The Added Value of Face-to-Face Supervision to a Therapeutic Exercise-Based App in the Management of Patients with Chronic Low Back Pain: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size Estimation
2.4. Interventions
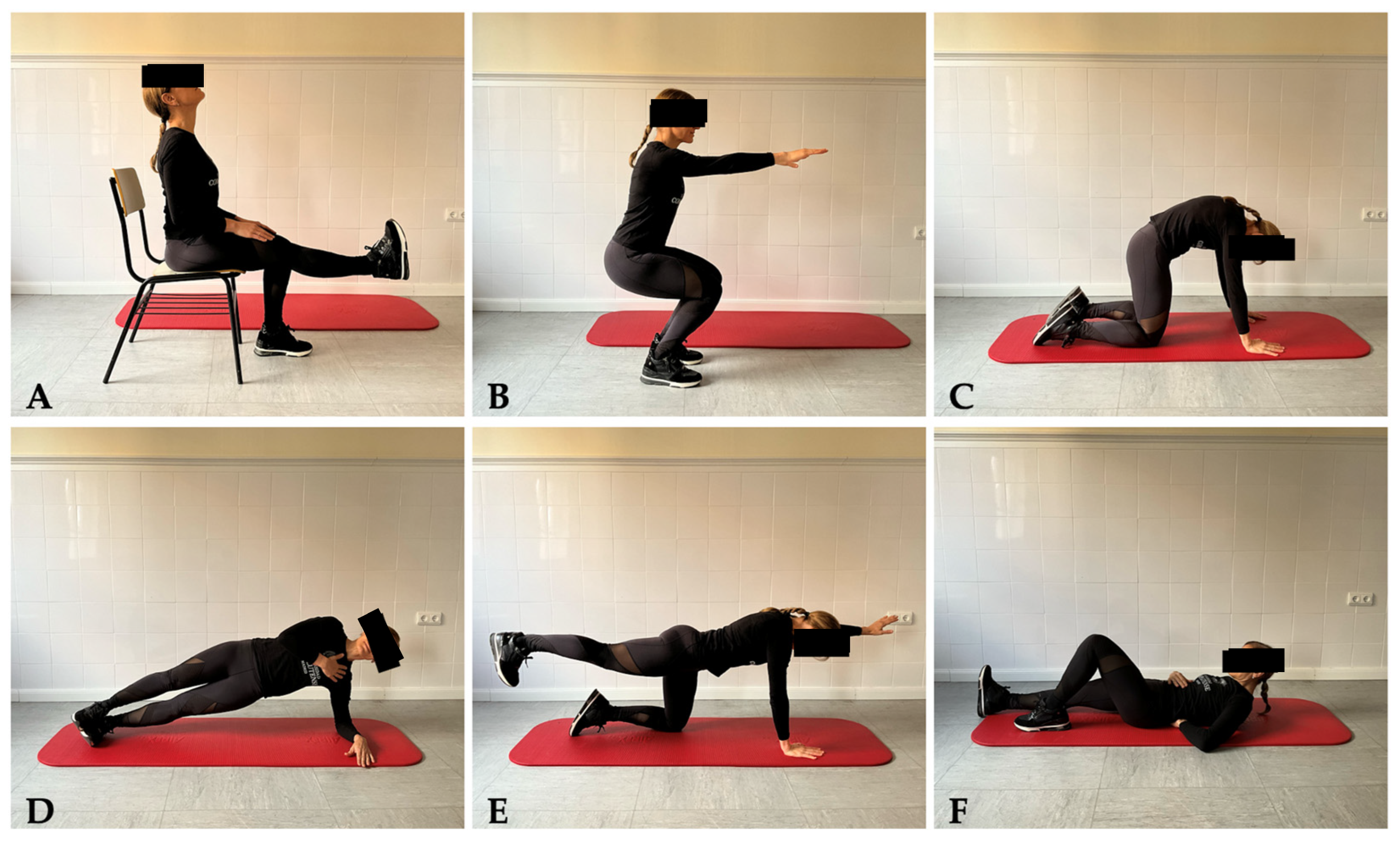
2.4.1. Self-Management Protocol: Therapeutic Exercise and Mobile Phone App Registry
2.4.2. Face-to-Face Sessions
2.5. Randomization and Masking
2.6. Primary Outcome
Pain Intensity
2.7. Secondary Outcomes
2.7.1. Pain-Related Disability
2.7.2. Quality of Life
2.7.3. Treatment Adherence and Satisfaction
2.8. Treatment Side Effects
2.9. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.B.; Ursin, H.; et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006, 15 (Suppl. S2), S192–S300. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Angarita-Fonseca, A.; Lacasse, A.; Pagé, M.G.; Tétreault, P.; Fortin, M.; Léonard, G.; Stone, L.S.; Roy, J.-S. Low back pain definitions: Effect on patient inclusion and clinical profiles. Pain Rep. 2022, 7, e997. [Google Scholar] [CrossRef]
- Baumeister, H.; Knecht, A.; Hutter, N. Direct and indirect costs in persons with chronic back pain and comorbid mental disorders—A systematic review. J. Psychosom. Res. 2012, 73, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Delitto, A.; George, S.Z.; Van Dillen, L.; Whitman, J.M.; Sowa, G.; Shekelle, P.; Denninger, T.R.; Godges, J.J. Orthopaedic Section of the American Physical Therapy Association. Low back pain. J. Orthop. Sports Phys. Ther. 2012, 42, A1–A57. [Google Scholar] [CrossRef]
- Urits, I.; Burshtein, A.; Sharma, M.; Testa, L.; Gold, P.A.; Orhurhu, V.; Viswanath, O.; Jones, M.R.; Sidransky, M.A.; Spektor, B.; et al. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr. Pain Headache Rep. 2019, 23, 23. [Google Scholar] [CrossRef]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Du, S.; Hu, L.; Dong, J.; Xu, G.; Chen, X.; Jin, S.; Zhang, H.; Yin, H. Self-management program for chronic low back pain: A systematic review and meta-analysis. Patient Educ. Couns. 2017, 100, 37–49. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Lin, C.C.; Chenot, J.F.; van Tulder, M.; Koes, B.W. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. Eur. Spine J. 2018, 27, 2791–2803. [Google Scholar] [CrossRef]
- Moffett, J.K.; Torgerson, D.; Bell-Syer, S.; Jackson, D.; Llewlyn-Phillips, H.; Farrin, A.; Barber, J. Randomised controlled trial of exercise for low back pain: Clinical outcomes, costs, and preferences. BMJ 1999, 319, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.A.; Scuffham, P.; Gupta, D.; Harch, T.M.; Borchi, J.; Richards, B. Going digital: A narrative overview of the effects, quality and utility of mobile apps in chronic disease self-management. Aust. Health Rev. 2020, 44, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Caiata Zufferey, M.; Schulz, P.J. Self-management of chronic low back pain: An exploration of the impact of a patient-centered website. Patient Educ. Couns. 