Stretch Sensor: Development of Biodegradable Film
Abstract
:1. Introduction
2. State of Art
2.1. Polysaccharides as a Biopolymers
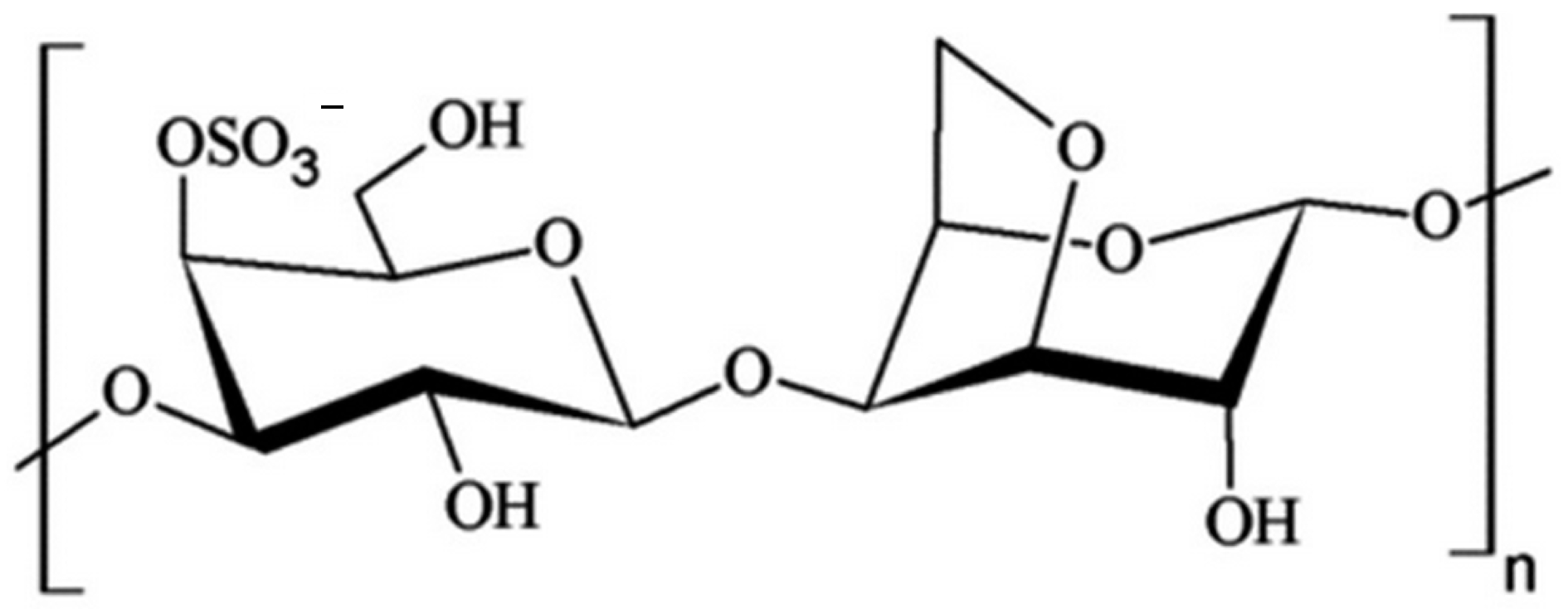
2.2. Natural Carrageenans
2.3. Natural Biopolymers in Industry and Sensor Manufacturing
2.4. Natural Biopolymers in Combination with Plasticizers
2.5. Natural Biopolymers in Modification and Combination with Other Polymers and Conductive Nanomaterials
3. Materials and Methods
3.1. Experiment Methodology
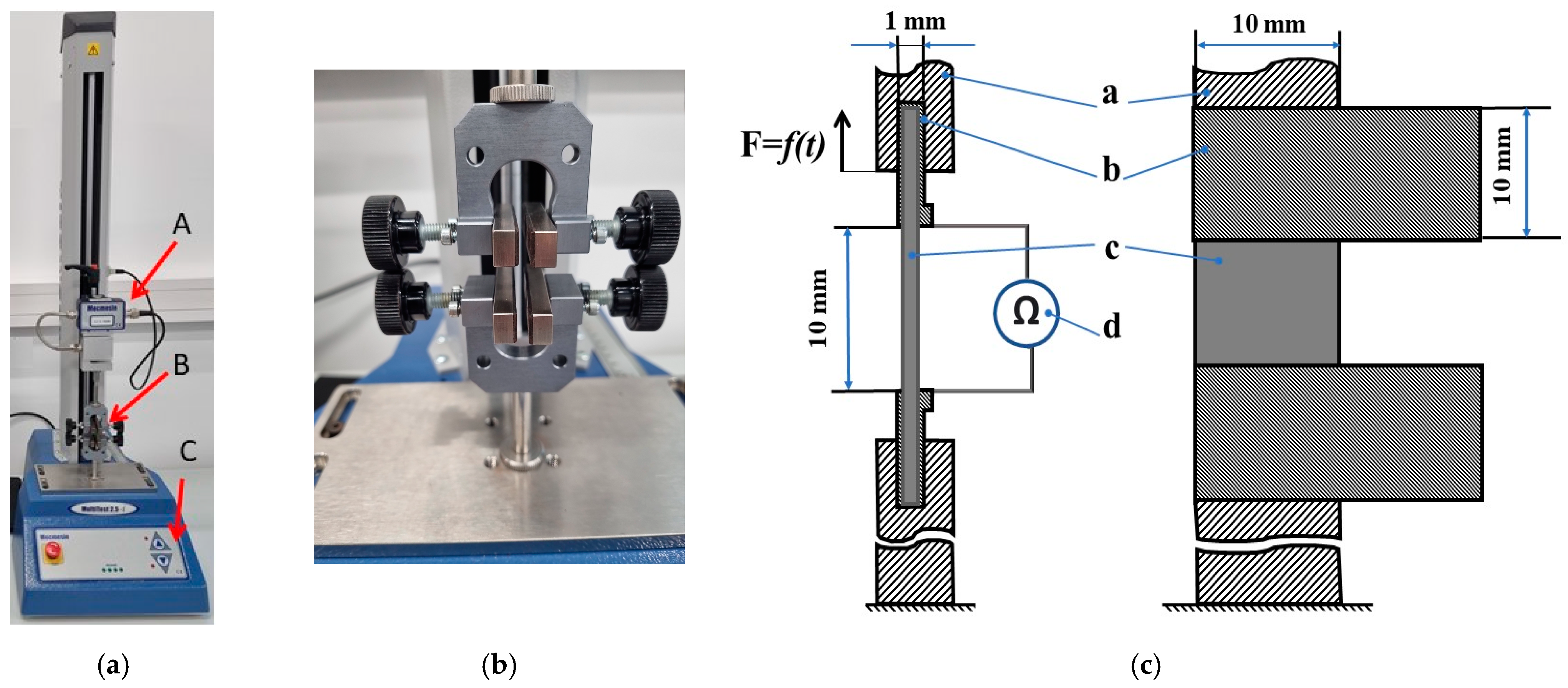
3.2. Equipment
3.3. Definition of Sensor Parameters
3.4. Development of a Sensor Prototype
3.4.1. Extracting of Carrageenan Biopolymer from Seaweed
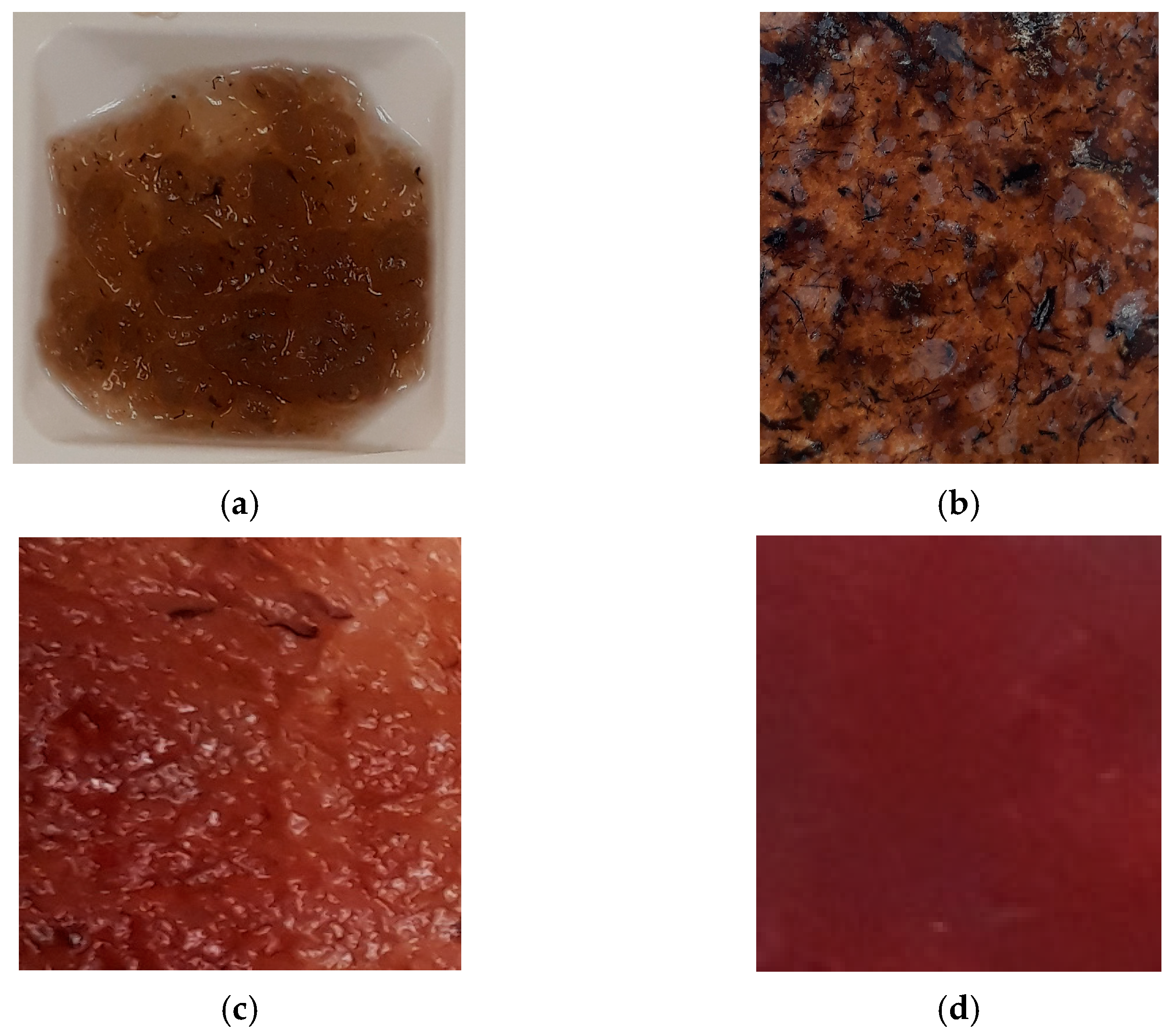
3.4.2. Sample for Stretch Sensor Preparation
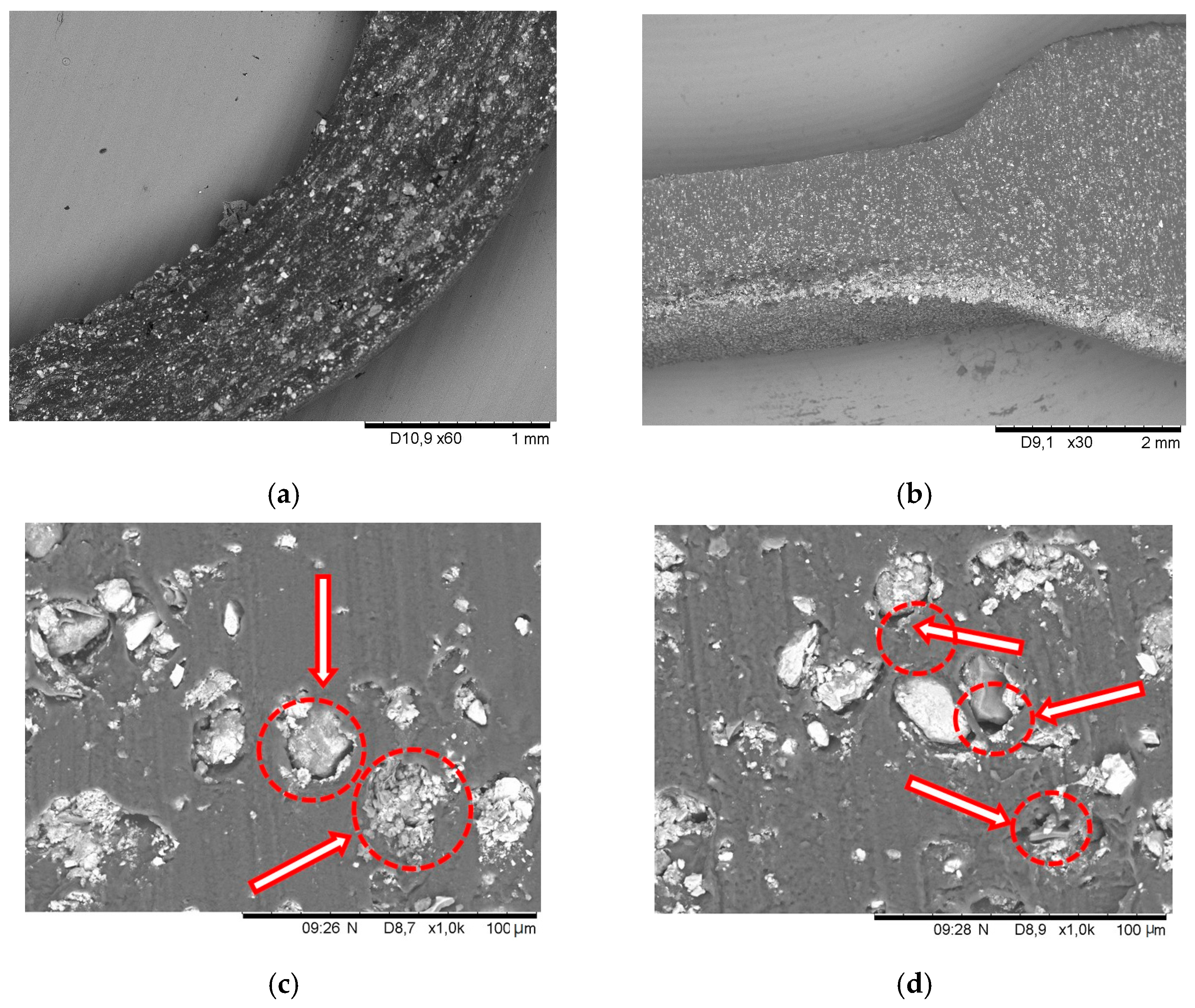
4. Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, Y.; Zhang, J.; Chang, L.; Zhang, X.; Liu, H.; Jiang, L. Preparation of High-Performance Ionogels with Excellent Transparency, Good Mechanical Strength, and High Conductivity. Adv. Mater. 2017, 29, 1704253. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Nguyen, T.; Phan, H.-P.; Nguyen, N.-T.; Dao, D.V.; Bell, J. Stretchable respiration sensors: Advanced designs and multifunctional platforms for wearable physiological monitoring. Biosens. Bioelectron. 2020, 166, 112460. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, D.; Zhang, H.; Tang, M.; Xu, Z.; Xia, H.; Li, K.; Wang, J. Trehalose-enhanced ionic conductive hydrogels with extreme stretchability, self-adhesive and anti-freezing abilities for both flexible strain sensor and all-solid-state supercapacitor. Chem. Eng. J. 2023, 472, 144849. [Google Scholar] [CrossRef]
