1. Introduction
On-field continuous monitoring of nitrate nitrogen and other nitrogen fertilizers of soil through advanced Internet of Things (IoT) technologies enables the optimization of crop growth, resource efficiency, and cost management by data-driven decision-making processes [
1,
2,
3]. It is also essentially important to environmental stewardship in precision and smart agriculture, as the overuse of nitrogen mineral fertilizers and the release of their residuals into the environment have led to groundwater pollution, stratospheric ozone depletion, and global warming over the past several decades [
4].
Among the recently presented technologies, Md. Azahar Ali et al. proposed an all-solid-state miniature potentiometric soil sensor with parts-per-million (ppm) resolution, but the degradation of the Ag/AgCl reference electrode may limit its long-term deployment over months [
5]. Mohammed A.E. et al. presented an ion-selective electrode (ISE) electrochemical soil nitrate sensor that utilizes electrochemical impedance spectroscopy (EIS) for direct real-time continuous soil nitrate measurement in the range from 8 ppm to 512 ppm without any soil pretreatment. However, the large size of the sensor probe posed integration challenges with IoT-oriented agricultural implements [
6]. Many other technologies have been reported as well, but up to today, none of them have moved into practical applications within distributed sensor networks in the IoT era.
Graphene, a hexagonal lattice of carbon-containing atom-thick layer that possesses remarkable electronic conductivity and thermal characteristics, is believed to be very promising for the detection of gaseous molecules due to its high sensitivity to electric charges in its vicinity and its extremely high surface-to-volume ratio [
7,
8,
9]. Graphene field-effect transistors (FETs) have been reported for detecting various biomolecules and gas molecules [
10,
11], making them a prominent research topic for precision and smart agriculture applications in recent years. Most graphene FETs employ solid-state gate materials that exhibit low sensitivity and selectivity to a certain molecular gas, necessitating high operating voltages in the range of several tens of volts [
12]. It is difficult to monitor soil fertilizers continuously through IoT technologies due to the challenges of both power supply and system integration in a distributed wireless sensor network since the lifetime of the sensor strongly depends on battery life. Additionally, as summarized and pointed out by Raj A. et al., harvesting techniques are largely insufficient for powering IoT nodes due to limited power densities or inconsistencies as to when power is harvested [
13].
Inaba A. et al. presented a graphene field-effect transistor gated by ionic liquid for ammonia gas sensing. They demonstrated the operation of the device at an ultra-low voltage of less than 1 V, by which the calculated detection limit was 130 ppb [
14]. In this sensor, the graphene channel is chemically modified by the ionic liquid, which selectively absorbs gas molecules. When a gate voltage (V
g) is applied to the ionic liquid, a non-conductive electric double layer forms near the graphene-FET channel. This results in a field-effect transistor with the ionic liquid as the gate. The absorbed target gas molecules by the ionic liquid are diffused to the surface of the graphene channel, resulting in the change of the drain-source current (I
ds). Consequently, the device’s sensitivity and selectivity are strongly dependent on the volume and type of the ionic liquid due to its diffusion speed, diffusion distances, and selectivity. Thus, a dedicated structure is needed to ensure its reliability for volume control in practical applications. The detailed device sensing mechanism can be found in Inaba A. et al. [
15].
In this study, a compact, multi-channel ionic liquid-gated graphene field-effect transistor (FET) has been proposed and developed for on-field continuous monitoring of nitrate nitrogen and other nitrogen fertilizers in smart agriculture [
16]. Nitrate nitrogen, which is easily soluble in water and exists in the soil solution, has a direct effect on crop growth. Our sensor may enable the measurement of nitrate nitrogen concentration from the water vapor emitted from the soil, which is extremely important for on-field continuous monitoring of soil conditions to improve agricultural practices [
17]. In this device, a hydrophobic ‘ring’-shaped pattern was designed around the graphene channel. An ultra-small droplet of ionic liquids, i.e., [PMIM][BF4], with controlled volume, was filled onto the surface of the graphene channel to selectively absorb target gas molecules and then change the drain-source current (I
ds) at different gate voltage (V
g). Compared to the solid-state graphene FETs, the proposed ionic liquid-gated graphene FETs offer the unique advantages of high sensitivity by ionic liquids with low-voltage operation to the atom-thick nature of graphene [
15,
17,
18]. It is believed to be promising for advancing and transforming future agriculture toward achieving sustainable and efficient farming practices. Compared to the electrolyte-gate GFET developed by P. D. Cabral et al. [
19] and the graphene field-effect transistor gated by ionic liquid developed by Inaba A. et al. [
14], our device exhibits excellent capability in controlling the volume and location of ionic liquids, making it suitable for sensing trace gas molecules with high resolution. Moreover, ionic liquid-gated graphene FETs are believed to excel in response time over solid electrolyte-gated FETs, which have high electrolyte resistance, quicker absorption and dis-absorption, and faster diffusion speed. However, developing a robust device structure with scalable and sensor-compatible fabrication processes that allow for easy filling of ultrasmall volumes of ionic liquids, minimal graphene defects, and high process yields is still necessary for commercial viability.
