The Development of a Multicommand Tactile Event-Related Potential-Based Brain–Computer Interface Utilizing a Low-Cost Wearable Vibrotactile Stimulator
Abstract
1. Introduction
2. Materials and Methods
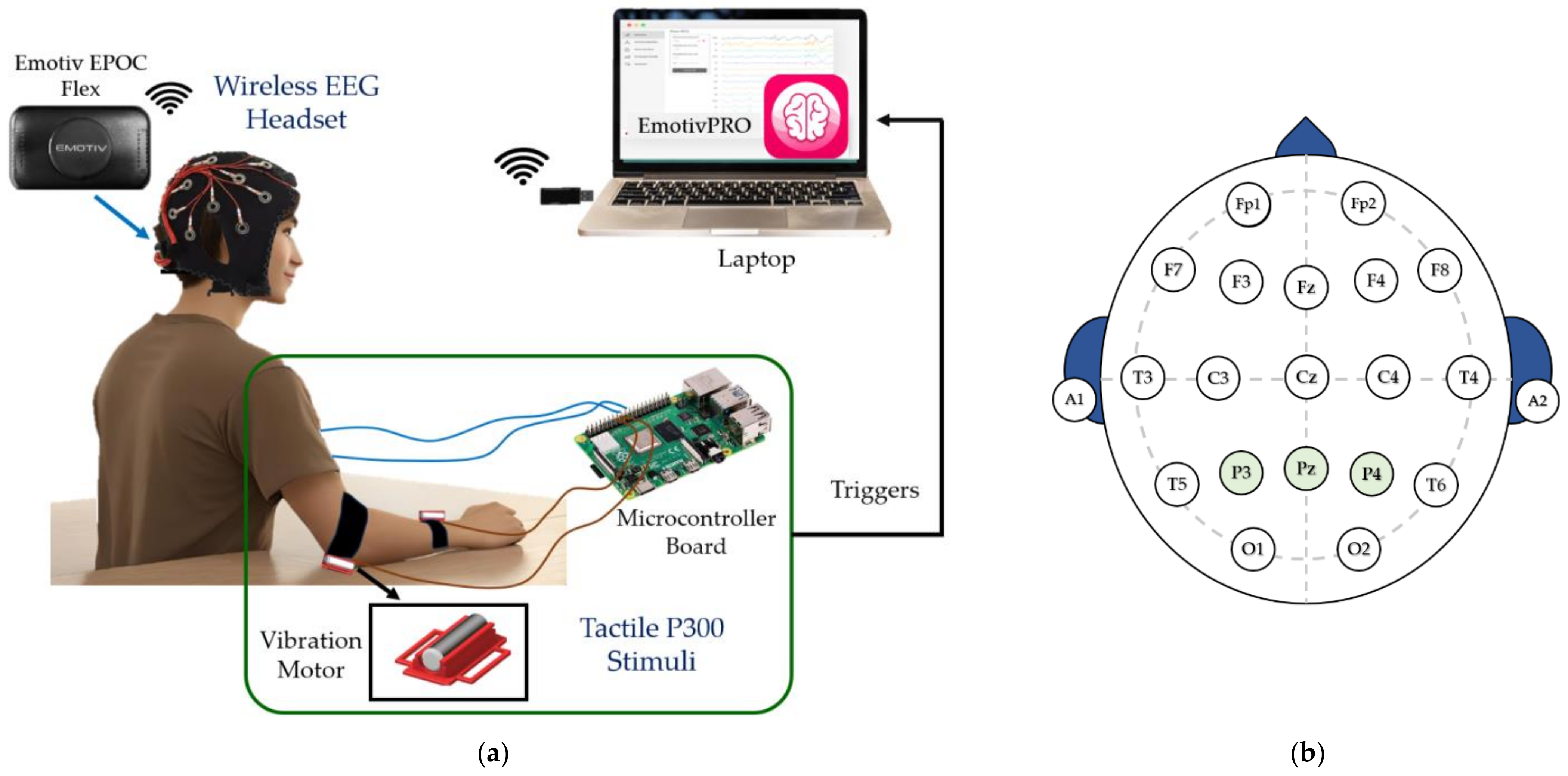
2.1. EEG Signal Acquisition
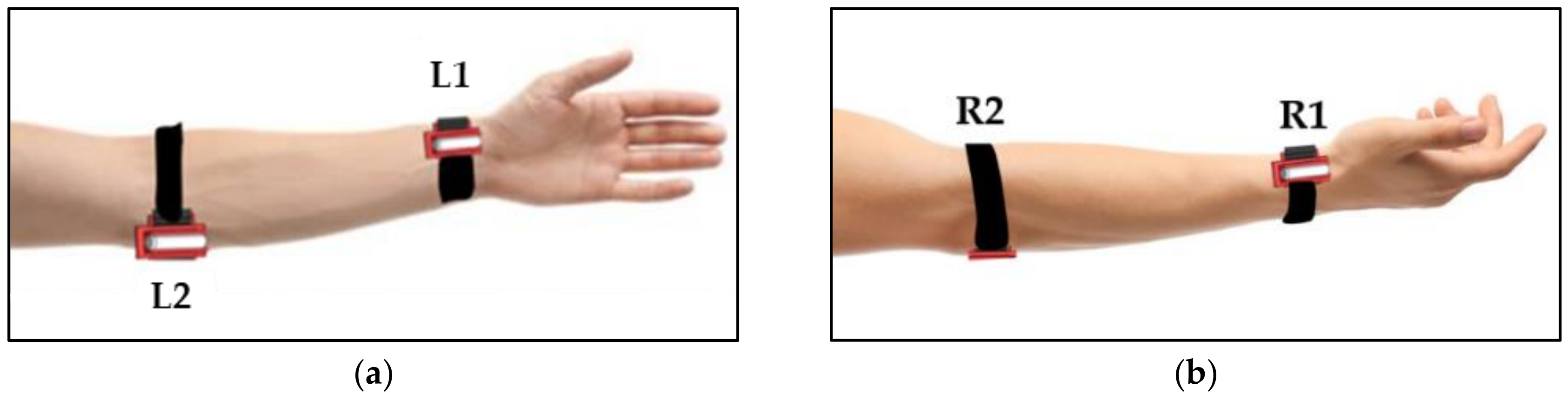
2.2. Proposed Vibrotactile Stimulation
2.3. ERP Feature Selection and Classification
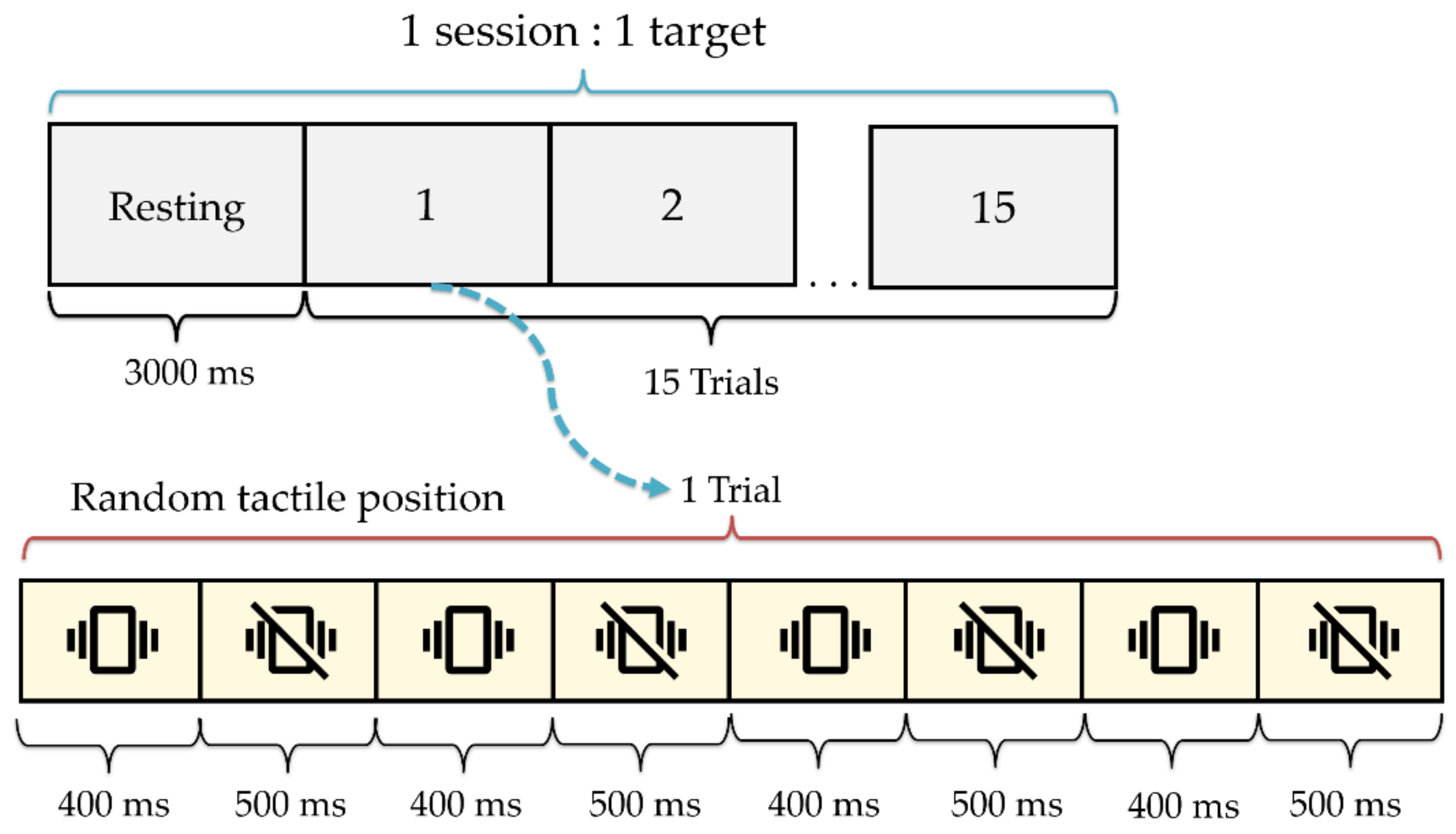
2.4. Experiments
3. Results
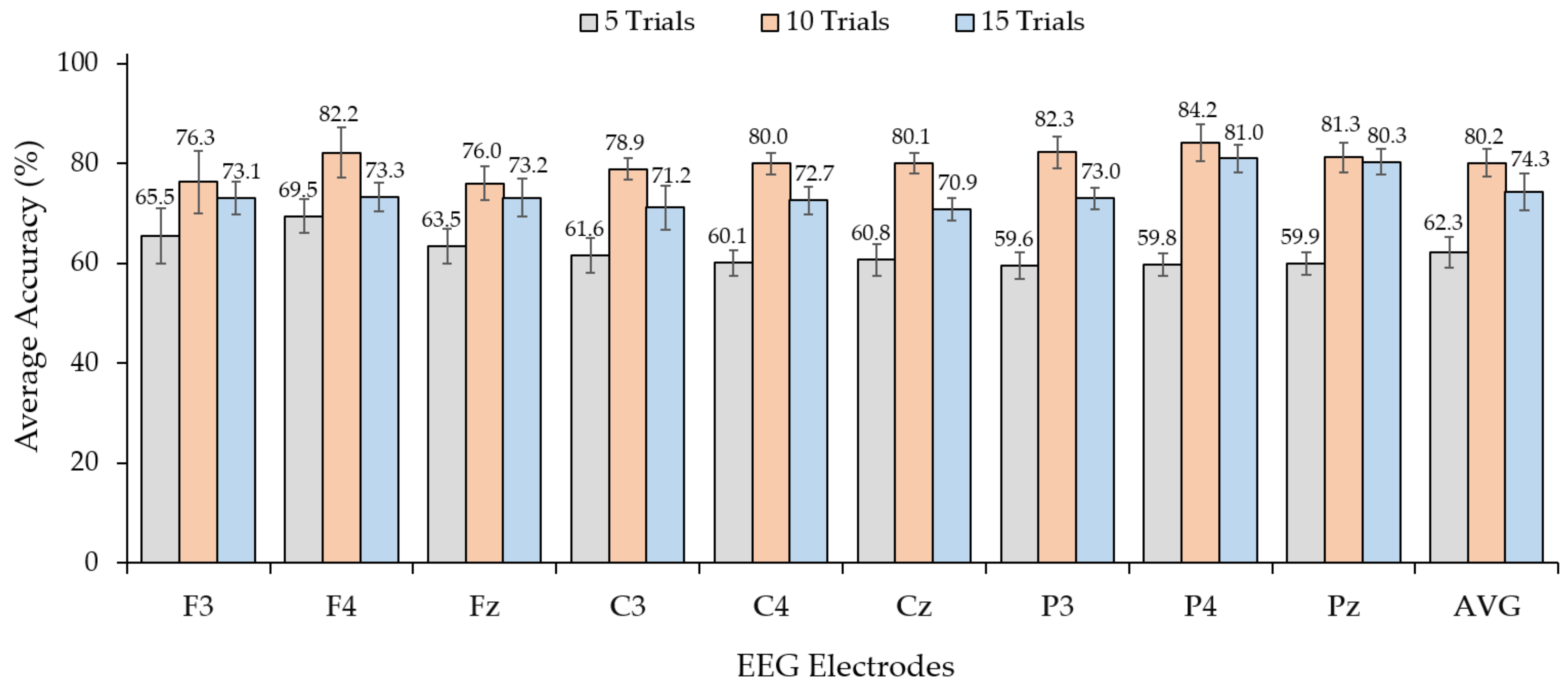
3.1. Observation of Trial of Use and EEG Electrodes for Vibrotactile Stimulus Pattern
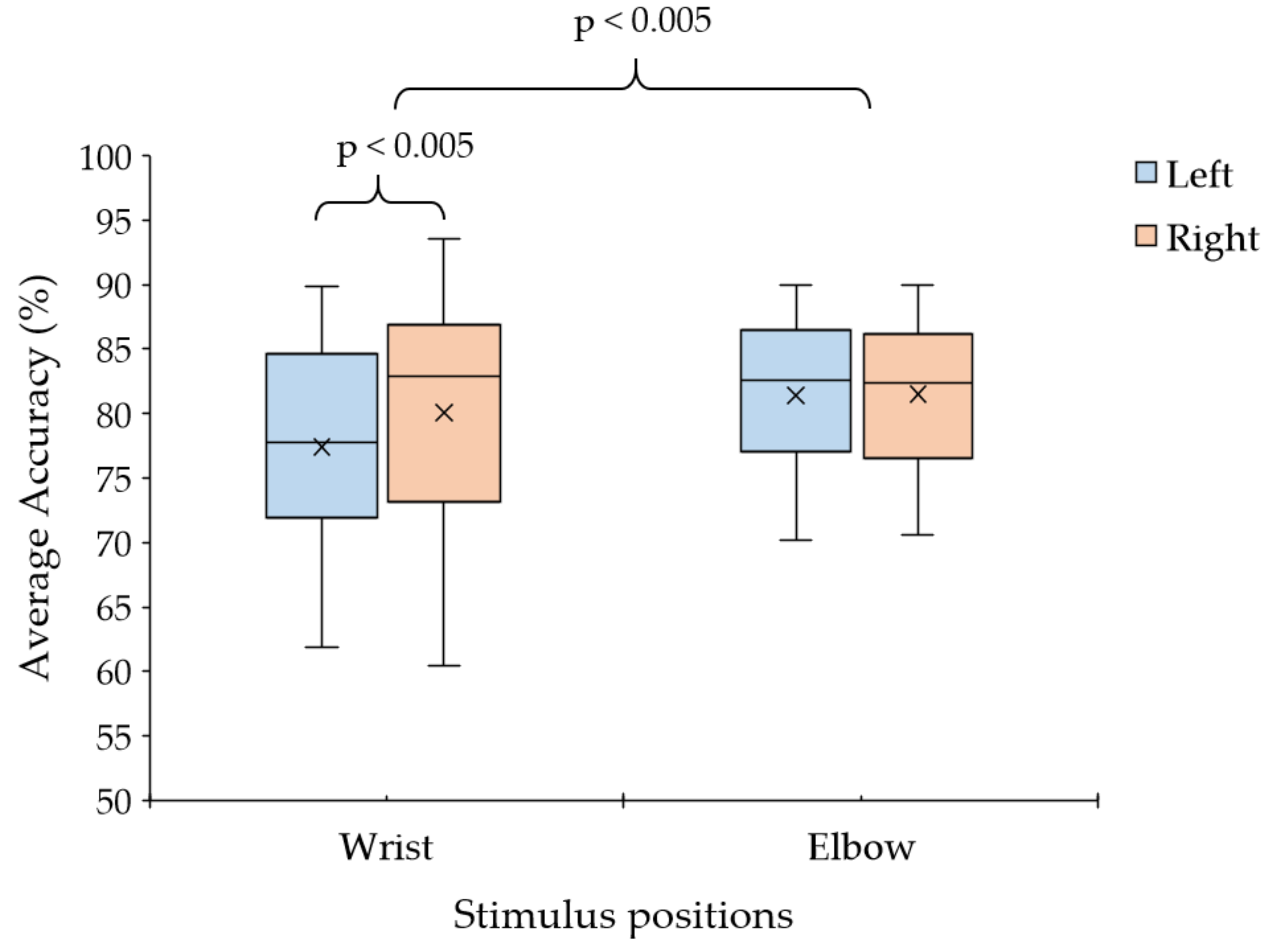
3.2. Observation of Stimulus Position for Vibrotactile Stimulus Pattern
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Compendium of Innovative Health Technologies for Low-Resource Settings 2024; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Zhuang, M.; Wu, Q.; Wan, F.; Hu, Y. State-of-the-art non-invasive brain–computer interface for neural rehabilitation: A review. J. Neurorestoratology 2020, 8, 12–25. [Google Scholar] [CrossRef]
