Wearable Sensor to Monitor Quality of Upper Limb Task Practice for Stroke Survivors at Home
Abstract
1. Introduction
| Reference | Participant | Method | Setting | Finding |
|---|---|---|---|---|
| Bochniewicz 2017 [27] | 10 healthy persons, 10 stroke survivors | RF | Lab | Classified functional vs. nonfunctional movement |
| David 2021 [28] | 5 healthy persons, 5 hemiparetic patients | Thresholding | Lab | Classified functional vs. nonfunctional movement |
| Gomez-Arrunategui 2022 [29] | 12 stroke survivors | RF, CNN | Lab | Detected reach time and number of reaching gestures during prescribed tasks. |
| Lee 2018 [25] | 9 healthy persons, 11 stroke survivors | RF | Lab | Classified quality of arm raise |
| Bhagat 2020 [30] | 2 persons with spinal cord injury | DTW, LSTM | Lab | Classified cylindrical vs. pincer grasp (to pick up a water bottle vs. pen) |
| Li 2023 [26] | 20 healthy persons | LSTM | Lab | Classified functional task practice quality |
| Lui 2019 [31] | 11 healthy persons | LDA, SVM, KNN, CT | Lab | Classified pre-selected upper limb movements |
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Analysis
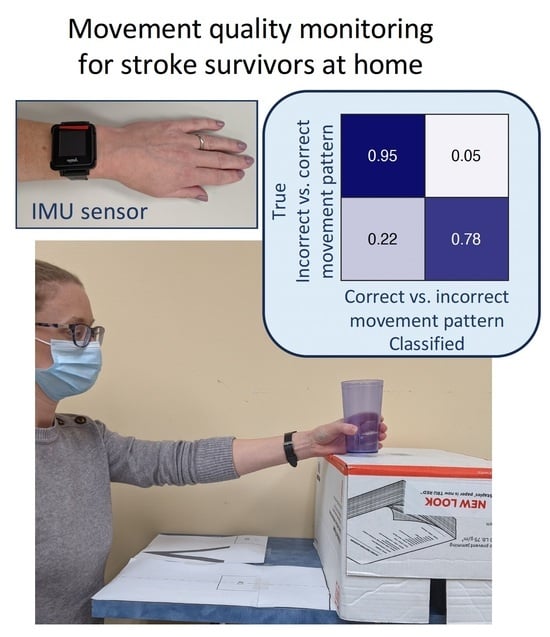
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Ovbiagele, B.; Goldstein, L.B.; Higashida, R.T.; Howard, V.J.; Johnston, S.C.; Khavjou, O.A.; Lackland, D.T.; Lichtman, J.H.; Mohl, S.; Sacco, R.L.; et al. Forecasting the future of stroke in the United States: A policy statement from the American Heart Association and American Stroke Association. Stroke 2013, 44, 2361–2375. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, H.T.; Van Limbeek, J.; Geurts, A.C.; Zwarts, M.J. Motor recovery after stroke: A systematic review of the literature. Arch. Phys. Med. Rehabil. 2002, 83, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E.S.; Coshall, C.; Dundas, R.; Stewart, J.; Rudd, A.G.; Howard, R.; Wolfe, C.D. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 2001, 32, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Sathian, K.; Buxbaum, L.J.; Cohen, L.G.; Krakauer, J.W.; Lang, C.E.; Corbetta, M.; Fitzpatrick, S.M. Neurological principles and rehabilitation of action disorders: Common clinical deficits. Neurorehabil. Neural. Repair 2011, 25 (Suppl. S5), 21S–32S. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H. Clinical practice. Rehabilitation after stroke. N. Engl. J. Med. 2005, 352, 1677–1684. [Google Scholar]
- Stewart, J.C.; Cramer, S.C. Patient-reported measures provide unique insights into motor function after stroke. Stroke 2013, 44, 1111–1116. [Google Scholar] [CrossRef]
- Dobkin, B.H. Strategies for stroke rehabilitation. Lancet Neurol. 2004, 3, 528–536. [Google Scholar] [CrossRef]
- Schaefer, S.Y.; Patterson, C.B.; Lang, C.E. Transfer of training between distinct motor tasks after stroke: Implications for task-specific approaches to upper-extremity neurorehabilitation. Neurorehabil. Neural. Repair 2013, 27, 602–612. [Google Scholar] [CrossRef]
