From the Modeling of an Electrochemical YSZ-Based Gas Sensor Used in Electrolysis Mode
Abstract
1. Introduction
2. Materials and Methods
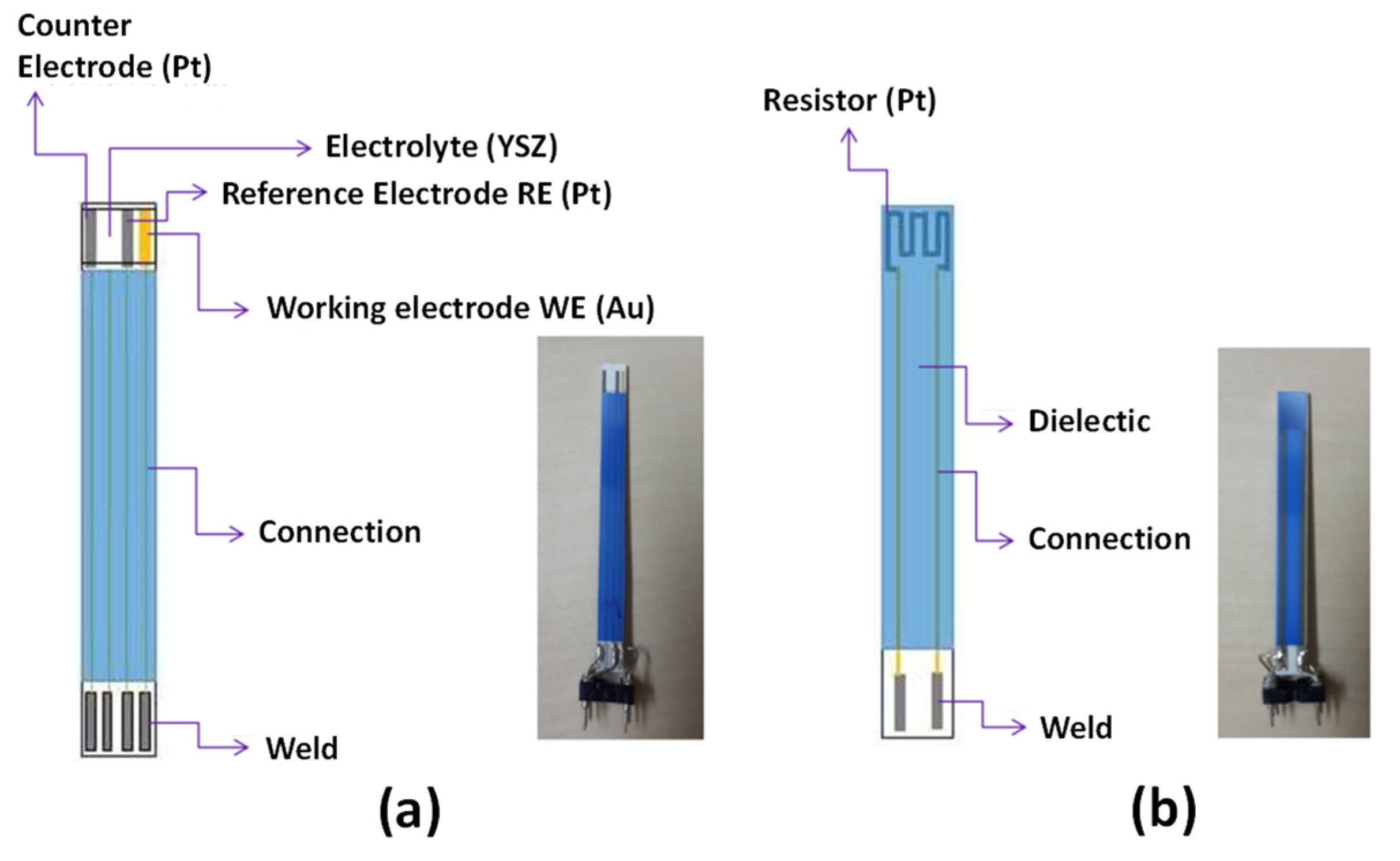
2.1. Sensor’s Physical and Electrochemical Description
- If is very low, the current value can be approximated by a first-order Taylor series expansion:
