Non-Invasive Estimation of Intracranial Pressure-Derived Cerebrovascular Reactivity Using Near-Infrared Spectroscopy Sensor Technology in Acute Neural Injury: A Time-Series Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Considerations
2.3. Data Collection
2.4. Data Cleaning and Processing
2.5. Statistical Data Analysis
2.5.1. Overview
2.5.2. Data Exploration
2.5.3. Linear Modeling of CVR
3. Results
3.1. Study Population
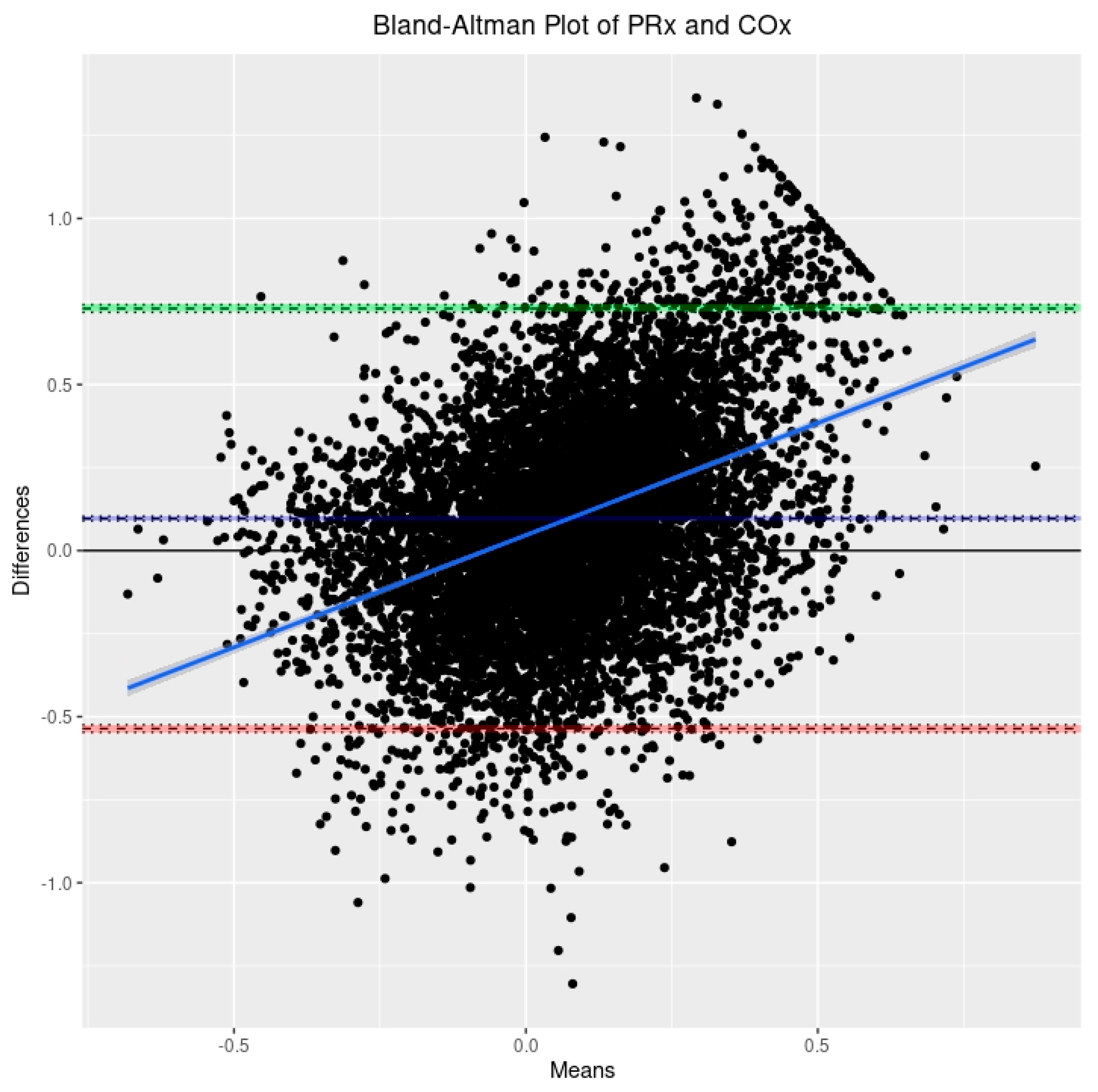
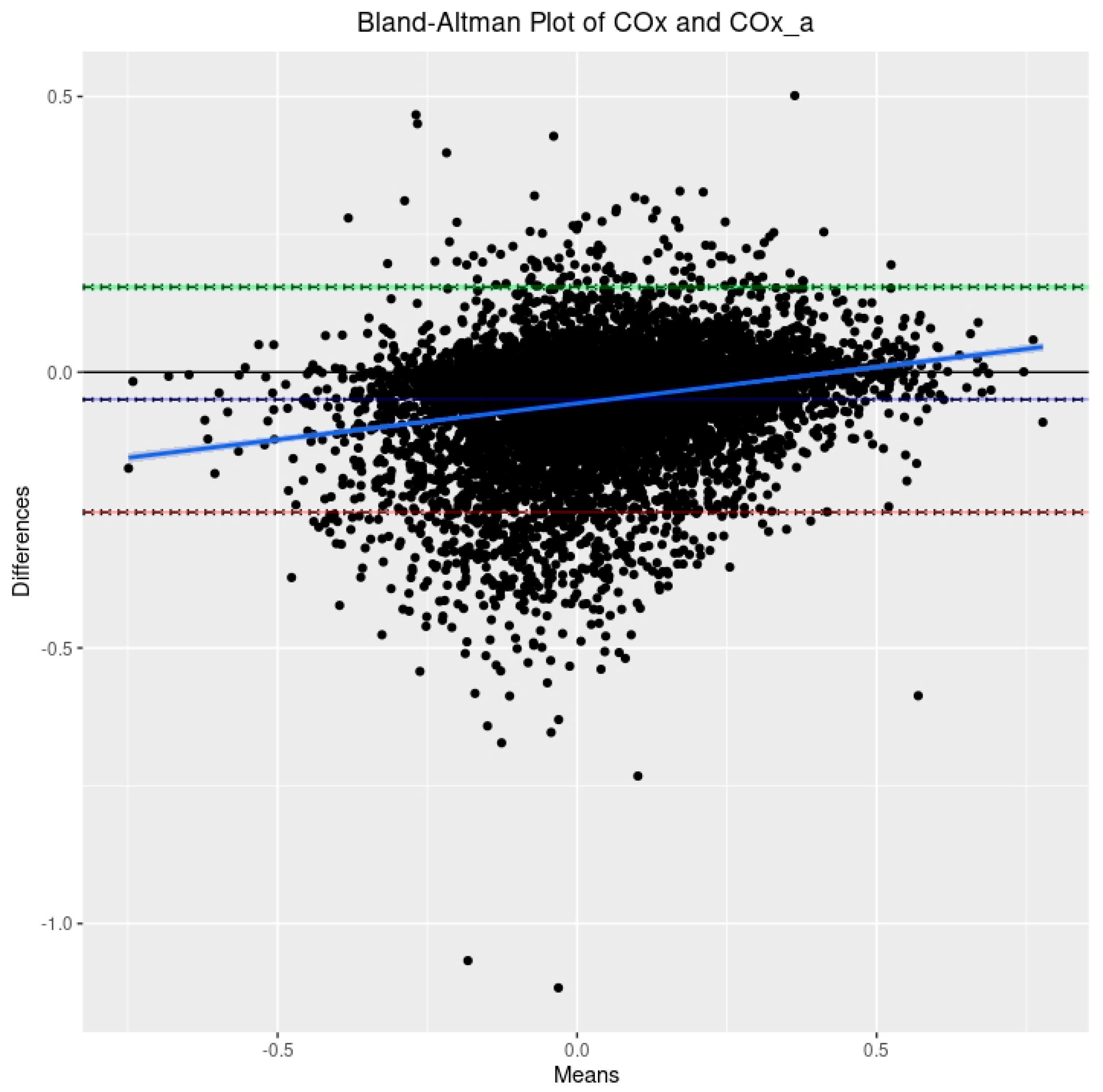
3.2. Relationship between PRx, COx, and COx_a
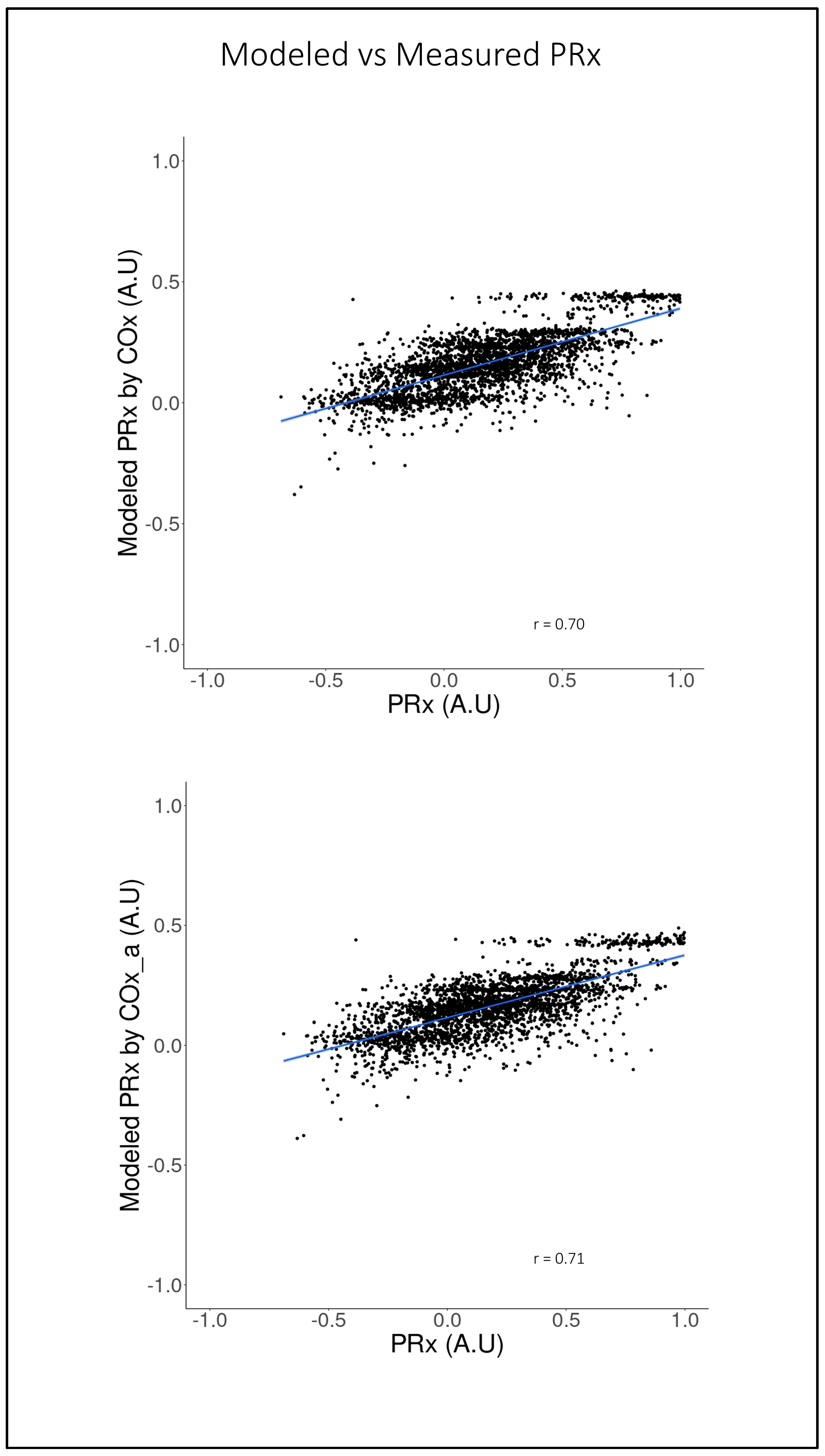
3.3. LME Modeling of PRx Using COx and COx_a
4. Discussion
4.1. Limitiation
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassen, N.A. Autoregulation of Cerebral Blood Flow. Circ. Res. 1964, 15, 201–204. [Google Scholar]
- Lassen, N.A. Cerebral Blood Flow and Oxygen Consumption in Man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Y.; Woodward, A.; Fan, A.P.; Chen, K.T.; Yu, Y.; Chen, D.Y.; Moseley, M.E.; Zaharchuk, G. Reproducibility of Cerebrovascular Reactivity Measurements: A Systematic Review of Neuroimaging Techniques*. J. Cereb. Blood Flow. Metab. 2022, 42, 700–717. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Miller, C. Monitoring of Cerebral Autoregulation. Neurocrit. Care 2014, 21, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Smielewski, P.; Piechnik, S.; Steiner, L.A.; Pickard, J.D. Cerebral Autoregulation Following Head Injury. J. Neurosurg. 2001, 95, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Smielewski, P.; Kirkpatrick, P.; Laing, R.J.; Menon, D.; Pickard, J.D. Continuous Assessment of the Cerebral Vasomotor Reactivity in Head Injury. Neurosurgery 1997, 41, 11–19. [Google Scholar] [CrossRef]
- Svedung Wettervik, T.; Howells, T.; Enblad, P.; Lewén, A. Temporal Neurophysiological Dynamics in Traumatic Brain Injury: Role of Pressure Reactivity and Optimal Cerebral Perfusion Pressure for Predicting Outcome. J. Neurotrauma 2019, 36, 1818–1827. [Google Scholar] [CrossRef]