- Boddohi, S.; Kipper, M.J.; Boddohi, S.; Collins, F.; Kipper, M.J. Engineering Nanoassemblies of Polysaccharides. Adv. Mater. 2010, 22, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Sandford, P.A.; Baird, J. Industrial Utilization of Polysaccharides. In The Polysaccharides; Academic Press: Cambridge, MA, USA, 1983; pp. 411–490. [Google Scholar] [CrossRef]
- Alipour, S.; Pourjavadi, A.; Hosseini, S.H. Magnetite embedded κ-carrageenan-based double network nanocomposite hydrogel with two-way shape memory properties for flexible electronics and magnetic actuators. Carbohydr. Polym. 2023, 310, 120610. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.M.S.; Mano, J.F. Extremely strong and tough hydrogels as prospective candidates for tissue repair—A review. Eur. Polym. J. 2015, 72, 344–364. [Google Scholar] [CrossRef]
- Tuvikene, R. Carrageenans. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2021; pp. 767–804. [Google Scholar] [CrossRef]
- Hugerth, A.; Sundelö, L.-O. The Effect of Polyelectrolyte Counterion Specificity, Charge Density, and Conformation on Polyelectrolyte-Amphiphile Interaction: The Carrageenan/Furcellaran-Amitriptyline System. Biopolym. Orig. Res. Biomol. 2001, 58, 186–194. [Google Scholar] [CrossRef]
- Bučas, M.; Daunys, D.; Olenin, S. Overgrowth patterns of the red algae Furcellaria lumbricalis at an exposed Baltic Sea coast: The results of a remote underwater video data analysis. Estuar. Coast. Shelf Sci. 2007, 75, 308–316. [Google Scholar] [CrossRef]