- Lohse, K.R.; Lang, C.E.; Boyd, L.A. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke 2014, 45, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.; Kim, B.; Kim, S.; Martinez, C.; Schweighofer, N. Dosage Matters: A phase IIb randomized controlled trial of motor therapy in the chronic phase after stroke. Stroke 2019, 50, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; MacDonald, J.R.; Reisman, D.S.; Boyd, L.; Kimberley, T.J.; Schindler-Ivens, S.M.; Hornby, T.G.; Ross, S.A.; Scheets, P.L. Observation of amounts of movement practice provided during stroke rehabilitation. Arch. Phys. Med. Rehabil. 2009, 90, 1692–1698. [Google Scholar] [CrossRef]
- Lang, C.E.; Strube, M.J.; Bland, M.D.; Waddell, K.J.; Cherry-Allen, K.M.; Nudo, R.J.; Dromerick, A.W.; Birkenmeier, R.L. Dose response of task-specific upper limb training in people at least 6 months poststroke: A phase II, single-blind, randomized, controlled trial. Ann. Neurol. 2016, 80, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.J.; Lannin, N.A.; Ada, L.; Schmidt, J. Increasing the amount of usual rehabilitation improves activity after stroke: A systematic review. J. Physiother. 2016, 62, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.K.; Porter, R.E.; DeBaun-Sprague, E.; Van Puymbroeck, M.; Schmid, A.A. Exercise after Stroke: Patient Adherence and Beliefs after Discharge from Rehabilitation. Top. Stroke Rehabil. 2017, 24, 142–148. [Google Scholar] [CrossRef]
- Gunnes, M.; Indredavik, B.; Langhammer, B.; Lydersen, S.; Ihle-Hansen, H.; Dahl, A.E.; Askim, T. Associations Between Adherence to the Physical Activity and Exercise Program Applied in the LAST Study and Functional Recovery after Stroke. Arch. Phys. Med. Rehabil. 2019, 100, 2251–2259. [Google Scholar] [CrossRef]
- Scronce, G.; Ramakrishnan, V.; Vatinno, A.A.; Seo, N.J. Effect of Self-Directed Home Therapy Adherence Combined with TheraBracelet on Poststroke Hand Recovery: A Pilot Study. Stroke Res. Treat. 2023, 2023, 3682898. [Google Scholar] [CrossRef]
- Levy, T.; Laver, K.; Killington, M.; Lannin, N.; Crotty, M. A systematic review of measures of adherence to physical exercise recommendations in people with stroke. Clin. Rehabil. 2019, 33, 535–545. [Google Scholar] [CrossRef]
- Waddell, K.J.; Lang, C.E. Comparison of Self-Report Versus Sensor-Based Methods for Measuring the Amount of Upper Limb Activity Outside the Clinic. Arch. Phys. Med. Rehabil. 2018, 99, 1913–1916. [Google Scholar] [CrossRef]
- Mahmood, A.; Solomon, J.M.; English, C.; Bhaskaran, U.; Menon, G.; Manikandan, N. Measurement of adherence to home-based exercises among community-dwelling stroke survivors in India. Physiother. Res. Int. 2020, 25, e1827. [Google Scholar] [CrossRef] [PubMed]
- Cirstea, M.C.; Levin, M.F. Improvement of arm movement patterns and endpoint control depends on type of feedback during practice in stroke survivors. Neurorehabil. Neural. Repair 2007, 21, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.A. Motor compensation and its effects on neural reorganization after stroke. Nat. Rev. Neurosci. 2017, 18, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H. Motor function-related maladaptive plasticity in stroke: A review. NeuroRehabilitation 2013, 32, 311–316. [Google Scholar] [CrossRef]
- Lee, S.I.; Adans-Dester, C.P.; Grimaldi, M.; Dowling, A.V.; Horak, P.C.; Black-Schaffer, R.M.; Bonato, P.; Gwin, J.T. Enabling Stroke Rehabilitation in Home and Community Settings: A Wearable Sensor-Based Approach for Upper-Limb Motor Training. IEEE J. Transl. Eng. Health Med. 2018, 6, 2100411. [Google Scholar] [CrossRef]