- Adams, H.; Donnelly, J.; Czosnyka, M.; Kolias, A.G.; Helmy, A.; Menon, D.K.; Smielewski, P.; Hutchinson, P.J. Temporal Profile of Intracranial Pressure and Cerebrovascular Reactivity in Severe Traumatic Brain Injury and Association with Fatal Outcome: An Observational Study. PLoS Med. 2017, 14, e1002353. [Google Scholar] [CrossRef]
- Sorrentino, E.; Diedler, J.; Kasprowicz, M.; Budohoski, K.P.; Haubrich, C.; Smielewski, P.; Outtrim, J.G.; Manktelow, A.; Hutchinson, P.J.; Pickard, J.D.; et al. Critical Thresholds for Cerebrovascular Reactivity After Traumatic Brain Injury. Neurocrit. Care 2012, 16, 258–266. [Google Scholar] [CrossRef]
- Budohoski, K.P.; Czosnyka, M.; de Riva, N.; Smielewski, P.; Pickard, J.D.; Menon, D.K.; Kirkpatrick, P.J.; Lavinio, A. The Relationship Between Cerebral Blood Flow Autoregulation and Cerebrovascular Pressure Reactivity After Traumatic Brain Injury. Neurosurgery 2012, 71, 652–661. [Google Scholar] [CrossRef]
- Aries, M.J.H.; Czosnyka, M.; Budohoski, K.P.; Kolias, A.G.; Radolovich, D.K.; Lavinio, A.; Pickard, J.D.; Smielewski, P. Continuous Monitoring of Cerebrovascular Reactivity Using Pulse Waveform of Intracranial Pressure. Neurocrit. Care 2012, 17, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Lavinio, A.; Steiner, L.A.; Radolovich, D.; Smielewski, P.; Timofeev, I.; Hiler, M.; Balestreri, M.; Kirkpatrick, P.J.; Pickard, J.D.; et al. Continuous Monitoring of Cerebrovascular Pressure Reactivity in Patients with Head Injury. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Hiler, M.; Czosnyka, M.; Hutchinson, P.; Balestreri, M.; Smielewski, P.; Matta, B.; Pickard, J.D. Predictive Value of Initial Computerized Tomography Scan, Intracranial Pressure, and State of Autoregulation in Patients with Traumatic Brain Injury. J. Neurosurg. 