (nm) | 30.3 | 25.1 | 13.1 | 9.2 | 58.2 | 13.2 | 51.0 | 87.3 | 91.5 |
| N | 29.5 | 40.3 | 14.0 | 12.3 | 39.8 | 10.5 | 37.6 | 84.6 | 66.3 |
| Source | Sum. Sq. 1 | d. f. 2 | Mean. Sq. 3 | F 4 | Prob > F(p) 5 |
|---|---|---|---|---|---|
| Graphite precursor | 161,631.1 | 2 | 80,815.6 | 5.01 | 0.0814 |
| Oxidation method | 341,428.9 | 2 | 170,714.4 | 10.58 | 0.0250 |
| Interaction | 64,513.5 | 4 | 16,128.4 | 6.05 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drewniak, Ł.; Drewniak, S.; Sajdak, M.; Muzyka, R. Statistical Analysis of the Influence of Various Types of Graphite Precursors and Oxidation Methods on the Gas Sensor Properties of Reduced Graphene Oxide. Sensors 2024, 24, 6346. https://doi.org/10.3390/s24196346
Drewniak Ł, Drewniak S, Sajdak M, Muzyka R. Statistical Analysis of the Influence of Various Types of Graphite Precursors and Oxidation Methods on the Gas Sensor Properties of Reduced Graphene Oxide. Sensors. 2024; 24(19):6346. https://doi.org/10.3390/s24196346
Chicago/Turabian StyleDrewniak, Łukasz, Sabina Drewniak, Marcin Sajdak, and Roksana Muzyka. 2024. "Statistical Analysis of the Influence of Various Types of Graphite Precursors and Oxidation Methods on the Gas Sensor Properties of Reduced Graphene Oxide" Sensors 24, no. 19: 6346. https://doi.org/10.3390/s24196346
APA StyleDrewniak, Ł., Drewniak, S., Sajdak, M., & Muzyka, R. (2024). Statistical Analysis of the Influence of Various Types of Graphite Precursors and Oxidation Methods on the Gas Sensor Properties of Reduced Graphene Oxide. Sensors, 24(19), 6346. https://doi.org/10.3390/s24196346