- Ariza, J.Á.; Pearce, J.M. Low-cost assistive technologies for disabled people using open-source hardware and software: A systematic literature review. IEEE Access 2022, 10, 124894–124927. [Google Scholar] [CrossRef]
- Vlaar, M.P.; Van Der Helm, F.C.; Schouten, A.C. Frequency Domain Characterization of the Somatosensory Steady State Response in Electroencephalography. IFAC-Pap. Online 2015, 28, 1391–1396. [Google Scholar] [CrossRef]
- Pokorny, C.; Breitwieser, C.; Müller-Putz, G.R. The Role of Transient Target Stimuli in a Steady-State Somatosensory Evoked Potential-Based Brain-Computer Interface Setup. Front. Neurosci. 2016, 10, 152. [Google Scholar] [CrossRef]
- Yao, L.; Meng, J.; Zhang, D.; Sheng, X.; Zhu, X. Selective Sensation Based Brain-Computer Interface via Mechanical Vibrotactile Stimulation. PLoS ONE 2013, 8, e64784. [Google Scholar] [CrossRef]
- Grigorev, N.A.; Savosenkov, A.O.; Lukoyanov, M.V.; Udoratina, A.; Shusharina, N.N.; Kaplan, A.Y.; Hramov, A.E.; Kazantsev, V.B.; Gordleeva, S. A BCI-Based Vibrotactile Neurofeedback Training Improves Motor Cortical Excitability During Motor Imagery. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1583–1592. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Kaufmann, T.; Herweg, A.; Kübler, A. Toward Brain-Computer Interface Based Wheelchair Control Utilizing Tactually-Evoked Event-Related Potentials. J. Neuroeng. Rehabil. 2014, 11, 7. [Google Scholar] [CrossRef]