In this paper, CYTOP® (CTL-809M, Asahi Glass Co., Ltd., Tokyo, Japan) and a photosensitive hydrophobic layer (LDW-N010, Tokyo Ohka Kogyo Co., Ltd., Kawasaki, Japan) were used to create the hydrophobic ‘ring’-shaped pattern. Correspondingly, two different processes using the CYTOP® and the photosensitive hydrophobic layer were developed, evaluated, and compared for the fabrication of our proposed devices. A 6-channel ionic liquid-gated graphene FET with typical FET characteristics was successfully obtained on a single chip to investigate the dependency of graphene channel size on response time, the selectivity of the ionic liquid for various gases, and the sensitivity of the device at different gas concentrations.
This paper demonstrates that the use of the ultra-thin photosensitive hydrophobic layer enables the successful filling of ionic liquids with volumes of 100 nL or less, while the CYTOP® allows for larger volumes of up to 600 nL but at the cost of reduced yields due to chemical residues etc. The process difficulties, major obstacles, and improvements are discussed in detail. Additionally, the fabricated devices were evaluated using a self-developed soil simulation system to confirm their functionality as a gas sensor after the filling of the ionic liquids.
2. Experimental
2.1. Device Layout and Structure
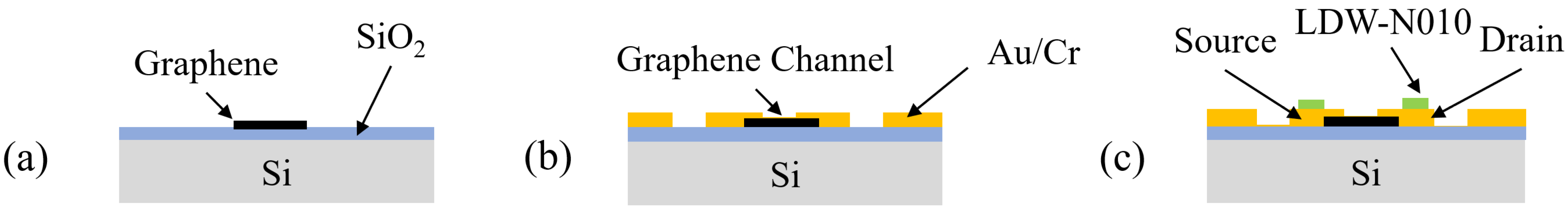
The detailed structure, layout, and cross-section views of each ionic liquid-gated graphene FET are shown in
Figure 1. A CVD-grown single graphene layer was used to create the graphene-FET channel. The SiO
2 on the backside of the silicon was removed to enable the applications when back gates are needed.
To investigate the selectivity of the ionic liquid for various gases, a hydrophobic ‘ring’-shaped pattern was designed around each graphene channel, as shown in the right panel of
Figure 1. This pattern restricts the position and controls the volume of the ionic liquid during the subsequent filling process. A thin layer of Au/Cr with a thickness of 36 nm/4 nm was deposited on each side of the graphene channel to serve as the source and drain electrodes. The gate electrode was designed on both sides of the graphene channel for better contact with ionic liquids and easy assembly.