- If and (As it will be seen later, for our tested polarization currents and gaseous compositions, overpotential at gold cathode has been measured between −1.1 V and −0.1 V):
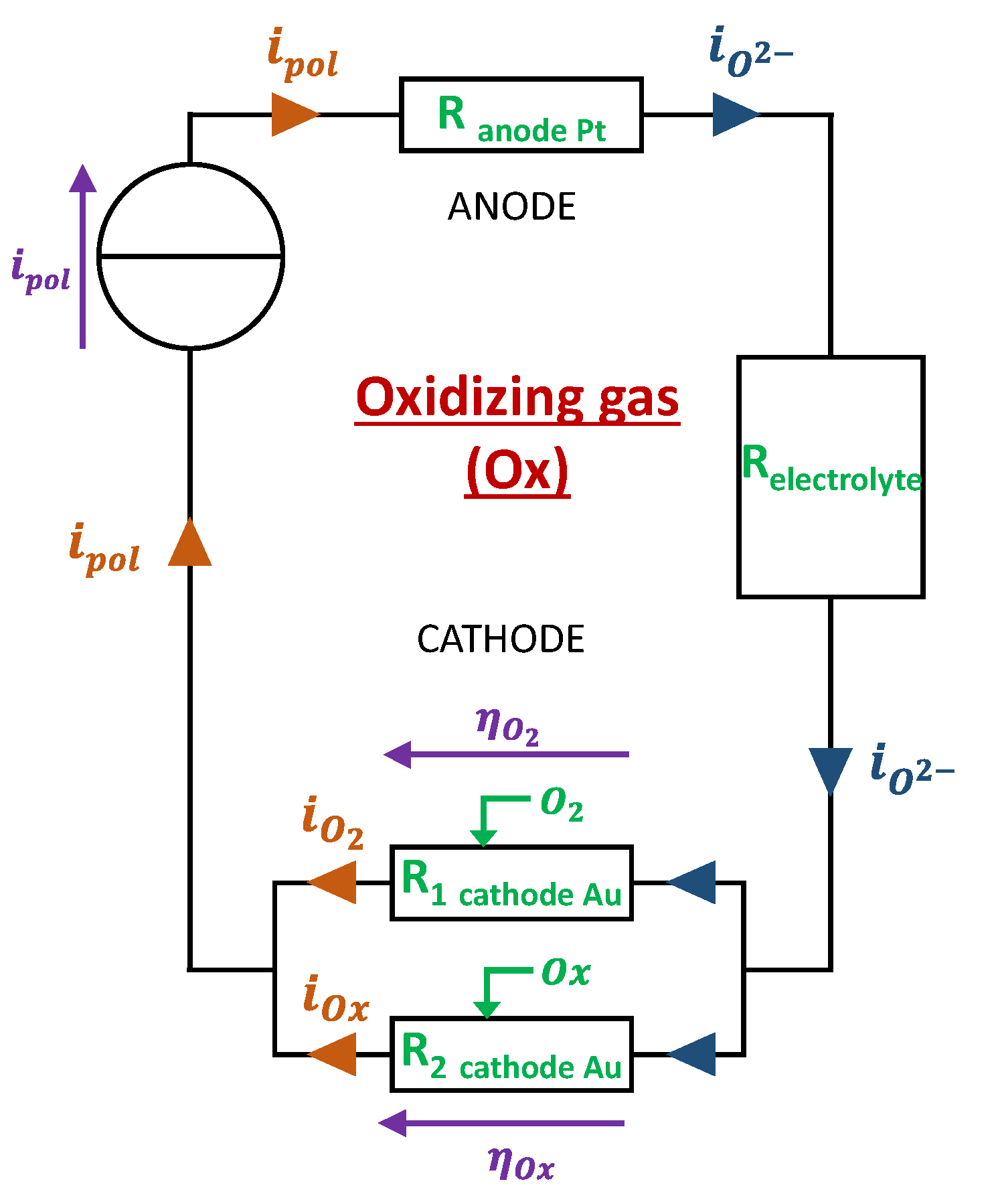
2.2. Electrochemical and Associated Electrical Models
- Model 1: “Base gas” case
- -
- at the cathode: O2 + 4e− → 2O2−
- -