2006, 104, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.A.; Czosnyka, M.; Piechnik, S.K.; Smielewski, P.; Chatfield, D.; Menon, D.K.; Pickard, J.D. Continuous Monitoring of Cerebrovascular Pressure Reactivity Allows Determination of Optimal Cerebral Perfusion Pressure in Patients with Traumatic Brain Injury. Crit. Care Med. 2002, 30, 733–738. [Google Scholar] [CrossRef]
- Brady, K.M.; Lee, J.K.; Kibler, K.K.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Koehler, R.C.; Shaffner, D.H. Continuous Time-Domain Analysis of Cerebrovascular Autoregulation Using near-Infrared Spectroscopy. Stroke 2007, 38, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.M.; Mytar, J.O.; Kibler, K.K.; Hogue, C.W., Jr.; Lee, J.K.; Czosnyka, M.; Smielewski, P.; Easley, R.B. Noninvasive Autoregulation Monitoring with and without Intracranial Pressure in the Naive Piglet Brain. Anesth. Analg. 2010, 111, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, Techniques, and Limitations of near Infrared Spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Smith, M. Shedding Light on the Adult Brain: A Review of the Clinical Applications of near-Infrared Spectroscopy. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4452–4469. [Google Scholar] [CrossRef]
- Murkin, J.M.; Arango, M. Near-Infrared Spectroscopy as an Index of Brain and Tissue Oxygenation. Br. J. Anaesth. 2009, 103 (Suppl. 1), i3–i13. [Google Scholar] [CrossRef]
- Gomez, A.; Sainbhi, A.S.; Stein, K.Y.; Vakitbilir, N.; Froese, L.; Zeiler, F.A. Statistical Properties of Cerebral near Infrared and Intracranial Pressure-Based Cerebrovascular Reactivity Metrics in Moderate and Severe Neural Injury: A Machine Learning and Time-Series Analysis. Intensive Care Med. Exp. 2023, 11, 57. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.J.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Howells, T.; Johnson, U.; McKelvey, T.; Enblad, P. An Optimal Frequency Range for Assessing the Pressure Reactivity Index in Patients with Traumatic Brain Injury. J. Clin. Monit. Comput. 