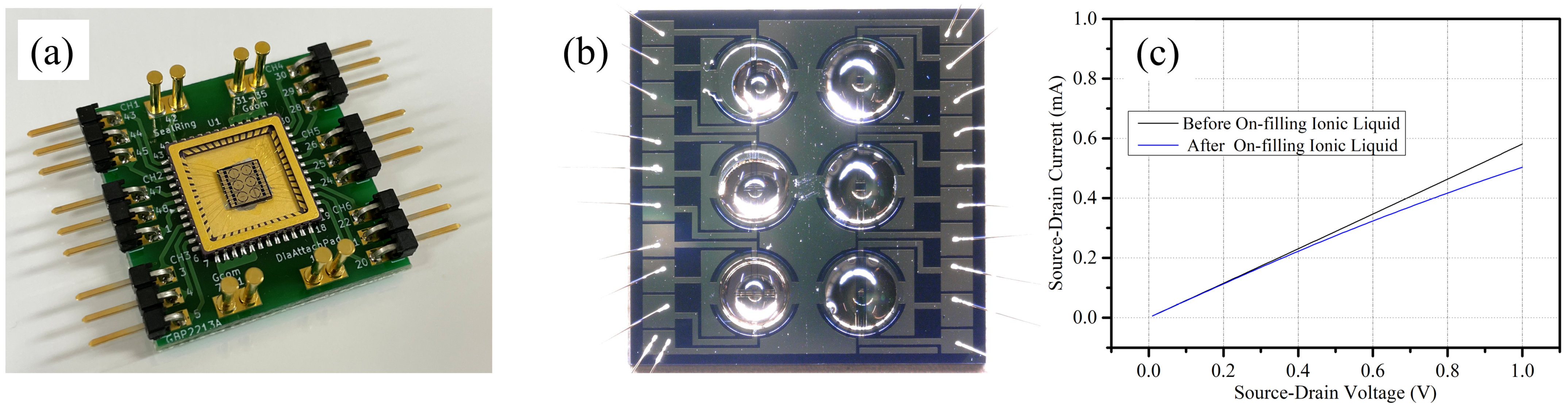
To study the dependency of the channel size on response time, we designed six ionic liquid-gated FETs with different channel sizes on the same chip. The channel length was designed to be 20 μm and 100 μm. The channel width was designed to be 50 μm, 100 μm, 250 μm, and 500 μm. The size of the chip with six channels of ionic liquid-gated graphene FETs was 5 mm × 5 mm.
2.2. Fabrication Process Using CYTOP®
The device fabrication started by using a 4-inch silicon wafer with a 100 nm-thick SiO
2 passivation layer on the top surface of the wafer. Since graphene is unsuitable to grow directly on SiO
2/Si substrate, a commercially available CVD-grown single graphene layer, sized 50 mm × 50 mm, was transferred from a copper foil onto the 4-inch SiO
2/Si wafer using a wet process. Raman spectroscopy was used to ensure fewer defects and contamination during the graphene transfer [
20].
CYTOP
® is a fluoropolymer that dissolves in exclusive fluorine-based solvents, making it suitable as a thin film coating with excellent hydrophobic properties.
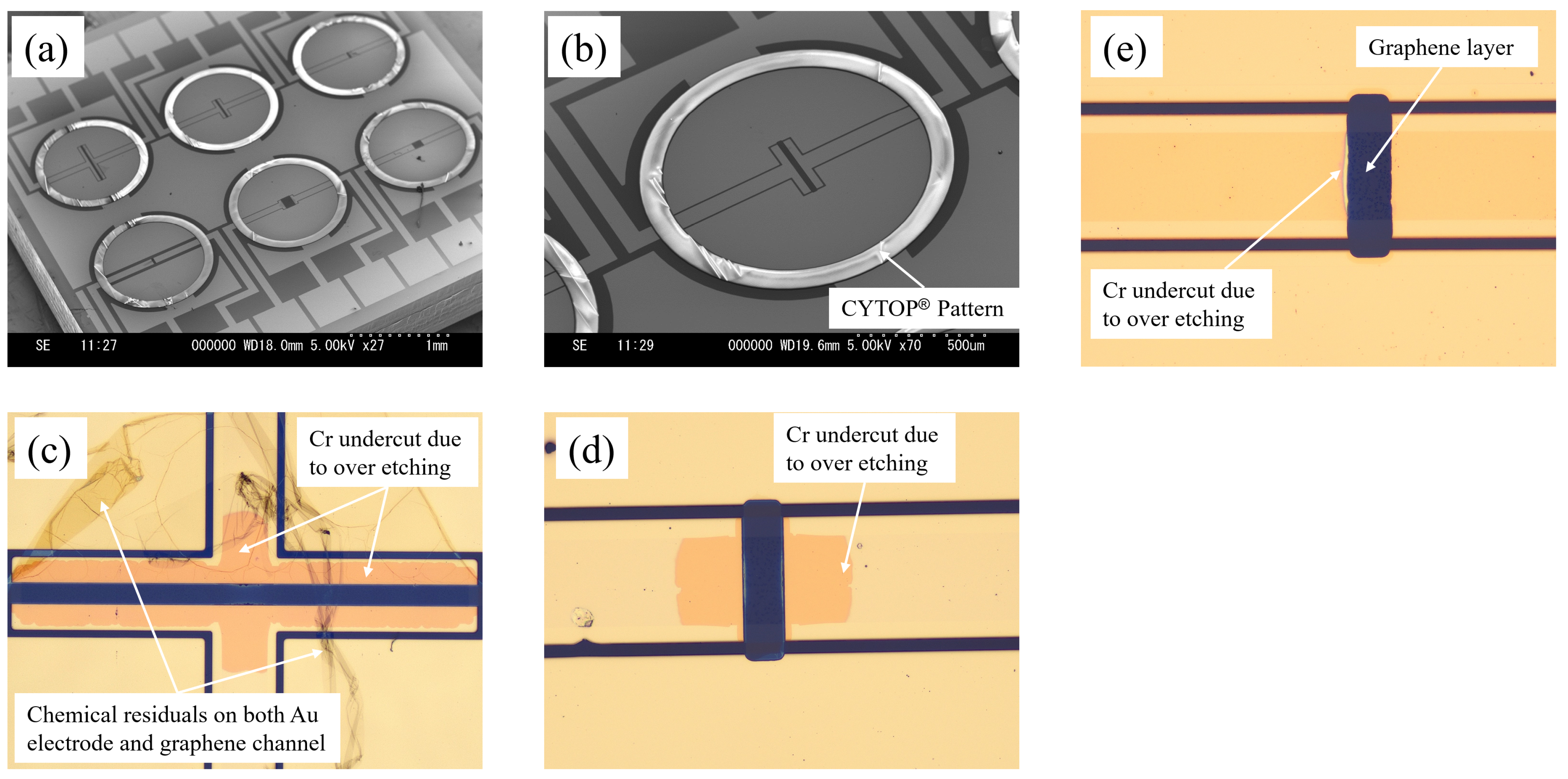
Figure 2 shows a detailed process flow of CYTOP
® being used to create a hydrophobic ‘ring’-shaped pattern surrounding the graphene channels to facilitate the easy deposition and filling of the ionic liquids. Firstly, as shown in
Figure 2a,f, the graphene layer was patterned using oxygen plasma reactive ion etching (RIE), followed by the Au/Cr electrode layer deposition via sputter and patterning by a lift-off process, as shown in