- Halder, S.; Raederscheidt, J.; Heß, R.; Eck, D.; Schilling, K.; Kübler, A. Tactile Brain-Computer Interface Control of a Mobile Platform in a Real World Environment Using a Low-Cost Electroencephalography Headset. In Proceedings of the GBCIC, Graz, Austria, 18–22 September 2017; Volume 2017. [Google Scholar]
- Zhang, W.; Song, A.; Zeng, H.; Xu, B.; Miao, M. Closed-Loop Phase-Dependent Vibration Stimulation Improves Motor Imagery-Based Brain-Computer Interface Performance. Front. Neurosci. 2021, 15, 638638. [Google Scholar] [CrossRef]
- Yajima, H.; Makino, S.; Rutkowski, T.M. Fingertip Stimulus Cue-Based Tactile Brain-Computer Interface. In Asia-Pacific Signal and Information Processing Association Annual Summit and Conference (APSIPA); IEEE Publications: Piscataway, NJ, USA, 2015; Volume 2015, pp. 1059–1064. [Google Scholar] [CrossRef]
- Kodama, T.; Makino, S.; Rutkowski, T.M. Tactile Brain-Computer Interface Using Classification of P300 Responses Evoked by Full Body Spatial Vibrotactile Stimuli. In Asia and the Pacific Signal and Information Processing Association Annual Summit and Conference (APSIPA); IEEE Publications: Piscataway, NJ, USA, 2016; Volume 2016, pp. 1–8. [Google Scholar] [CrossRef]
- Guger, C.; Spataro, R.; Allison, B.Z.; Heilinger, A.; Ortner, R.; Cho, W.; La Bella, V. Complete Locked-In and Locked-In Patients: Command Following Assessment and Communication With Vibro-tactile P300 and Motor Imagery Brain-Computer Interface Tools. Front. Neurosci. 2017, 11, 251. [Google Scholar] [CrossRef]
- Li, J.; Pu, J.; Cui, H.; Xie, X.; Xu, S.; Li, T.; Hu, Y. An Online P300 Brain-Computer Interface Based on Tactile Selective Attention of Somatosensory Electrical Stimulation. J. Med. Biol. Eng. 2019, 39, 732–738. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, J.; Daly, I.; Zuo, C.; Wang, X.; Cichocki, A. Effects of Visual Attention on Tactile P300 BCI. Comp. Intell. Neurosci. 2020, 2020, 6549189. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Luo, J.; Tian, X.; Han, X.; Guo, S. A P300 Brain-Computer Interface Paradigm Based on Electric and Vibration Simple Command Tactile Stimulation. Front. Hum. Neurosci. 2021, 15, 641357. [Google Scholar] [CrossRef]