- Vahtmäe, E.; Kutser, T.; Martin, G.; Kotta, J. Feasibility of hyperspectral remote sensing for mapping benthic macroalgal cover in turbid coastal waters—A Baltic Sea case study. Remote Sens. Environ. 2006, 101, 342–351. [Google Scholar] [CrossRef]
- Eha, K.; Pehk, T.; Heinmaa, I.; Kaleda, A.; Laos, K. Impact of short-term heat treatment on the structure and functional properties of commercial furcellaran compared to commercial carrageenans. Heliyon 2021, 7, e06640. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.A.; Scott, W.E.; Williamson, F.B. Correlation of Optical Activity with Polysaccharide Conformation. Nature 1970, 227, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Ata, S.; Islam, A.; Khan, R.U.; Carrageenan, K. Fabrication of novel carrageenan based stimuli responsive injectable hydrogels for controlled release of cephradine. RSC Adv. 2019, 9, 12282–12290. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Xia, S.; Fu, W.; Liu, J.; Gao, G. Recyclable hydrogel for human-machine interface of multi-mode human vital signal acquisition. Sci. China Mater. 2023, 66, 2843–2851. [Google Scholar] [CrossRef]
- Chortos, A.; Liu, J.; Bao, Z. Pursuing prosthetic electronic skin. Nat. Mater. 2016, 15, 937–950. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, B.; Duan, L.; Gao, G. Tough, recyclable and biocompatible carrageenan-modified polyvinyl alcohol ionic hydrogel with physical cross-linked for multimodal sensing. Int. J. Biol. Macromol. 2023, 253, 126954. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Hou, Y.; Wang, X.; Li, X.; Chen, T.; Xu, X. Tough and stretchable all-κ-carrageenan hydrogel based on the cooperative effects between chain conformation transition and stepwise mechanical training. Carbohydr. Polym. 