Figure 2b. Then, the hydrophobic CYTOP
® layer was deposited by spin-coating, baked at 80 °C for 30 min and 200 °C for 30 min, and patterned by oxygen plasma RIE as shown in
Figure 2c,d. Finally, as shown in
Figure 2e, the graphene channel was released by removing Au/Cr on the top of the channel via wet etching.
The dry etching of a graphene single layer has been reported by P. D. Cabral et al. [
19], and the detailed process for CYTOP
® coating, baking, and RIE etching has been published elsewhere [
21,
22]. The photoresist was used as the etching mask for oxygen plasma RIE in both the graphene etching and CYTOP
® patterning. Before coating the photoresist on CYTOP
®, oxygen plasma RIE was applied to the surface of CYTOP
® for 10 s to enhance the adhesive force between the photoresist and the CYTOP
®.
A two-step process was used in the oxygen plasma RIE of CYTOP®. The process parameters were as follows: RF power 300 W, pressure 10 Pa, O2 flow rate 50 sccm, and process time 2 min 40 s for the first step; RF power 100 W, pressure 15 Pa, O2 flow rate 100 sccm, and process time 1 min for the second step. Prior to releasing the graphene channel, an O2 plasma ashing process (power 500 W, pressure 15 Pa, O2 flow rate 200 sccm, process time 3 min) was performed to ensure that no residues were left on the surface of the electrodes, ensuring optimal contact of the ionic liquids with the source, drain, and gate electrodes.