- Mao, Y.; Jin, J.; Li, S.; Miao, Y.; Cichocki, A. Effects of Skin Friction on Tactile P300 Brain-Computer Interface Performance. Comp. Intell. Neurosci. 2021, 2021, 6694310. [Google Scholar] [CrossRef]
- Eidel, M.; Kübler, A. Identifying Potential Training Factors in a Vibrotactile P300-BCI. Sci. Rep. 2022, 12, 14006. [Google Scholar] [CrossRef]
- Savić, A.M.; Novičić, M.; Ðorđević, O.; Konstantinović, L.; Miler-Jerković, V. Novel electrotactile brain-computer interface with somatosensory event-related potential-based control. Front. Hum. Neurosci. 2023, 17, 1096814. [Google Scholar] [CrossRef]
- Savić, A.M.; Novičić, M.; Miler-Jerković, V.; Djordjević, O.; Konstantinović, L. Electrotactile BCI for Top-Down Somatosensory Training: Clinical Feasibility Trial of Online BCI Control in Subacute Stroke Patients. Biosensors 2024, 14, 368. [Google Scholar] [CrossRef]
- Novičić, M.; Savić, A.M. Somatosensory event-related potential as an electrophysiological correlate of endogenous spatial tactile attention: Prospects for electrotactile brain-computer interface for sensory training. Brain Sci. 2023, 13, 766. [Google Scholar] [CrossRef]
- Williams, N.S.; McArthur, G.M.; de Wit, B.; Ibrahim, G.; Badcock, N.A. A Validation of Emotiv EPOC Flex Saline for EEG and ERP Research. PeerJ 2020, 8, e9713. [Google Scholar] [CrossRef]
- Silvoni, S.; Konicar, L.; Prats-Sedano, M.A.; Garcia-Cossio, E.; Genna, C.; Volpato, C.; Cavinato, M.; Paggiaro, A.; Veser, S.; De Massari, D.; et al. Tactile event-related potentials in amyotrophic lateral sclerosis (ALS): Implications for brain-computer interface. Clin. Neurophysiol. 2016, 127, 936–945. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Calderon, J.; Luck, S.J. ERPLAB: An Open-Source Toolbox for the Analysis of Event-Related Potentials. Front. Hum. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef]
- Council for International Organizations of Medical Sciences. International ethical guidelines for biomedical research involving human subjects. Bull. Med. Ethics 2002, 182, 17–23. [Google Scholar]
- World Health Organization. Ensuring Ethical Standards and Procedures for Research with Human Beings. 2023. Available online: https://www.who.int/activities/ensuring-ethical-standards-and-procedures-for-research-with-human-beings (accessed on 22 February 2023).