- Li, M.; Scronce, G.; Finetto, C.; Coupland, K.; Zhong, M.; Lambert, M.E.; Baker, A.; Luo, F.; Seo, N.J. Application of Deep Learning Algorithm to Monitor Upper Extremity Task Practice. Sensors 2023, 23, 6110. [Google Scholar] [CrossRef]
- Bochniewicz, E.M.; Emmer, G.; McLeod, A.; Barth, J.; Dromerick, A.W.; Lum, P. Measuring Functional Arm Movement after Stroke Using a Single Wrist-Worn Sensor and Machine Learning. J. Stroke Cerebrovasc. Dis. 2017, 26, 2880–2887. [Google Scholar] [CrossRef]
- David, A.; ReethaJanetSureka, S.; Gayathri, S.; Annamalai, S.J.; Samuelkamleshkumar, S.; Kuruvilla, A.; Magimairaj, H.P.; Varadhan, S.K.; Balasubramanian, S. Quantification of the relative arm use in patients with hemiparesis using inertial measurement units. J. Rehabil. Assist. Technol. Eng. 2021, 8, 20556683211019694. [Google Scholar] [CrossRef]
- Gomez-Arrunategui, J.P.; Eng, J.J.; Hodgson, A.J. Monitoring Arm Movements Post-Stroke for Applications in Rehabilitation and Home Settings. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, N.; King, K.; Ramdeo, R.; Stein, A.; Bouton, C. Determining grasp selection from arm trajectories via deep learning to enable functional hand movement in tetraplegia. Bioelectron. Med. 2020, 6, 17. [Google Scholar] [CrossRef]
- Lui, J.; Menon, C. Would a thermal sensor improve arm motion classification accuracy of a single wrist-mounted inertial device? Biomed. Eng. Online 2019, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; Birkenmeier, R.L. Upper-Extremity Task-Specific Training after Stroke or Disability: A Manual for Occupational Therapy and Physical Therapy; AOTA Press: Bethesda, MD, USA, 2013. [Google Scholar]
- Yurtman, A.; Barshan, B. Automated evaluation of physical therapy exercises using multi-template dynamic time warping on wearable sensor signals. Comput. Methods Programs Biomed. 2014, 117, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.E.; Hebert, J.S.; Pilarski, P.M.; Shehata, A.W. A Case Series in Position-Aware Myoelectric Prosthesis Control Using Recurrent Convolutional Neural Network Classification with Transfer Learning. IEEE Int. Conf. Rehabil. Robot. 2023, 2023, 1–6. [Google Scholar]
- Totty, M.S.; Wade, E. Muscle Activation and Inertial Motion Data for Noninvasive Classification of Activities of Daily Living. IEEE Trans. Biomed. Eng. 2018, 65, 1069–1076. [Google Scholar]
- Seo, N.J.; Enders, L.R.; Fortune, A.; Cain, S.; Vatinno, A.A.; Schuster, E.; Ramakrishnan, V.; Feng, W. Phase I Safety Trial: Extended Daily Peripheral Sensory Stimulation Using a Wrist-Worn Vibrator in Stroke Survivors. Transl. Stroke Res. 2020, 11, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.J.; Crocher, V.; Spaho, E.; Ewert, C.R.; Fathi, M.F.; Hur, P.; Lum, S.A.; Humanitzki, E.M.; Kelly, A.L.; Ramakrishnan, V.; et al. Capturing Upper Limb Gross Motor Categories Using the Kinect(R) Sensor. Am. J. Occup. Ther. 2019, 73, p1–p7304205090. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.M.; Johnson, E.; Simpson, K.N.; Seo, N.J. Determining Factors that Influence Adoption of New Post-Stroke Sensorimotor Rehabilitation Devices in the USA. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Donoso Brown, E.V.; Nolfi, D.; Wallace, S.E.; Eskander, J.; Hoffman, J.M. Home program practices for supporting and measuring adherence in post-stroke rehabilitation: A scoping review. Top. Stroke Rehabil. 2020, 27, 377–400. [Google Scholar] [CrossRef]