2015, 29, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.D.; Brady, K.M.; Rhee, C.J.; Easley, R.B.; Kibler, K.; Smielewski, P.; Czosnyka, M.; Kaczka, D.W.; Andropoulos, D.B.; Rusin, C. The Frequency Response of Cerebral Autoregulation. J. Appl. Physiol. 2013, 115, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Czosnyka, Z.; Smielewski, P. Pressure Reactivity Index: Journey through the Past 20 Years. Acta Neurochir. 2017, 159, 2063–2065. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kibler, K.K.; Benni, P.B.; Easley, R.B.; Czosnyka, M.; Smielewski, P.; Koehler, R.C.; Shaffner, D.H.; Brady, K.M. Cerebrovascular Reactivity Measured by Near-Infrared Spectroscopy. Stroke 2009, 40, 1820–1826. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Smielewski, P.; Stevens, A.; Czosnyka, M.; Menon, D.K.; Ercole, A. Non-Invasive Pressure Reactivity Index Using Doppler Systolic Flow Parameters: A Pilot Analysis. J. Neurotrauma 2019, 36, 713–720. [Google Scholar] [CrossRef]
- Lütkepohl, H. New Introduction to Multiple Time Series Analysis; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005; ISBN 978-3-540-40172-8. [Google Scholar]
- Chatfield, C.; Xing, H. The Analysis of Time Series: An Introduction with R, 7th ed.; Chapman & Hall/CRC Texts in Statistical Science Series; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2019; ISBN 978-1-351-25944-6. [Google Scholar]
- Mills, T.C. Applied Time Series Analysis: A Practical Guide to Modeling and Forecasting; Academic Press: London, UK, 2019; ISBN 978-0-12-813117-6. [Google Scholar]
- Percival, D.B.; Walden, A.T. Spectral Analysis for Univariate Time Series, 1st ed.; Cambridge University Press: Cambridge, UK, 2020; ISBN 978-1-139-23572-3. [Google Scholar]