2009, 77, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, N.J.; Shah, A.; Shah, S.; Phatak, S.; Viswanathan, V. Improved Medication Adherence and Frequency of Blood Glucose Self-Testing Using an m-Health Platform Versus Usual Care in a Multisite Randomized Clinical Trial Among People with Type 2 Diabetes in India. Telemed. J. E Health 2017, 23, 733–740. [Google Scholar] [CrossRef]
- Chao, D.Y.; Lin, T.M.; Ma, W.Y. Enhanced Self-Efficacy and Behavioral Changes Among Patients With Diabetes: Cloud-Based Mobile Health Platform and Mobile App Service. JMIR Diabetes 2019, 4, e11017. [Google Scholar] [CrossRef]
- Xu, T.; Pujara, S.; Sutton, S.; Rhee, M. Telemedicine in the Management of Type 1 Diabetes. Prev. Chronic Dis. 2018, 15, E13. [Google Scholar] [CrossRef]
- Contreras, E.M.; Rivero, S.M.; García, E.R.; López-García-Ramos, L.; Vilas, J.C.P.; Suárez, A.B.; Diez, C.G.; Gil Guillén, V.; Claros, N.M.; Compliance Group of Spanish Society of Hypertension (SEH-LELHA). Specific hypertension smartphone application to improve medication adherence in hypertension: A cluster-randomized trial. Curr. Med. Res. Opin. 2019, 35, 167–173. [Google Scholar] [CrossRef]
- Santo, K.; Singleton, A.; Chow, C.K.; Redfern, J. Evaluating Reach, Acceptability, Utility, and Engagement with an App-Based Intervention to Improve Medication Adherence in Patients with Coronary Heart Disease in the MedApp-CHD Study: A Mixed-Methods Evaluation. Med. Sci. 2019, 7, 68. [Google Scholar] [CrossRef]
- Bedson, J.; Hill, J.; White, D.; Chen, Y.; Wathall, S.; Dent, S.; Cooke, K.; van der Windt, D. Development and validation of a pain monitoring app for patients with musculoskeletal conditions (The Keele pain recorder feasibility study). BMC Med. Inform. Decis. Mak. 2019, 19, 24. [Google Scholar] [CrossRef]
- Chhabra, H.S.; Sharma, S.; Verma, S. Smartphone app in self-management of chronic low back pain: A randomized controlled trial. Eur. Spine J. 2018, 27, 2862–2874. [Google Scholar] [CrossRef]
- Machado, G.C.; Pinheiro, M.B.; Lee, H.; Ahmed, O.H.; Hendrick, P.; Williams, C.; Kamper, S.J. Smartphone apps for the self-management of low back pain: A systematic review. Best Pract. Res. Clin. Rheumatol. 2016, 30, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Simera, I.; Moher, D.; Hoey, J.; Schulz, K.F.; Altman, D.G. A catalogue of reporting guidelines for health research. Eur. J. Clin. Investig. 2010, 40, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.; Laslett, M.; Juhl, C. Clinical classification in low back pain: Best-evidence diagnostic rules based on systematic reviews. BMC Musculoskelet. Disord. 2017, 18, 188. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates Inc.: Hillsdale, NJ, USA, 1988; pp. 131–132. [Google Scholar]