2023, 313, 120869. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, M.; Sun, D.; Hou, Y.; Li, Y.; Dong, T.; Wang, X.; Zhang, L.; Yang, W. Dual Physically Cross-Linked κ-Carrageenan-Based Double Network Hydrogels with Superior Self-Healing Performance for Biomedical Application. ACS Appl. Mater. Interfaces 2018, 10, 37544–37554. [Google Scholar] [CrossRef]
- Liu, F.; Duan, G.; Yang, H. Recent advances in exploiting carrageenans as a versatile functional material for promising biomedical applications. Int. J. Biol. Macromol. 2023, 235, 123787. [Google Scholar] [CrossRef]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. A Review of Property Enhancement Techniques for Carrageenan-based Films and Coatings. Carbohydr. Polym. 2019, 216, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Tranquilan-Aranilla, C.; Yoshii, F.; Rosa, A.M.D.; Makuuchi, K. Kappa-carrageenan–polyethylene oxide hydrogel blends prepared by gamma irradiation. Radiat. Phys. Chem. 1999, 55, 127–131. [Google Scholar] [CrossRef]
- Li, X.; Xiang, S.; Ling, D.; Zhang, S.; Li, C.; Dai, R.; Zhu, P.; Liu, X.; Pan, Z. Stretchable, self-healing, transparent macromolecular elastomeric gel and PAM/carrageenan hydrogel for self-powered touch sensors. Mater. Sci. Eng. B 2022, 283, 115832. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Wu, C.; Cong, Y.; Zhang, R.; Fu, J. Highly Sensitive Pressure and Strain Sensors Based on Stretchable and Recoverable Ion-Conductive Physically Cross-Linked Double-Network Hydrogels. ACS Appl. Mater. Interfaces 2020, 12, 51969–51977. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Xu, H.; Wu, Q.; Liu, C.; Yang, B.