- Manyakov, N.V.; Chumerin, N.; Combaz, A.; Van Hulle, M. Comparison of Linear Classification Methods for P300 Brain-Computer Interface on Disabled Subjects. Comput. Intell. Neurosci. 2011, 519868. [Google Scholar] [CrossRef]
- Aloise, F.; Schettini, F.; Aricò, P.; Salinari, S.; Babiloni, F.; Cincotti, F. A Comparison of Classification Techniques for a Gaze-Independent P300-Based Brain-Computer Interface. J. Neural Eng. 2012, 9, 045012. [Google Scholar] [CrossRef]
- Billinger, M.; Daly, I.; Kaiser, V.; Jin, J.; Allison, B.Z.; Müller-Putz, G.R.; Brunner, C. Is It Significant? Guidelines for Reporting BCI Performance. Towards Pract. Brain-Comput. Interfaces Bridg. Gap Res. Real-World Appl. 2013, 2013, 333–354. [Google Scholar]
- Jin, J.; Chen, Z.; Xu, R.; Miao, Y.; Wang, X.; Jung, T.P. Developing a Novel Tactile P300 Brain-Computer Interface With a Cheeks-stim Paradigm. IEEE Trans. Biomed. Eng. 2020, 67, 2585–2593. [Google Scholar] [CrossRef]
- Huang, X.; Liang, S.; Li, Z.; Lai, C.Y.Y.; Choi, K.S. EEG-Based Vibrotactile Evoked Brain-Computer Interfaces System: A Systematic Review. PLoS ONE 2022, 17, e0269001. [Google Scholar] [CrossRef]


| Author | Proposed Methods | Tactile Stimulus | Stimulus Areas | Electrodes | Result(s) |
|---|---|---|---|---|---|
| Yajima et al. (2015) [12] | A tactile glove fingertips’ stimulator for a BCI | Five vibrotactile transducers | Fingertips | Cz, CPz, P3, P4, C3, C4, CP5, and CP6, | Average accuracy is 80.0% |
| Kodama et al. (2016) [13] | A tactile P300 BCI using full-body spatial vibrotactile stimuli (full-body BCI (fbBCI)) | Six spatial vibrotactile stimulus patterns | Arms, shoulders, waist, and legs | Cz, Pz, P3, P4, C3, C4, CP5, and CP6 | Average accuracy of 98.18%, whereas the real-time accuracy is 53.67% |
| Guger et al. (2017) [14] | Vibro-tactile P300 and a motor imagery brain—computer interface for LIS patients | Vibrotactile stimulation with 2 and 3 tactors (VT2 and VT3) | VT2: Left and right wrists VT3: Left wrist, right wrist, and shoulder | Fz, C3, Cz, C4, CP1, CPz, CP2, and Pz | Health subject: mean accuracy of 100% in VT2, 93% in VT3 LIS patient: mean accuracy of 76.6% in VT2, 63.1% in VT3 |
| Li et al. (2019) [15] | An online P300 BCI based on somatosensory stimulation paradigm | Electrical muscle stimulation device | Fingertips | Fz, C3, Cz, and C4 | Average accuracy is 79.81% |
| Chen et al. (2020) [16] | Visual attention on tactile P300 BCI for users who only need to focus their attention on a single-target stimulus within a stream of tactile stimuli | Five vibrotactile stimulator (g.VIBROstims) | Left wrist, right wrist, abdomen, left ankle, and right ankle | Fz, FC1, FC2, C3, Cz, C4, CP3, CP1, CP2, CP4, P3, Pz, P4, and Oz | Average accuracy of 90.91% for VA and 62.73% for NVA |
| Chu et al. (2021) [17] | A novel tactile stimuli P300 paradigm for people with less learning ability or difficulty in maintaining attention | Electric and vibration stimulators | Finger pads and the wrist | C3, C4, CP1, CP2, Cz, F3, F4, FC1, FC2, Fz, P3, P4, POz, and Pz | An average accuracy of 94.88% for electrical stimuli and 95.21% for vibration stimuli |