- Takebayashi, T.; Koyama, T.; Amano, S.; Hanada, K.; Tabusadani, M.; Hosomi, M.; Marumoto, K.; Takahashi, K.; Domen, K. A 6-month follow-up after constraint-induced movement therapy with and without transfer package for patients with hemiparesis after stroke: A pilot quasi-randomized controlled trial. Clin. Rehabil. 2013, 27, 418–426. [Google Scholar] [CrossRef]
- Taub, E.; Uswatte, G.; Mark, V.W.; Morris, D.M.; Barman, J.; Bowman, M.H.; Bryson, C.; Delgado, A.; Bishop-McKay, S. Method for enhancing real-world use of a more affected arm in chronic stroke: Transfer package of constraint-induced movement therapy. Stroke 2013, 44, 1383–1388. [Google Scholar] [CrossRef]
- Seo, N.J.; Ramakrishnan, V.; Woodbury, M.L.; Bonilha, L.; Finetto, C.; Schranz, C.; Scronce, G.; Coupland, K.; Blaschke, J.; Baker, A.; et al. Concomitant sensory stimulation during therapy to enhance hand functional recovery post stroke. Trials 2022, 23, 262. [Google Scholar] [CrossRef] [PubMed]
- Swanson, V.A.; Chan, V.; Cruz-Coble, B.; Alcantara, C.M.; Scott, D.; Jones, M.; Zondervan, D.K.; Khan, N.; Ichimura, J.; Reinkensmeyer, D.J. A Pilot Study of a Sensor Enhanced Activity Management System for Promoting Home Rehabilitation Exercise Performed during the COVID-19 Pandemic: Therapist Experience, Reimbursement, and Recommendations for Implementation. Int. J. Environ. Res. Public Health 2021, 18, 10186. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, S.M.; Dannenbaum, R.; Levin, M.F. Task-specific training with trunk restraint on arm recovery in stroke: Randomized control trial. Stroke 2006, 37, 186–192. [Google Scholar] [CrossRef]
- Guadagnoli, M.A.; Lee, T.D. Challenge point: A framework for conceptualizing the effects of various practice conditions in motor learning. J. Mot. Behav. 2004, 36, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Held, J.P.O.; Luft, A.R.; Veerbeek, J.M. Encouragement-Induced Real-World Upper Limb Use after Stroke by a Tracking and Feedback Device: A Study Protocol for a Multi-Center, Assessor-Blinded, Randomized Controlled Trial. Front. Neurol. 2018, 9, 13. [Google Scholar] [CrossRef]
- Signal, N.E.; McLaren, R.; Rashid, U.; Vandal, A.; King, M.; Almesfer, F.; Henderson, J.; Taylor, D. Haptic Nudges Increase Affected Upper Limb Movement During Inpatient Stroke Rehabilitation: Multiple-Period Randomized Crossover Study. JMIR Mhealth Uhealth 2020, 8, e17036. [Google Scholar] [CrossRef]
- Wei, W.X.; Fong, K.N.; Chung, R.C.; Cheung, H.K.; Chow, E.S. “Remind-to-Move” for Promoting Upper Extremity Recovery Using Wearable Devices in Subacute Stroke: A Multi-Center Randomized Controlled Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.J.; Woodbury, M.L.; Bonilha, L.; Ramakrishnan, V.; Kautz, S.A.; Downey, R.J.; Dellenbach, B.H.; Lauer, A.W.; Roark, C.M.; Landers, L.E.; et al. TheraBracelet Stimulation During Task-Practice Therapy to Improve Upper Extremity Function After Stroke: A Pilot Randomized Controlled Study. Phys. Ther. 2019, 99, 319–328. [Google Scholar] [CrossRef]