- at the anode: 2O2− → O2 + 4e−
- Model 2: Presence of an oxidizing gas (NO2, NO)
- -
- at the cathode: O2 + 4e− → 2O2−
- -
- at the cathode: 2O2− → O2 + 4e−
- Model 3: Presence of a reducing gas (NH3, CO)
- -
- at the cathode: O2 + 4e− → 2O2−
- -
- at the anode: 2O2− → O2 + 4e−
2.3. Multivariate Fitting Methods
3. Results and Discussions
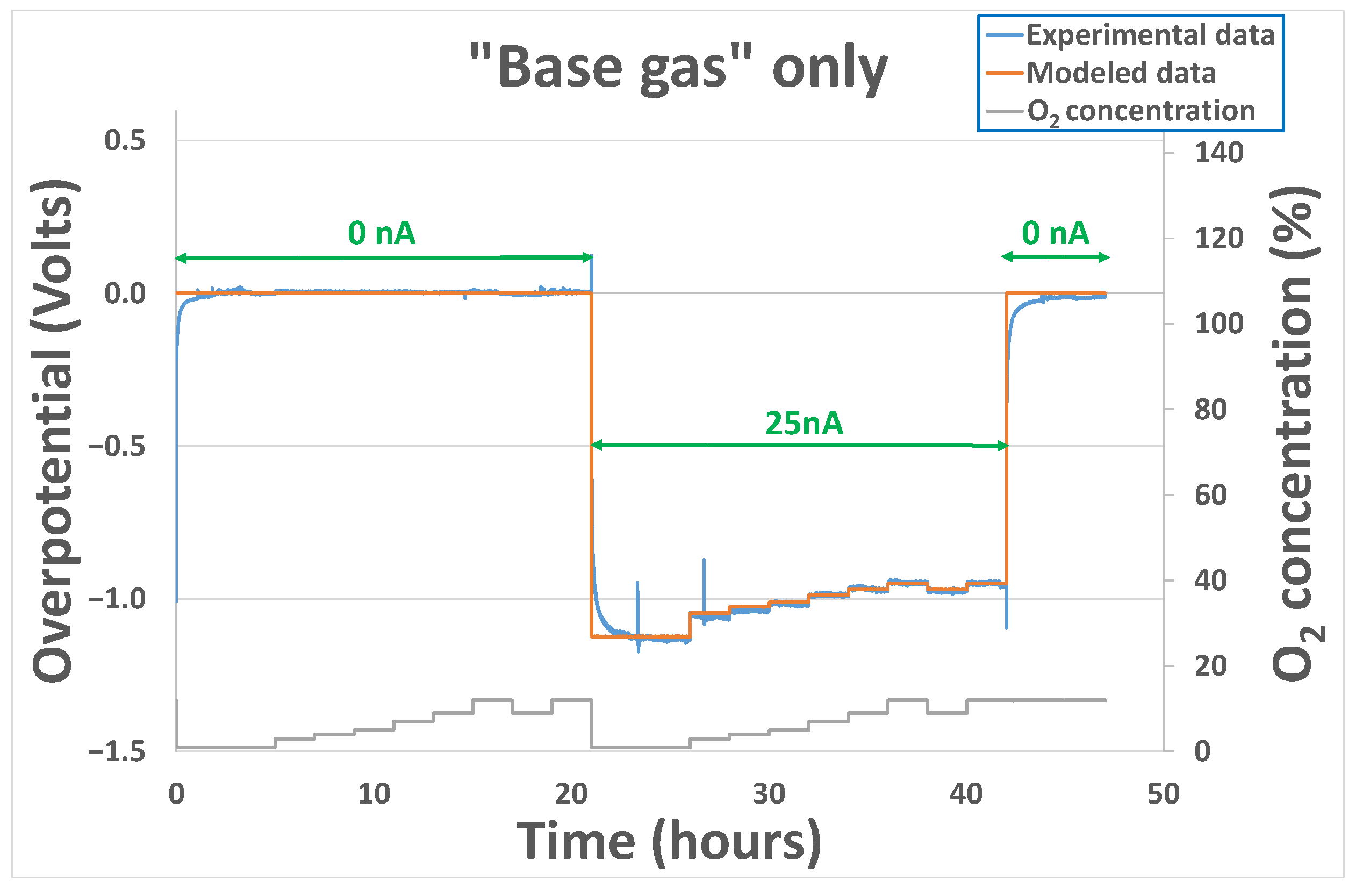
3.1. “Base Gas” Alone Case
3.2. Introduction of Pollutant Gases Together with “Base Gas”
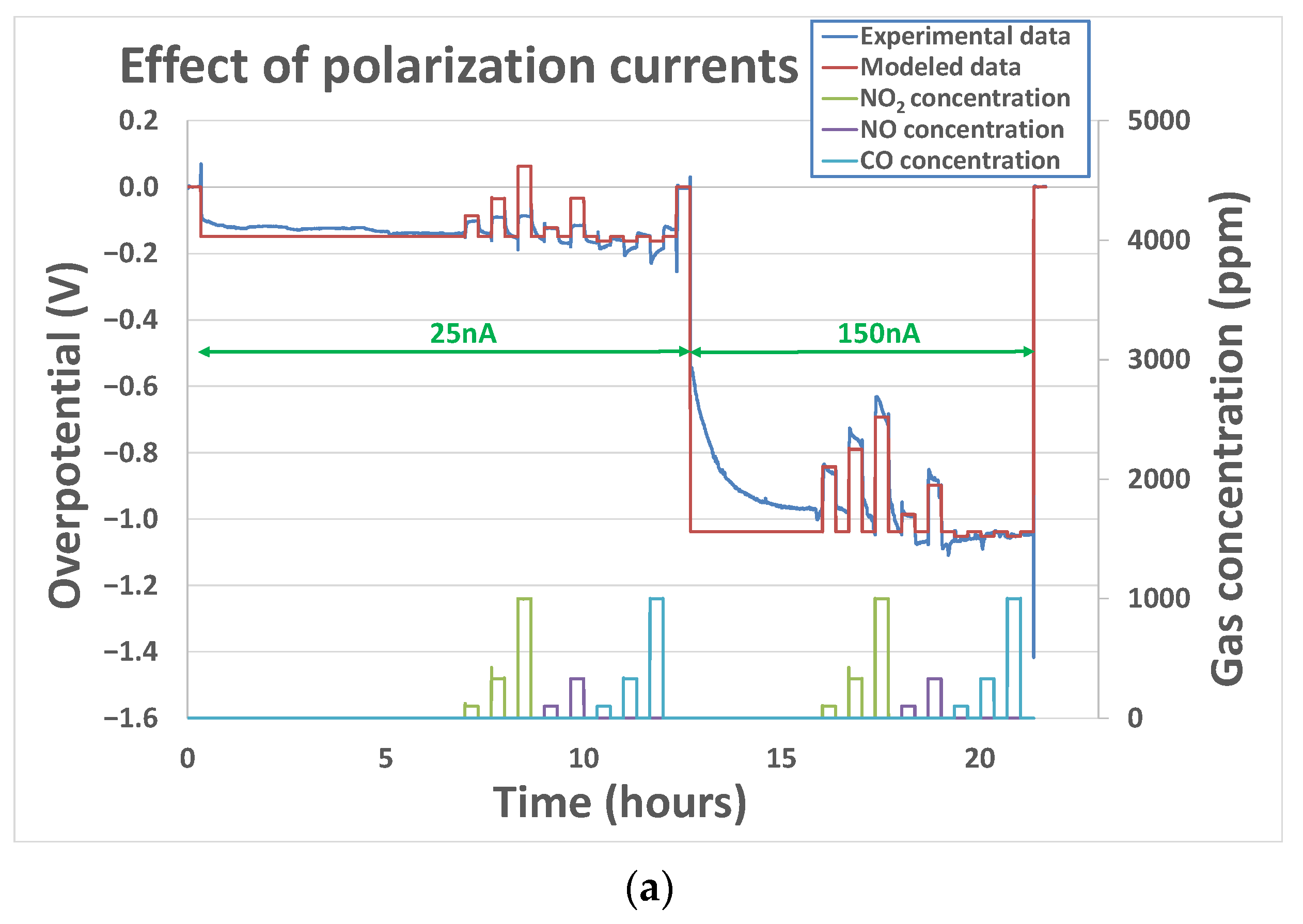
3.2.1. Effect of Polarization Current
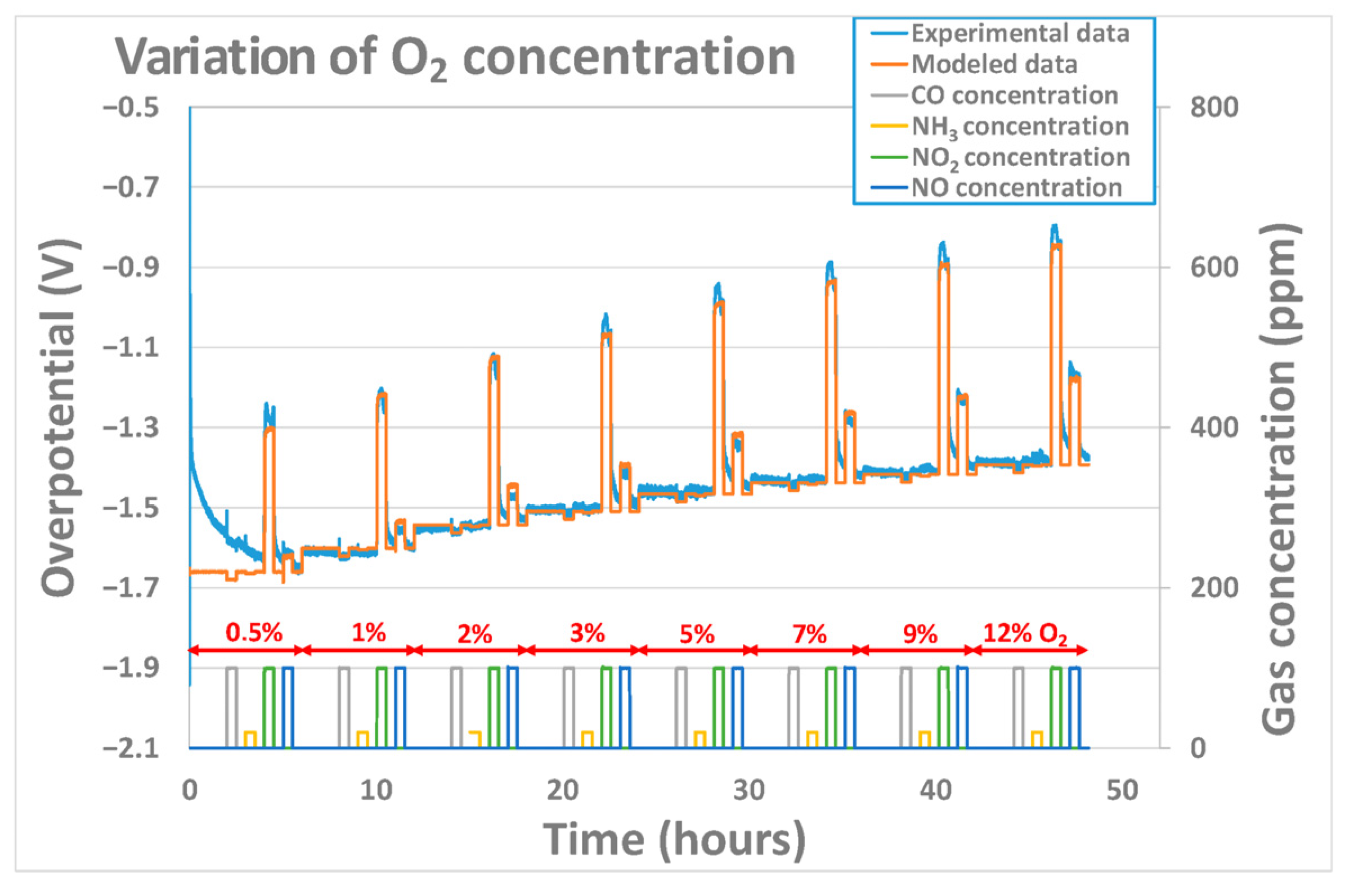
3.2.2. Effect of Oxygen Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, J.; Nazarenko, L. Soot climate forcing via snow and ice albedos. Proc. Natl. Acad. Sci. USA 2004, 101, 423–428. [Google Scholar] [CrossRef] [PubMed]
- EEA Report, National Emissions Reported to the Convention on Long-Range Transboundary Air Pollution (LRTAP Convention). 2023. Available online: http://www.eea.europa.eu (accessed on 1 July 2023).