2.3. Fabrication Process Using LDW-N010
LDW-N010 offers similar hydrophobic properties to CYTOP
® with better process compatibility, but at a much thinner layer, only a few tens of nanometers, which facilitates process integration with less contamination. Another attractive property of LDW-N010 is its photosensitivity and good chemical resistance to ionic liquids.
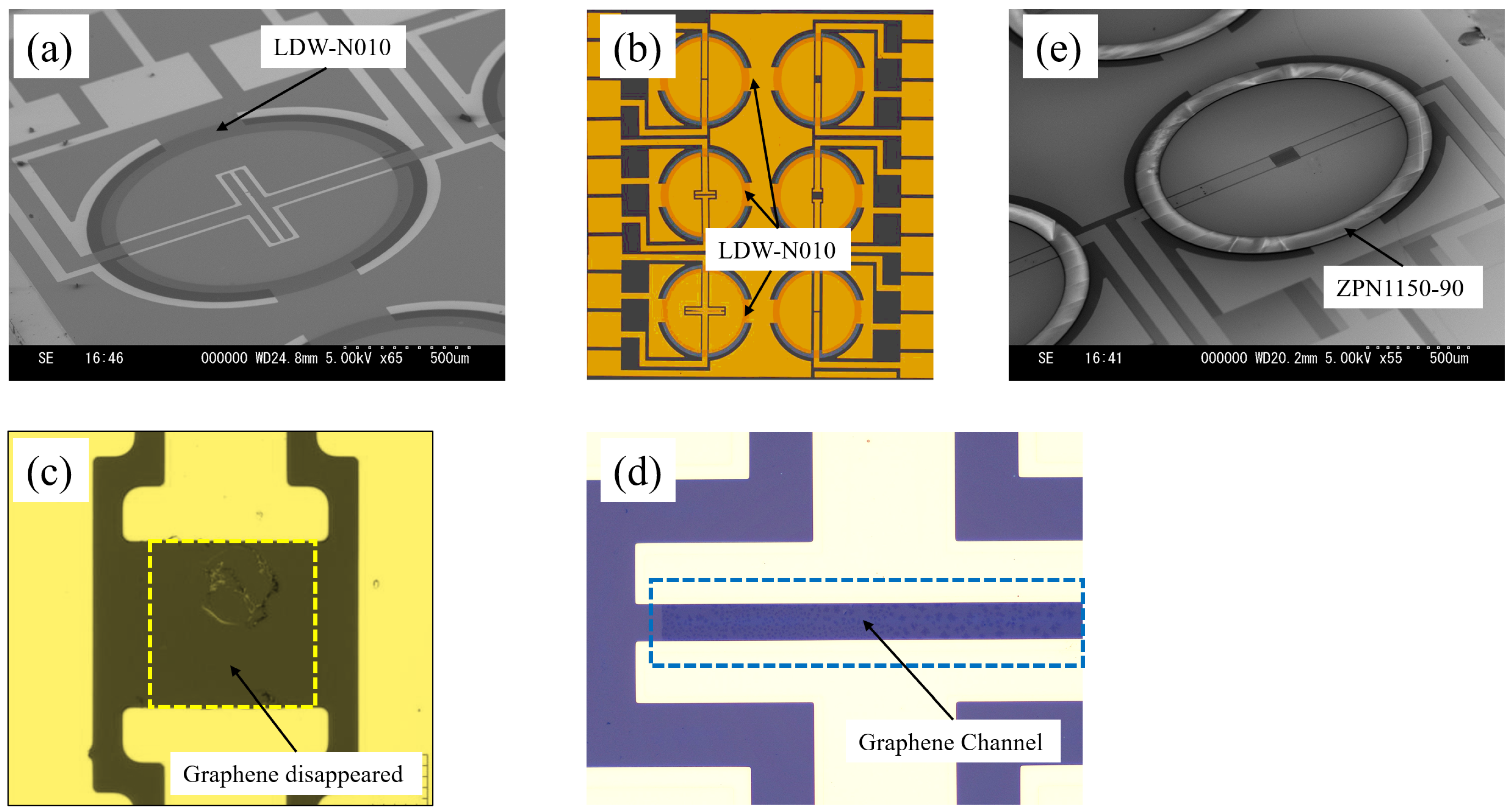
Figure 3 illustrates the detailed process flow when using LDW-N010 to create a hydrophobic ‘ring’-shaped pattern surrounding the graphene channels. The first and second processes were the same as those used in CYTOP
®. Then, the wafer surface was coated with LDW-N010 via spin-coating (1000 rpm, 60 s). After pre-baking the LDW-N010 at 100 °C for 60 s, exposing it at 300 mJ/cm², pre-baking again at 100 °C for 60 s, and developing it in TMAH for 60 s, the hydrophobic LDW-N010 “ring”-shaped pattern was obtained, as shown in
Figure 3c.