- Sainbhi, A.S.; Vakitbilir, N.; Gomez, A.; Stein, K.Y.; Froese, L.; Zeiler, F.A. Time Series Autocorrelative Structure of Cerebrovascular Reactivity Metrics in Severe Neural Injury: An Evaluation of the Impact of Data Resolution. Biomed. Signal Process. Control. 2023; submitted for publication. [Google Scholar]
- Fisher, J.A.; Venkatraghavan, L.; Mikulis, D.J. Magnetic Resonance Imaging–Based Cerebrovascular Reactivity and Hemodynamic Reserve: A Review of Method Optimization and Data Interpretation. Stroke 2018, 49, 2011–2018. [Google Scholar] [CrossRef]
- Sleight, E.; Stringer, M.S.; Marshall, I.; Wardlaw, J.M.; Thrippleton, M.J. Cerebrovascular Reactivity Measurement Using Magnetic Resonance Imaging: A Systematic Review. Front. Physiol. 2021, 12, 643468. [Google Scholar] [CrossRef]
- Stein, K.Y.; Froese, L.; Sekhon, M.; Griesdale, D.; Thelin, E.P.; Raj, R.; Tas, J.; Aries, M.; Gallagher, C.; Bernard, F.; et al. Intracranial Pressure-Derived Cerebrovascular Reactivity Indices and Their Critical Thresholds: A Canadian High Resolution-Traumatic Brain Injury Validation Study. J. Neurotrauma 2023, 16, 258–266. [Google Scholar] [CrossRef]
- Gomez, A.; Dian, J.; Zeiler, F.A. Continuous and Entirely Non-Invasive Method for Cerebrovascular Reactivity Assessment: Technique and Implications. J. Clin. Monit. Comput. 2020, 35, 307–315. [Google Scholar] [CrossRef] [PubMed]



| Demographic Parameter | Median (IQR) or N (%) | |
|---|---|---|
| Age | 42 (28.5–59.25) | |
| Sex | Male | 65 (79.3) |
| Female | 17 (20.7) | |
| Admission GCS | 6.5 (4–8) | |
| Follow-up GOSE at 6 Months | 6 (1–7) | |
| Admission Pupil Exam | Bilaterally Unreactive | 13 (15.9) |
| Unilaterally Unreactive | 16 (19.5) | |
| Bilaterally Reactive | 53 (64.6) | |
| Admission Marshall CT Score | I | 0 (0.0) |
| II | 3 (3.7) | |
| III | 23 (28.0) | |
| IV | 15 (18.3) | |
| V | 41 (50.0) | |
| VI | 0 (0.0) | |
| Largest Lesion Type | SDH | 47 (57.3) |
| EDH | 4 (4.9) | |
| Cerebral Contusion | 10 (12.2) | |
| DAI | 6 (7.3) | |
| tSAH | 15 (18.3) | |
| Surgical Intervention | Yes | 50 (61.0) |
| No | 32 (39.0) | |