- Fergus, J.W. Sensing mechanism of non-equilibrium solid-electrolyte-based chemical sensors. J. Solid State Electrochem. 2011, 15, 971–984. [Google Scholar] [CrossRef]
- Ryu, H.; Thompson, D.; Huang, Y.; Li, B.; Lei, Y. Electrochemical sensors for nitrogen species: A review. Sens. Actuators Rep. 2020, 2, 100022. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, X.; Du, H.; Zhu, H.; Li, D.; Ao, D.; Guo, Y.; Fu, Y.; Zu, X. Cellulose nano-crystals as a sensitive and selective layer for high performance surface acoustic wave HCl gas sensors. Sens. Actuators A Phys. 2020, 301, 111792. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wu, J.-X.; Chen, H.-I. High-selectivity NOx sensors based on an Au/InGaP Schottky diode functionalized with self-assembled monolayer of alkanedithiols. Sens. Actuators B Chem. 2020, 305, 127269. [Google Scholar] [CrossRef]
- Pushpanjali, P.A.; Manjunatha, J.G.; Srinivas, M.T. Highly sensitive platform utilizing poly(l-methionine) layered carbon nanotube paste sensor for the determination of voltaren. FlatChem 2020, 24, 100207. [Google Scholar] [CrossRef]
- Staszek, K.; Szkudlarek, A.; Kawa, M.; Rydosz, A. Microwave system with sensor utilizing GO-based gas-sensitive layer and its application to acetone detection. Sens. Actuators B Chem. 2019, 297, 126699. [Google Scholar] [CrossRef]
- Gao, J.; Viricelle, J.-P.; Breuil, P.; Pijolat, C.; Vernoux, P.; Boreave, A.; Giroir-Fendler, A. Improvement of the NOx selectivity for a planar YSZ. Sens. Actuators B 2011, 154, 106–110. [Google Scholar] [CrossRef]
- Liang, A.A.; Hou, B.H.; Tang, C.S.; Sun, D.L.; Luo, E.A. An advanced molecularly imprinted electrochemical sensor for the highly sensitive and selective detection and determination of Human IgG. Bioelectrochemistry 2021, 137, 107671. [Google Scholar]
- Wang, Q.; Xiao, X.; Hu, X.; Huang, L.; Li, T.; Yang, M. Molecularly imprinted electrochemical sensor for ascorbic acid determination based on MXene modified electrode. Mater. Lett. 2021, 285, 129158. [Google Scholar] [CrossRef]
- Alamry, K.A.; Hussein, M.A.; Choi, J.-W.; El-Said, W.A. Non-enzymatic electrochemical sensor to detect γ-aminobutyric acid with ligand-based on graphene oxide modified gold electrode. J. Electroanal. Chem. 2020, 879, 114789. [Google Scholar] [CrossRef]
- Alsaedi, B.S.O.; McGraw, C.M.; Schaerf, T.M.; Dillingham, P.W. Multivariate limit of detection for non-linear sensor arrays. Chemom. Intell. Lab. Syst. 2020, 201, 104016. [Google Scholar] [CrossRef]
- Ferro, L.M.M.; Lemos, S.G.; Ferreira, M.; Trivinho-Strixino, F. Use of multivariate analysis on Fabry-Pérot interference spectra of nanoporous anodic alumina (NAA) for optical sensors purposes. Sens. Actuators B Chem. 2017, 248, 718–723. [Google Scholar] [CrossRef]
- Song, L.; Yang, L.; Wang, Z.; Liu, D.; Luo, L.; Zhu, X.; Xi, Y.; Yang, Z.; Han, N.; Wang, F.; et al. One-step electrospun SnO2/MOx heterostructured nanomaterials for highly selective gas sensor array integration. Sens. Actuators B Chem. 2019, 283, 793–801. [Google Scholar] [CrossRef]
- Yousefi-Darani, A.; Babor, M.; Paquet-Durand, O.; Hitzmann, B. Model-based calibration of a gas sensor array for on-line monitoring of ethanol concentration in Saccharomyces cerevisiae batch cultivation. Biosyst. Eng. 2020, 198, 198–209. [Google Scholar] [CrossRef]