| Mao et al. (2021) [18] | The effects of skin friction on tactile P300 BCI performance | Five vibrators with silk-stim paradigm (SSP) and linen-stim paradigm (LSP) | Left palm, right palm, abdomen, left ankle, and right ankle | Fz, FC1, FC2, C3, Cz, C4, CP3, CP1, CP2, CP4, P3, Pz, P4, and Oz | Average accuracy of 64.50% for SSP and 75.50% for LSP |
| Eidel, and Kübler (2022) [19] | To determine the potential training factors pre/post and assess the robustness of the tactile P300 BCI | Vibrotactor devices (C-2 tactor) | Right and left thigh, abdomen, and lower neck | Fz, FC1, FC2, C3, Cz, C4, P3, Pz, P4, O1, Oz, and O2 | Accuracy in the range of 79.2–92.0% |
| Savić et al. (2023) [20] | A novel electrotactile stimulus with a control paradigm based on tactile attention tasks and sERP | Electrotactile stimuli with two stimulation channels | Radial styloid and medial epicondyle for right forearm | C3, Cz, C4, CP3, Pz, and Fp1 | Average accuracy in the range of 75.1 to 88.1% |
| Session | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target | L1 | R1 | R2 | L2 | R2 | L1 | R1 | R1 | L2 | L1 | L2 | R2 |
| Participants | Average Classification Accuracy (%) | |||
|---|---|---|---|---|
| L1 (Left Wrist) | R1 (Right Wrist) | L2 (Left Elbow) | R2 (Right Elbow) | |
| 1 | 76.59 | 78.66 | 82.23 | 80.79 |
| 2 | 74.79 | 80.47 | 80.14 | 84.74 |
| 3 | 78.39 | 85.04 | 78.71 | 81.18 |
| 4 | 80.81 | 82.06 | 78.47 | 82.75 |
| 5 | 80.82 | 79.01 | 83.10 | 80.93 |
| 6 | 78.88 | 80.89 | 84.17 | 83.16 |
| 7 | 78.08 | 79.75 | 78.77 | 80.79 |
| 8 | 74.72 | 79.65 | 80.66 | 80.14 |
| 9 | 73.76 | 79.28 | 83.86 | 78.97 |
| 10 | 81.34 | 80.71 | 81.00 | 81.01 |
| 11 | 72.80 | 76.64 | 84.36 | 80.41 |
| 12 | 77.95 | 80.13 | 79.41 | 85.34 |
| Mean ± SD | 77.41 ± 2.89 | 80.19 ± 2.03 | 81.24 ± 2.23 | 81.68 ± 1.91 |
| Targets | Precision (PS) | Recall (RC) | F-Measure (F1) |
|---|---|---|---|
| L1 | 0.78 | 0.94 | 0.85 |
| R1 | 0.82 | 0.95 | 0.88 |
| L2 | 0.83 | 0.95 | 0.89 |
| R2 | 0.84 | 0.95 | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borirakarawin, M.; Siribunyaphat, N.; Aung, S.T.; Punsawad, Y. The Development of a Multicommand Tactile Event-Related Potential-Based Brain–Computer Interface Utilizing a Low-Cost Wearable Vibrotactile Stimulator. Sensors 2024, 24, 6378. https://doi.org/10.3390/s24196378
Borirakarawin M, Siribunyaphat N, Aung ST, Punsawad Y. The Development of a Multicommand Tactile Event-Related Potential-Based Brain–Computer Interface Utilizing a Low-Cost Wearable Vibrotactile Stimulator. Sensors. 2024; 24(19):6378. https://doi.org/10.3390/s24196378
Chicago/Turabian StyleBorirakarawin, Manorot, Nannaphat Siribunyaphat, Si Thu Aung, and Yunyong Punsawad. 2024. "The Development of a Multicommand Tactile Event-Related Potential-Based Brain–Computer Interface Utilizing a Low-Cost Wearable Vibrotactile Stimulator" Sensors 24, no. 19: 6378. https://doi.org/10.3390/s24196378
APA StyleBorirakarawin, M., Siribunyaphat, N., Aung, S. T., & Punsawad, Y. (2024). The Development of a Multicommand Tactile Event-Related Potential-Based Brain–Computer Interface Utilizing a Low-Cost Wearable Vibrotactile Stimulator. Sensors, 24(19), 6378. https://doi.org/10.3390/s24196378