-R.; Gui, X.; Xie, X.; Tao, K.; Shen, Y.; et al. An intrinsically stretchable humidity sensor based on anti-drying, self-healing and transparent organohydrogels. Mater. Horizons 2019, 6, 595–603. [Google Scholar] [CrossRef]
- Liu, S.; Li, L. Ultrastretchable and Self-Healing Double-Network Hydrogel for 3D Printing and Strain Sensor. ACS Appl. Mater. Interfaces 2017, 9, 26429–26437. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Z.; Wei, Y.; Ding, H.; Li, Z.; Tao, K.; Wu, J. Anti-Freezing and Anti-Drying Organohydrogel Coated with Graphene for Highly Sensitive and Ultrastretchable Strain Sensing. In Proceedings of the 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Orlando, FL, USA, 20–24 June 2021; pp. 1231–1234. [Google Scholar] [CrossRef]
- Sun, H.; Li, S.; Li, K.; Liu, Y.; Tang, C.; Liu, Z.; Zhu, L.; Yang, J.; Qin, G.; Chen, Q. Tough and self-healable carrageenan-based double network microgels enhanced physical hydrogels for strain sensor. J. Polym. Sci. 2022, 60, 2720–2732. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, K.; Zhang, J.; Tang, J.; Jiang, H. Integration of high strength, resilience and stretchability into the nanocomposite hydrogel sensor for a wide working range detection and underwater sensing. J. Mater. Res. Technol. 2023, 24, 3524–3533. [Google Scholar] [CrossRef]
- Wang, L.; Fei, T.; Lou, Z.; Zhang, T. Three-dimensional hierarchical flowerlike α-Fe2O3 nanostructures: Synthesis and ethanol-sensing properties. ACS Appl. Mater. Interfaces 2011, 3, 4689–4694. [Google Scholar] [CrossRef]