- McGill, S.M. Low Back Stability: From Formal Description to Issues for Performance and Rehabilitation. Exerc. Sport Sci. Rev. 2001, 29, 26–31. [Google Scholar] [CrossRef]
- Yao, M.; Xu, B.P.; Li, Z.J.; Zhu, S.; Tian, Z.R.; Li, D.H.; Cen, J.; Cheng, S.D.; Wang, Y.J.; Guo, Y.M.; et al. A comparison between the low back pain scales for patients with lumbar disc herniation: Validity, reliability, and responsiveness. Health Qual. Life Outcomes 2020, 18, 175. [Google Scholar] [CrossRef]
- Childs, J.D.; Piva, S.R.; Fritz, J.M. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 2005, 30, 1331–1334. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952, discussion 2952. [Google Scholar] [CrossRef]
- Garg, A.; Pathak, H.; Churyukanov, M.V.; Uppin, R.B.; Slobodin, T.M. Low back pain: Critical assessment of various scales. Eur. Spine J. 2020, 29, 503–518. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, Y.; Zeng, J.; Zhao, X.; Wan, C. Comparing the reliability and validity of the SF-36 and SF-12 in measuring quality of life among adolescents in China: A large sample cross-sectional study. Health Qual. Life Outcomes 2020, 18, 360. [Google Scholar] [CrossRef]
- Escatlar-Gonzalez, M. Adaptación y Validación de un Cuestionario Para Medir la Satisfacción del Tratamiento de Fisioterapia en Atención Primaria. Ph.D. Thesis, Universitat Rovira i Virgili, Tarragona, Spain, 2017. [Google Scholar]
- Capili, B.; Anastasi, J.K.; Geiger, J.N. Adverse event reporting in acupuncture clinical trials focusing on pain. Clin. J. Pain 2010, 26, 43–48. [Google Scholar] [CrossRef]
- Yu, Z.; Guindani, M.; Grieco, S.F.; Chen, L.; Holmes, T.C.; Xu, X. Beyond t test and ANOVA: Applications of mixed-effects models for more rigorous statistical analysis in neuroscience research. Neuron 2022, 110, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Alhussein, G.; Hadjileontiadis, L. Digital Health Technologies for Long-term Self-management of Osteoporosis: Systematic Review and Meta-analysis. JMIR Mhealth Uhealth 2022, 10, e32557. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Sánchez-Laulhé, P.; Luque-Romero, L.G.; Barrero-García, F.J.; Biscarri-Carbonero, Á.; Blanquero, J.; Suero-Pineda, A.; Heredia-Rizo, A.M. An Exercise and Educational and Self-management Program Delivered With a Smartphone App (CareHand) in Adults With Rheumatoid Arthritis of the Hands: Randomized Controlled Trial. JMIR Mhealth Uhealth 2022, 10, e35462. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Or, C.K.; Chen, J. Effects of technology-supported exercise programs on the knee pain, physical function, and quality of life of individuals with knee osteoarthritis and/or chronic knee pain: A systematic review and meta-analysis of randomized controlled trials. J. Am. Med. Inform. Assoc. 