The use of LDW-N010 eliminates the need for the oxygen plasma RIE of the CYTOP
®, as seen in
Figure 2d. Consequently, the graphene channels can be released simultaneously using the lift-off process, depicted in
Figure 3b, thereby omitting the Au/Cr wet etching process, as shown in
Figure 2e. The process depicted in
Figure 3 simplified the processes and eliminated the need for high-temperature baking and oxygen plasma RIE of the CYTOP
®, thereby increasing the process compatibility and yields.
A hydrophobic negative photoresist (ZPN1150-90, ZEON Crop., Tokyo Japan) was used in this work for comparison with the results of using the CYTOP® and the LDW-N010, as well as to investigate the thickness effects of the hydrophobic pattern. The ZPN1150-90 was spin-coated at 3000 rpm for 60 s to achieve a thickness similar to that of the CYTOP®.
2.4. Device Evaluation
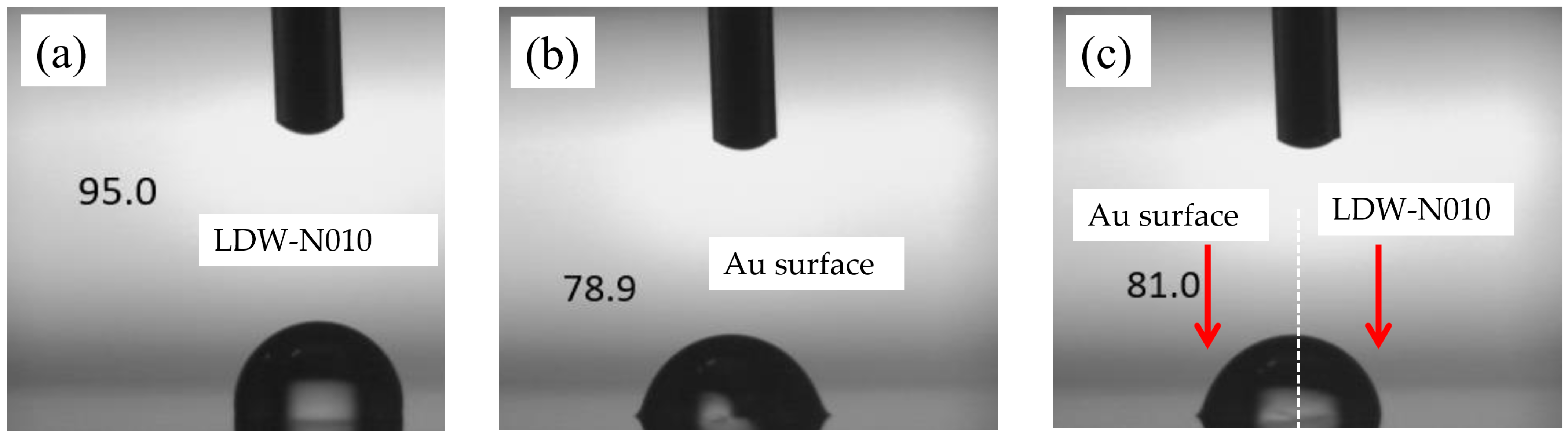
The wettability of the hydrophobic patterns was evaluated using a contact angle meter (PCA11, Kyowa Interface Science Co., Ltd., Niiza, Japan). The thickness of the hydrophobic patterns was measured using a wafer surface profiler (Tencor P-16, KLA Corp., Milpitas, CA, USA). Since the graphene is easily removed by the electron beam when using a scanning electron microscope, the geometry of the fabricated devices was observed using a scanning electron microscope (S-3000H, Hitachi Co., Ltd., Tokyo, Japan), and the graphene channel of the fabricated devices was observed by using a 3D laser scanning microscope (VK-X3000, Keyence Crop., Osaka, Japan). The ionic liquid filling was evaluated using a pipette with a controllable volume range of 100 nL to 2 μL (Nichipet EXII, Nichiryo Co., Ltd., Koshigaya, Japan) and then observed using the 3D laser scanning microscope.