- Park, H.J.; Hong, S.Y.; Chun, D.H.; Kang, S.W.; Park, J.C.; Lee, D.S. A highly susceptive mesoporous hematite microcube architecture for sustainable P-type formaldehyde gas sensors. Sensors Actuators B Chem. 2019, 287, 437–444. [Google Scholar] [CrossRef]
- Gardner, R.F.G.; Sweett, F.; Tanner, D.W. The electrical properties of alpha ferric oxide—I: The impure oxide. J. Phys. Chem. Solids 1963, 24, 1175–1181. [Google Scholar] [CrossRef]
- Chicot, D.; Mendoza, J.; Zaoui, A.; Louis, G.; Lepingle, V.; Roudet, F.; Lesage, J. Mechanical properties of magnetite (Fe3O4), hematite (α-Fe2O3) and goethite (α-FeO·OH) by instrumented indentation and molecular dynamics analysis. Mater. Chem. Phys. 2011, 129, 862–870. [Google Scholar] [CrossRef]
- Maleki, F.K.; Nasution, M.K.M.; Gok, M.S.; Maleki, V.A. An experimental investigation on mechanical properties of Fe2O3 microparticles reinforced polypropylene. J. Mater. Res. Technol. 2022, 16, 229–237. [Google Scholar] [CrossRef]
- Kulal, P.M.; Dubal, D.P.; Lokhande, C.D.; Fulari, V.J. Chemical synthesis of Fe2O3 thin films for supercapacitor application. J. Alloys Compd. 2011, 509, 2567–2571. [Google Scholar] [CrossRef]
- Gurlo, A.; Sahm, M.; Oprea, A.; Barsan, N.; Weimar, U. A p- to n-transition on α-Fe2O3-based thick film sensors studied by conductance and work function change measurements. Sens. Actuators B Chem. 2004, 102, 291–298. [Google Scholar] [CrossRef]
- Hao, Q.; Li, L.; Yin, X.; Liu, S.; Li, Q.; Wang, T. Anomalous conductivity-type transition sensing behaviors of n-type porous α-Fe2O3 nanostructures toward H2S. Mater. Sci. Eng. B 2011, 176, 600–605. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Cong, Y.; Zhang, H.; Xu, T.; Nie, L.; Fu, J. Ultrastretchable Strain Sensors and Arrays with High Sensitivity and Linearity Based on Super Tough Conductive Hydrogels. Chem. Mater. 2018, 30, 8062–8069. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, L.; Cao, L. Stimuli-responsive properties of double network hydrogels derived from κ-carrageenan and poly (acrylamide). Polym. Adv. Technol. 2024, 35, e6226. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Wu, Q.; Xiao, H.; Han, J. Self-Recovery, Fatigue-Resistant, and Multifunctional Sensor Assembled by a Nanocellulose/Carbon Nanotube Nanocomplex-Mediated Hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 50281–50297. [Google Scholar] [CrossRef]
- Kulkarni, S.; Khan, K.A.; Alhammadi, K.; Cantwell, W.J.; Umer, R. A visco-hyperelastic approach to model rate dependent compaction response of a 3D woven fabric. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107229. [Google Scholar] [CrossRef]
- Zaimis, U.; Ozolina, S.; Kukuskins, A. Recycled algae paper density control system for quality screening with adjustable light source wavelength. In Proceedings of the 21st International Scientific Conference on Engineering for Rural Development, Jelgava, Latvia, 25–27 May 2022; pp. 818–823. [Google Scholar]
- Ozolina, S.; Zaimis, U. Some aspects of extraction and application of seaweed furcellaria lumbricalis carrageenan in production of recycled paper. In Proceedings of the 21st International Scientific Conference on Engineering for Rural Development, Jelgava, Latvia, 25–27 May 2022; pp. 618–622. [Google Scholar]