2021, 28, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, B.; Bagala, M.; Creighton, A.; Leavey, T.; Nicholls, S.; Wood, C.; Longman, J.; Barker, J.; Pit, S. Mobile phone applications and their use in the self-management of Type 2 Diabetes Mellitus: A qualitative study among app users and non-app users. Diabetol. Metab. Syndr. 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Akodu, A.; Tella, B.; Olujobi, O. Effect of stabilization exercise on pain and quality of life of patients with non-specific chronic low back pain. Afr. J. Physiother. Rehabil. Sci. 2015, 7, 7–11. [Google Scholar] [CrossRef]
- Fritz, J.M.; Lane, E.; Minick, K.I.; Bardsley, T.; Brennan, G.; Hunter, S.J.; McGee, T.; Rassu, F.S.; Wegener, S.T.; Skolasky, R.L. Perceptions of Telehealth Physical Therapy Among Patients with Chronic Low Back Pain. Telemed. Rep. 2021, 2, 258–263. [Google Scholar] [CrossRef]
- Thanawat, T.; Nualnetr, N. Effects of an intervention based on the Transtheoretical Model on back muscle endurance, physical function and pain in rice farmers with chronic low back pain. J. Back. Musculoskelet. Rehabil. 2017, 30, 847–856. [Google Scholar] [CrossRef]
- Fritz, J.M.; Minick, K.I.; Brennan, G.P.; McGee, T.; Lane, E.; Skolasky, R.L.; Thackeray, A.; Bardsley, T.; Wegener, S.T.; Hunter, S.J. Outcomes of Telehealth Physical Therapy Provided Using Real-Time, Videoconferencing for Patients with Chronic Low Back Pain: A Longitudinal Observational Study. Arch. Phys. Med. Rehabil. 2022, 103, 1924–1934. [Google Scholar] [CrossRef]
- Albaladejo, C.; Kovacs, F.M.; Royuela, A.; del Pino, R.; Zamora, J.; Spanish Back Pain Research Network. The efficacy of a short education program and a short physiotherapy program for treating low back pain in primary care: A cluster randomized trial. Spine 2010, 35, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Koppenaal, T.; Pisters, M.F.; Kloek, C.J.; Arensman, R.M.; Ostelo, R.W.; Veenhof, C. The 3-Month Effectiveness of a Stratified Blended Physiotherapy Intervention in Patients with Nonspecific Low Back Pain: Cluster Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e31675. [Google Scholar] [CrossRef]
- Weise, H.; Zenner, B.; Schmiedchen, B.; Benning, L.; Bulitta, M.; Schmitz, D.; Weise, K. The Effect of an App-Based Home Exercise Program on Self-reported Pain Intensity in Unspecific and Degenerative Back Pain: Pragmatic Open-label Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e41899. [Google Scholar] [CrossRef] [PubMed]
- Sitges, C.; Terrasa, J.L.; García-Dopico, N.; Segur-Ferrer, J.; Velasco-Roldán, O.; Crespí-Palmer, J.; González-Roldán, A.M.; Montoya, P. An Educational and Exercise Mobile Phone-Based Intervention to Elicit Electrophysiological Changes and to Improve Psychological Functioning in Adults with Nonspecific Chronic Low Back Pain (BackFit App): Nonrandomized Clinical Trial. JMIR Mhealth Uhealth 2022, 10, e29171. [Google Scholar] [CrossRef]