- Jones, M.; Collier, G.; Reinkensmeyer, D.J.; DeRuyter, F.; Dzivak, J.; Zondervan, D.; Morris, J. Big Data Analytics and Sensor-Enhanced Activity Management to Improve Effectiveness and Efficiency of Outpatient Medical Rehabilitation. Int. J. Environ. Res. Public Health 2020, 17, 748. [Google Scholar] [CrossRef] [PubMed]
- Judy, L.M.; Morrow, C.; Seo, N.J. Development and evaluation of an efficient training program to facilitate the adoption of a novel neurorehabilitation device. J. Rehabil. Assist. Technol. Eng. 2023, 10, 20556683231158552. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Descriptive Statistics |
|---|---|
| Age (mean ± SD years) | 61 ± 12 |
| Sex (M/F) | 12/7 |
| Time since stroke (mean ± SD years) | 4 ± 3 |
| Stroke type (ischemic/hemorrhagic) | 14/5 |
| Fugl-Meyer Assessment of Motor Recovery after Stroke—Upper Extremity (mean ± SD out of 66) | 45 ± 9 |
| Task | Description |
|---|---|
| 1. Cup to shelf | Reach to grasp a cup on a table in front of the body, lift the cup to a shelf on top of the table, release the cup on the shelf, and bring the hand back to the table. |
| 2. Cup to mouth | Reach to grasp a cup on the table in front of the body, bring the cup to the mouth, and return the cup to the table, simulating a drinking motion. |
| 3. Tongs use | Reach to grasp tongs on the table, use the tongs to grasp a block and move the block to a destination on the other side of the table crossing the body, and release the tongs back on the table. |
| 4. Finger food | Reach to grasp a block on the table using the pincer or 3-jaw chuck grasp, move the block to a destination away from the body, and bring the hand back to the table. |
| Movement Type | Sensitivity | Observations |
|---|---|---|
| Compensatory trunk and/or shoulder involvement | 86% | Trunk flexion for reaching and shoulder hike or shoulder abduction for lifting were identified. |
| Unable to complete | 68% | Classification errors were from earlier object drop and repeated grip attempts in tasks 3–4 and object drop after release in task 1. |
| Use of the nonparetic hand to assist | 62% | Classification errors were from the participants who exhibited this movement type at home but not in the lab. This movement type occurred most for task 3, the most difficult task. |
| Compensatory grip | 83% | Use of key grip or whole hand grip instead of precision grip for task 4 and noncylindrical grip for task 1 were identified. Classification errors were from dragging of the object for task 3 misclassified as correct. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, N.J.; Coupland, K.; Finetto, C.; Scronce, G. Wearable Sensor to Monitor Quality of Upper Limb Task Practice for Stroke Survivors at Home. Sensors 2024, 24, 554. https://doi.org/10.3390/s24020554
Seo NJ, Coupland K, Finetto C, Scronce G. Wearable Sensor to Monitor Quality of Upper Limb Task Practice for Stroke Survivors at Home. Sensors. 2024; 24(2):554. https://doi.org/10.3390/s24020554
Chicago/Turabian StyleSeo, Na Jin, Kristen Coupland, Christian Finetto, and Gabrielle Scronce. 2024. "Wearable Sensor to Monitor Quality of Upper Limb Task Practice for Stroke Survivors at Home" Sensors 24, no. 2: 554. https://doi.org/10.3390/s24020554
APA StyleSeo, N. J., Coupland, K., Finetto, C., & Scronce, G. (2024). Wearable Sensor to Monitor Quality of Upper Limb Task Practice for Stroke Survivors at Home. Sensors, 24(2), 554. https://doi.org/10.3390/s24020554