- Chu, J.; Li, W.; Yang, X.; Wu, Y.; Wang, D.; Yang, A.; Yuan, H.; Wang, X.; Li, Y.; Rong, M. Identification of gas mixtures via sensor array combining with neural networks. Sens. Actuators B Chem. 2020, 329, 129090. [Google Scholar] [CrossRef]
- Singh, V. Quantum dot decorated multi-walled carbon nanotube modified electrochemical sensor array for single drop insulin detection. Mater. Lett. 2019, 254, 415–418. [Google Scholar] [CrossRef]
- Gornall, D.D.; Collyer, S.D.; Higson, S.P.J. Investigations into the use of screen-printed carbon electrodes as templates for electrochemical sensors and sonochemically fabricated microelectrode arrays. Sens. Actuators B Chem. 2009, 141, 581–591. [Google Scholar] [CrossRef]
- Iglesias, L.; Boudjiet, M.T.; Dufour, I. Discrimination and concentration measurement of different binary gas mixtures with a simple resonator through viscosity and mass density. Sens. Actuators B Chem. 2019, 285, 487–494. [Google Scholar] [CrossRef]
- Janardhanan, V.M.; Deutschmann, O. Modeling of Solid-Oxide Fuel Cells. Z. Für Phys. Chem. 2007, 221, 443–478. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Ottaviano, A. Solid oxide fuel cell modelling: Electrochemical performance and thermal management during load-following operation. Energy 2016, 115 Pt 1, 107–119. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Rao, M.; Chen, Z.; Xiong, K.; Hou, L.; Wang, X.; Chen, C.; Li, S.; Li, X. Research on Solid Oxide Fuel Cell System Model Building and 3D Testing Based on the Nodal Idea. Atmosphere 2023, 14, 1261. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, X.; Liu, Z.; Qiu, G.; Zhang, X.; Han, Y.; Ding, J.; Wang, X. A limiting current oxygen sensor with (CuO)0.1(8YSZ)0.9 dense diffusion barrier. Phys. B Condens. Matter 2022, 630, 413701. [Google Scholar] [CrossRef]
- Halley, S.; Ramaiyan, K.P.; Tsui, L.-K.; Garzon, F. A review of zirconia oxygen, NOx, and mixed potential gas sensors—History and current trends. Sens. Actuators B Chem. 2022, 370, 132363. [Google Scholar] [CrossRef]
- Ritter, T.; Zosel, J.; Guth, U. Solid electrolyte gas sensors based on mixed potential principle—A review. Sens. Actuators B Chem. 2023, 382, 133508. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Cheng, P.; Sun, S.; Xu, L.; Liang, X.; Lu, G.; Hao, Y. Sub-ppb mixed potential H2S gas sensor based on YSZ and Nd2AO4 (A=Cu, Ba and Ni) as a new type of sensing electrode. Sens. Actuators B Chem. 2023, 382, 133500. [Google Scholar] [CrossRef]
- Brosha, E.L.; Mukundan, R.; Brown, D.R.; Garzon, F.H.; Visser, J.H. Development of ceramic mixed potential sensors for automotive applications. Solid State Ion. 2002, 148, 61–69. [Google Scholar] [CrossRef]
- Liu, T.; Guan, H.; Wang, T.; Liang, X.; Liu, F.; Zhang, C.; Lu, G. Mixed potential type acetone sensor based on GDC used for breath analysis. Sens. Actuators B Chem. 2021, 326, 128846. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 92–105. [Google Scholar]
- Romanytsia, I.; Viricelle, J.-P.; Vernoux, P.; Pijolat, C. Application of advanced morphology Au–X (X=YSZ, ZrO2) composites as sensing electrode for solid state mixed-potential exhaust NOx sensor. Sens. Actuators B Chem. 2015, 207 Pt A, 391–397. [Google Scholar] [CrossRef]
- Viricelle, J.-P.; Vernoux, P.; Gao, J.; Romanytsia, I.; Breuil, P. NO2-selective electrochemical sensors for Diesel exhausts, EUROSENSORS 2016, Budapest. Procedia Eng. 2016, 168, 7–10. [Google Scholar] [CrossRef]