- Shebib, R.; Bailey, J.F.; Smittenaar, P.; Perez, D.A.; Mecklenburg, G.; Hunter, S. Randomized controlled trial of a 12-week digital care program in improving low back pain. NPJ Digit. Med. 2019, 2, 1. [Google Scholar] [CrossRef]
- Ulger, O.; Demirel, A.; Oz, M.; Tamer, S. The effect of manual therapy and exercise in patients with chronic low back pain: Double blind randomized controlled trial. J. Back. Musculoskelet. Rehabil. 2017, 30, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Rintala, A.; Rantalainen, R.; Kaksonen, A.; Luomajoki, H.; Kauranen, K. mHealth Apps for Low Back Pain Self-management: Scoping Review. JMIR Mhealth Uhealth 2022, 10, e39682. [Google Scholar] [CrossRef]
- de Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Kini, V.; Ho, P.M. Interventions to Improve Medication Adherence: A Review. JAMA 2018, 320, 2461–2473. [Google Scholar] [CrossRef]
- Mannion, A.F.; Helbling, D.; Pulkovski, N.; Sprott, H. Spinal segmental stabilisation exercises for chronic low back pain: Programme adherence and its influence on clinical outcome. Eur. Spine J. 2009, 18, 1881–1891. [Google Scholar] [CrossRef]
- Lemmers, G.P.G.; Bier, J.D.; van Lankveld, W.; Westert, G.P.; Staal, J.B.; van der Wees, P.J. Guideline adherence of physiotherapists in the treatment of patients with low back pain: A qualitative study. J. Eval. Clin. Pract. 2022, 28, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Saner, J.; Bergman, E.M.; de Bie, R.A.; Sieben, J.M. Low back pain patients’ perspectives on long-term adherence to home-based exercise programmes in physiotherapy. Musculoskelet. Sci. Pract. 2018, 38, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mbada, C.E.; Olaoye, M.I.; Dada, O.O.; Ayanniyi, O.; Johnson, O.E.; Odole, A.C.; Ishaya, G.P.; Omole, O.J.; Makinde, M.O. Comparative Efficacy of Clinic-Based and Telerehabilitation Application of Mckenzie Therapy in Chronic Low-Back Pain. Int. J. Telerehabil. 2019, 11, 41–58. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Azghani, M.R.; Taghipour, M.; Salahzadeh, Z.; Ghaderi, F.; Oskouei, A.E. Effects of McGill stabilization exercises and conventional physiotherapy on pain, functional disability and active back range of motion in patients with chronic non-specific low back pain. J. Phys. Ther. Sci. 2018, 30, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, M.M. Effects of progressive neuromuscular stabilization exercise on the support surface on patients with high obesity with lumbar instability. A double-blinded randomized controlled trial. Medicine 2021, 100, e23285. [Google Scholar] [CrossRef]


| Experimental Group (n = 45) | Control Group (n = 45) | p Value | |
|---|---|---|---|
| Demographic Information | |||
| Females (%) | 76.5 | 89.3 | 0.189 |
| Age (years) | 52.2 ± 9.8 | 49.9 ± 10.0 | 0.338 |
| Weekly Physical Activity | |||
| 1 day a week (%) | 29.4 | 25.0 | 0.923 |
| 2 days a week (%) | 26.5 | 32.1 | |
| 3 days a week (%) | 14.7 | 17.9 | |
| +4 days a week (%) | 29.4 | 25.0 | |