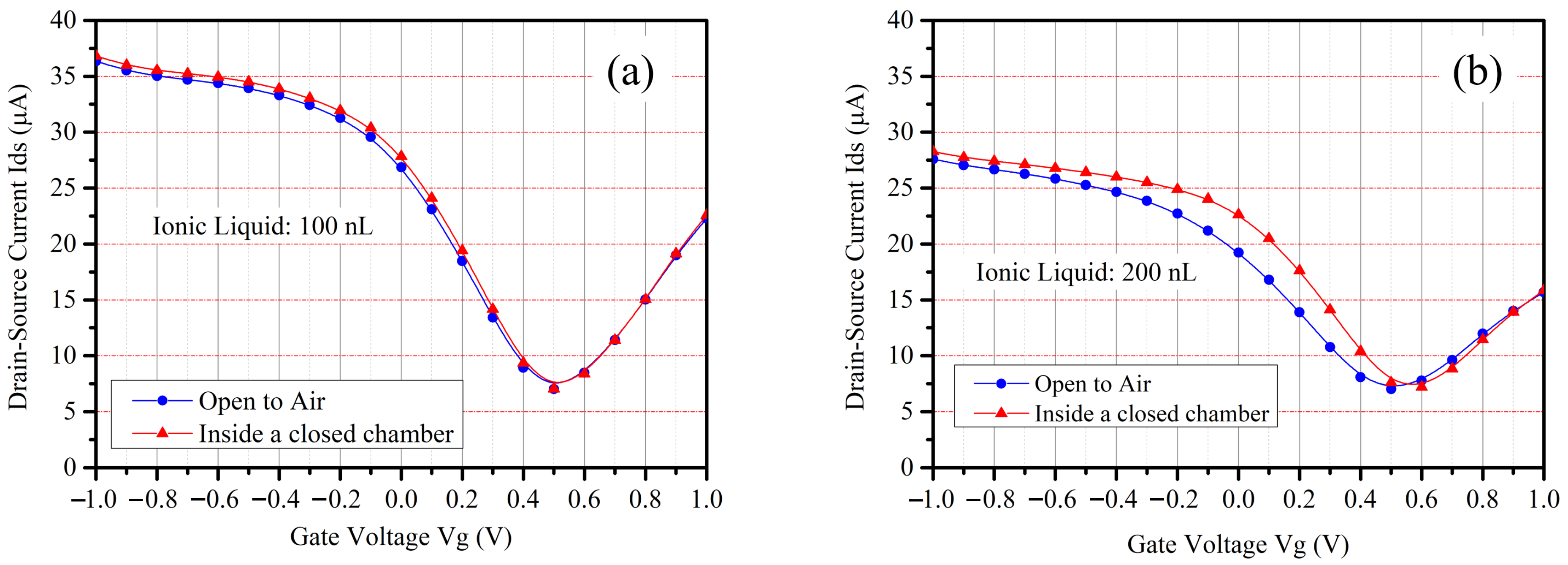
A soil simulation system was developed to investigate the electrical characteristics of the device and its functionalities as a gas sensor. The self-developed soil simulation system includes a sealed chamber with a size of 300 mm × 300 mm × 170 mm, a vaporizer using a heater and a Peltier to artificially mix the target gas components, an electrical fan to circulate the target molecular gas inside the chamber for better reliability and repeatability, and a signal processing module with a source measure unit (Keithley 2450, Tektronix, Inc., Beaverton, OR, USA). To confirm the response of the device and their characteristics for NO
3-N detection, the fabricated device was set inside the self-developed soil simulation system after the filling of the ionic liquids, and then the current-voltage characteristics of the source-drain electrodes (I
ds-V
ds) of the device was measured and recorded. The working principles and the design of the soil simulation system have been published elsewhere [
15,
16]. More detailed information about the self-developed soil simulation system will be presented, together with our device evaluation results by using various gases, in our future publications.