- Viricelle, J.-P.; Vernoux, P.; Pijolat, C.; Breuil, P. Capteur NOx à Filtre Catalytique et Polarisation. WO2016202997 (A1) 22 December 2016. [Google Scholar]
- Cui, J.; Hao, Z.; Wang, Y.; Xue, L.; Xue, H.; Tu, L.; Hao, L.; Tian, M.; Guo, J.; Zhao, D.; et al. Mechanism of ozone-assisted catalytic oxidation of isopropanol over single-atom platinum catalysts at ambient temperature. Chem. Eng. J. 2022, 446 Pt 2, 136989. [Google Scholar] [CrossRef]
- Wei, H.; Wei, T.; Li, L.; Zhang, T.; Seidi, F.; Jin, Y.; Xiao, H. Morphological effect of ceria-supported platinum catalyst on low-temperature ethylene oxidation. Appl. Catal. B Environ. 2023, 324, 122242. [Google Scholar] [CrossRef]
- Krause, C.; Mirsky, V.M.; Heckmann, K.D. Capacitive Detection of Surfactant Adsorption on Hydrophobized Gold Electrodes. Langmuir 1996, 12, 6059–6064. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, N. A general mechanistic model of solid oxide fuel cells. Tsinghua Sci. Technol. 2006, 11, 701–711. [Google Scholar] [CrossRef]
- Aliramezani, M.; Koch, C.R.; Secanell, M.; Hayes, R.E.; Patrick, R. An electrochemical model of an amperometric NOx sensor. Sens. Actuators B Chem. 2019, 290, 302–311. [Google Scholar] [CrossRef]
- Bagotzky, V.S. Fundamentals of Electrochemistry, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]






| Sensor | Temperature (°C) | |||
|---|---|---|---|---|
| 1 | 450 | 3.2 | 0.026 | 0.09 |
| 500 | 11 | 0.025 | 0.13 | |
| 550 | 25 | 0.027 | 0.13 | |
| 2 | 450 | 3.5 | 0.025 | 0.10 |
| 500 | 5.5 | 0.034 | 0.10 | |
| 550 | 14.8 | 0.027 | 0.09 |
| Gas | Polarization Current (nA) | |||
|---|---|---|---|---|
| Base gas | 25–50–150 | 5–12 | 0.03–0.04 | 0.1–0.15 |
| 25 | 3–5 | 0.03–0.04 | 0.25–0.3 | |
| 50 | 30–70 | 0.03–0.04 | 0.4–0.6 | |
| 150 | 30–130 | 0.03–0.04 | 0.4–0.7 | |
| 25 | 4–8 | 0.06–0.08 | 0.5 | |
| 50 | 70–170 | 0.06–0.08 | 0.5–0.7 | |
| 150 | 120–400 | 0.06–0.08 | 0.5–0.8 | |
| 25 | 7–25 | 0.03–0.04 | 0.09 | |
| 50 | <0.1 | Not assessable | Not assessable | |
| 150 | <0.1 | Not assessable | Not assessable |
| Gas | ||||
|---|---|---|---|---|
| Base gas | 1.5–3.5 | 0.02–0.035 | 0.09–0.16 | |
| Oxidant gas | NO2 | 60–100 | 0.0014–0.015 | 0.3–0.4 |
| NO | 50–470 | 0.003–0.05 | 0.4–0.5 | |
| Reductant gas | CO | 2–7 | 0.02–0.035 | 0.09–0.16 |
| NH3 | <0.1 | Not assessable | Not assessable | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakhmi, R.; Viricelle, J.-P.; Alrammouz, R.; Rieu, M. From the Modeling of an Electrochemical YSZ-Based Gas Sensor Used in Electrolysis Mode. Sensors 2024, 24, 658. https://doi.org/10.3390/s24020658
Lakhmi R, Viricelle J-P, Alrammouz R, Rieu M. From the Modeling of an Electrochemical YSZ-Based Gas Sensor Used in Electrolysis Mode. Sensors. 2024; 24(2):658. https://doi.org/10.3390/s24020658
Chicago/Turabian StyleLakhmi, Riadh, Jean-Paul Viricelle, Rouba Alrammouz, and Mathilde Rieu. 2024. "From the Modeling of an Electrochemical YSZ-Based Gas Sensor Used in Electrolysis Mode" Sensors 24, no. 2: 658. https://doi.org/10.3390/s24020658
APA StyleLakhmi, R., Viricelle, J.-P., Alrammouz, R., & Rieu, M. (2024). From the Modeling of an Electrochemical YSZ-Based Gas Sensor Used in Electrolysis Mode. Sensors, 24(2), 658. https://doi.org/10.3390/s24020658