| Professional Situation | |||
| Working (%) | 55.9 | 71.4 | 0.397 |
| Sick Leave (%) | 32.4 | 14.3 | |
| Laboral inhability (%) | 2.9 | 0.0 | |
| Not applicable (%) | 8.8 | 14.3 | |
| Clinical Information | |||
| Symptoms Duration (years) | 3.2 ± 1.1 | 3.3 ± 1.3 | 0.646 |
| Experimental Group (n = 45) | Control Group (n = 45) | Between-Group Differences | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 Weeks | 12 Weeks | Baseline | 4 Weeks | 12 Weeks | Group × Time | Group | Time | |
| Pain Intensity | |||||||||
| NPRS (0–10) | 7.0 ± 1.8 | 4.1 ± 2.0 | 4.3 ± 1.6 | 6.3 ± 1.5 | 4.7±1.6 | 4.6 ± 1.4 | F = 2.818 = 0.030 p = 0.062 | F = 0.073 = 0.000 p = 0.788 | F = 35.084 = 0.280 p < 0.001 |
| Quality of Life | |||||||||
| SF-12 Physical (0–100) | 35.1 ± 8.4 | 41.0 ± 6.9 | 41.7 ± 6.4 | 35.8 ± 7.3 | 37.1 ± 5.9 | 38.2 ± 4.6 | F = 2.124 = 0.023 p = 0.123 | F = 4.555 = 0.025 p = 0.034 | F = 7.912 = 0.081 p < 0.001 |
| SF-12 Mental (0–100) | 40.6 ± 10.4 | 41.4 ± 7.7 | 52.9 ± 5.9 | 40.1 ± 9.5 | 49.3 ± 6.5 | 53.2 ± 6.1 | F = 5.301 = 0.056 p = 0.006 | F = 4.775 = 0.026 p = 0.030 | F = 40.068 = 0.308 p < 0.001 |
| Pain-Related Disability | |||||||||
| ODI (0–100) | 30.6 ± 16.3 | 20.8 ± 12.9 | 16.5 ± 13.7 | 29.8 ± 9.3 | 18.9 ± 13.3 | 18.1 ± 15.0 | F = 0.254 = 0.776 p = 0.003 | F = 0.005 = 0.942 p = 0.000 | F = 14.637 = 0.140 p < 0.001 |
| Experimental Group (n = 45) | Control Group (n = 45) | Difference | |
|---|---|---|---|
| % Sessions Completed | |||
| Face-to-Face (%) | 100.0 ± 0.0 | - | |
| Self-Management (%) | 78.1 ± 13.1 | 64.4 ± 9.2 | 13.6 (7.8; 19.7) p < 0.001 |
| PSQ (0–70) | 47.7 ± 4.2 | 35.7 ± 2.9 | 12.0 (10.1;13.9) p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Marcos, J.J.; Díaz-Arribas, M.J.; Valera-Calero, J.A.; Navarro-Santana, M.J.; Izquierdo-García, J.; Ortiz-Gutiérrez, R.M.; Plaza-Manzano, G. The Added Value of Face-to-Face Supervision to a Therapeutic Exercise-Based App in the Management of Patients with Chronic Low Back Pain: A Randomized Clinical Trial. Sensors 2024, 24, 567. https://doi.org/10.3390/s24020567
López-Marcos JJ, Díaz-Arribas MJ, Valera-Calero JA, Navarro-Santana MJ, Izquierdo-García J, Ortiz-Gutiérrez RM, Plaza-Manzano G. The Added Value of Face-to-Face Supervision to a Therapeutic Exercise-Based App in the Management of Patients with Chronic Low Back Pain: A Randomized Clinical Trial. Sensors. 2024; 24(2):567. https://doi.org/10.3390/s24020567
Chicago/Turabian StyleLópez-Marcos, José Javier, María José Díaz-Arribas, Juan Antonio Valera-Calero, Marcos José Navarro-Santana, Juan Izquierdo-García, Rosa María Ortiz-Gutiérrez, and Gustavo Plaza-Manzano. 2024. "The Added Value of Face-to-Face Supervision to a Therapeutic Exercise-Based App in the Management of Patients with Chronic Low Back Pain: A Randomized Clinical Trial" Sensors 24, no. 2: 567. https://doi.org/10.3390/s24020567