| ICP monitoring method | Intraparenchymal Probe | 77 (93.9) |
| External Ventricular drains | 5 (6.1) | |
| Admission HgB (g/L) | 135 (113–147) | |
| Admission Serum Glucose (mmol/L) | 8.05 (7–10.95) | |
| Average PaO2 (mmHg) Over Course of Recording | 109 (87–138) | |
| Average PaCO2 (mmHg) Over Course of Recording | 37 (34–40) | |
| Average Blood Gas pH Over Course of Recording | 7.43 (7.39–7.47) | |
| Side of rSO2 Used | Right | 66 (80.5) |
| Left | 16 (19.5) | |
| Frontal Contusion Present | Right | 9 (11.0) |
| Left | 7 (8.5) | |
| Frontal Scalp Hematoma Present | Right | 7 (8.5) |
| Left | 6 (7.3) | |
| PRx | 0.11 (−0.08–0.31) | |
| COx | 0.02 (−0.09–0.15) | |
| COx_a | 0.07 (−0.03–0.18) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, A.; Froese, L.; Bergmann, T.J.G.; Sainbhi, A.S.; Vakitbilir, N.; Islam, A.; Stein, K.Y.; Marquez, I.; Ibrahim, Y.; Zeiler, F.A. Non-Invasive Estimation of Intracranial Pressure-Derived Cerebrovascular Reactivity Using Near-Infrared Spectroscopy Sensor Technology in Acute Neural Injury: A Time-Series Analysis. Sensors 2024, 24, 499. https://doi.org/10.3390/s24020499
Gomez A, Froese L, Bergmann TJG, Sainbhi AS, Vakitbilir N, Islam A, Stein KY, Marquez I, Ibrahim Y, Zeiler FA. Non-Invasive Estimation of Intracranial Pressure-Derived Cerebrovascular Reactivity Using Near-Infrared Spectroscopy Sensor Technology in Acute Neural Injury: A Time-Series Analysis. Sensors. 2024; 24(2):499. https://doi.org/10.3390/s24020499
Chicago/Turabian StyleGomez, Alwyn, Logan Froese, Tobias J. G. Bergmann, Amanjyot Singh Sainbhi, Nuray Vakitbilir, Abrar Islam, Kevin Y. Stein, Izabella Marquez, Younis Ibrahim, and Frederick A. Zeiler. 2024. "Non-Invasive Estimation of Intracranial Pressure-Derived Cerebrovascular Reactivity Using Near-Infrared Spectroscopy Sensor Technology in Acute Neural Injury: A Time-Series Analysis" Sensors 24, no. 2: 499. https://doi.org/10.3390/s24020499