- Therkelsen, G.H. Carrageenan. In Industrial Gums: Polysaccharides and Their Derivatives, 3rd ed.; Academic Press: Cambridge, MA, USA, 1993; pp. 145–180. [Google Scholar] [CrossRef]
- Šutinys, E.; Dzedzickis, A.; Samukaitė-Bubnienė, U.; Bučinskas, V. Novel synthetic iron (III) oxide-based force sensor. Sensors Actuators A Phys. 2021, 331, 113043. [Google Scholar] [CrossRef]
- Wu, X.; He, C.; Wu, Y.; Chen, X. Synergistic therapeutic effects of Schiff’s base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model. Biomaterials 2016, 75, 148–162. [Google Scholar] [CrossRef] [PubMed]





| Composition of Film | Gauge Factor | Elongation | Conductivity | Ref. |
|---|---|---|---|---|
| magnetic nanocomposite hydrogels: N, N-methylene bisacrylamide (MBA) + N, N, N′, N″, N″-pentamethyldiethylenetriamine bonded carrageenan | PAA/kCG—1.19 MPAA/kCG—1.41 | 8% 6% | [6] | |
| PVA. CaCl2, yota-carrageenan, | 1.33 | 20% | [19] | |
| K-CG/P(Aam-co-AAC) Fe3 + hydrogel from κ-carrageenan + acrylamide (AAm), acrylic acid (AAc), and acryloyl chloride | 0.78 to 2.8 | 1400% | 1.15 Sm−1 | [26] |
| κ-carrageenan + polyacrylamide (PAAm) double networks | 0.63 | 1000% | n/a | [29] |
| κ-carrageenan (K + C) microgel (MG) composite hydrophobically cross-linked polyacrylamide (HPAAm) gels or K + C-MG/HPAAm gels | 0.745 | n/a | n/a | [31] |
| Hydrogel based films | 0.6 to 11 | 100% | 8.14 ± 0.50 Sm−1 | [41] |
| rGO + polyacrylamide (PAM) + carrageenan dual network | 4.6 to 90.5 | n/a | n/a | [30] |
| acrylamide (AM) and N, N-dimethyl acrylamide (DMAA) (AMD gel) | 1.0 to 3.1 | 1400% | [32] | |
| polyacrylamide Li +/carrageenan | 1.83 | 200% to 567.7% | 1.9 Sm−1 | [42] |
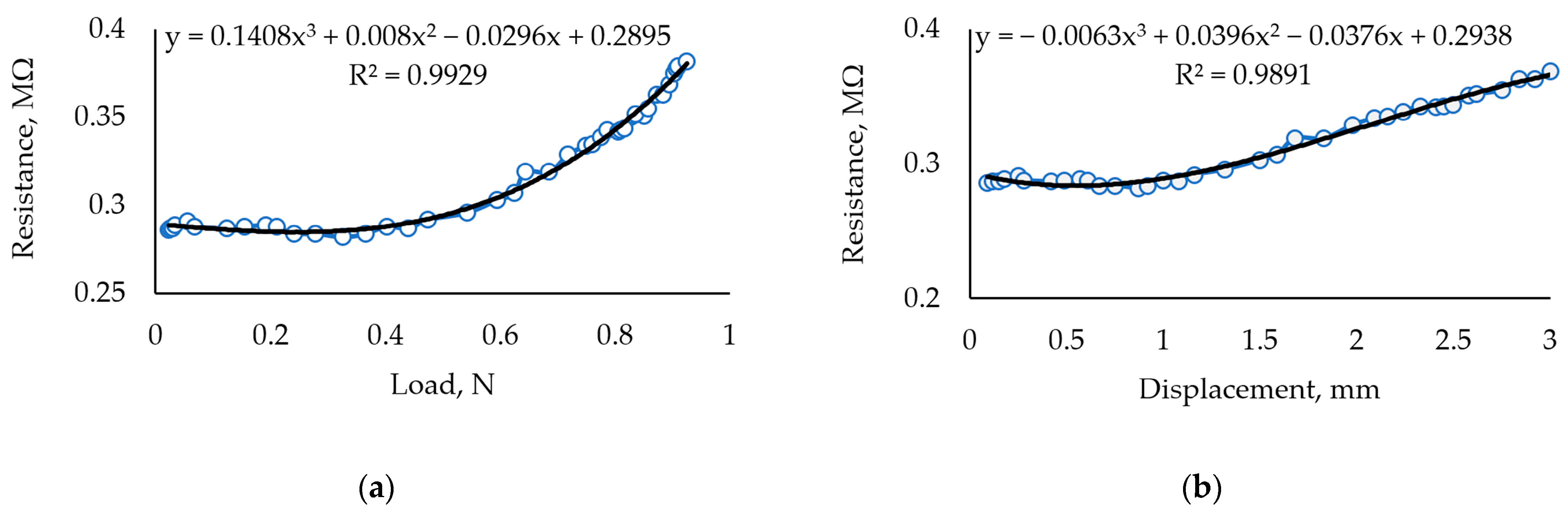
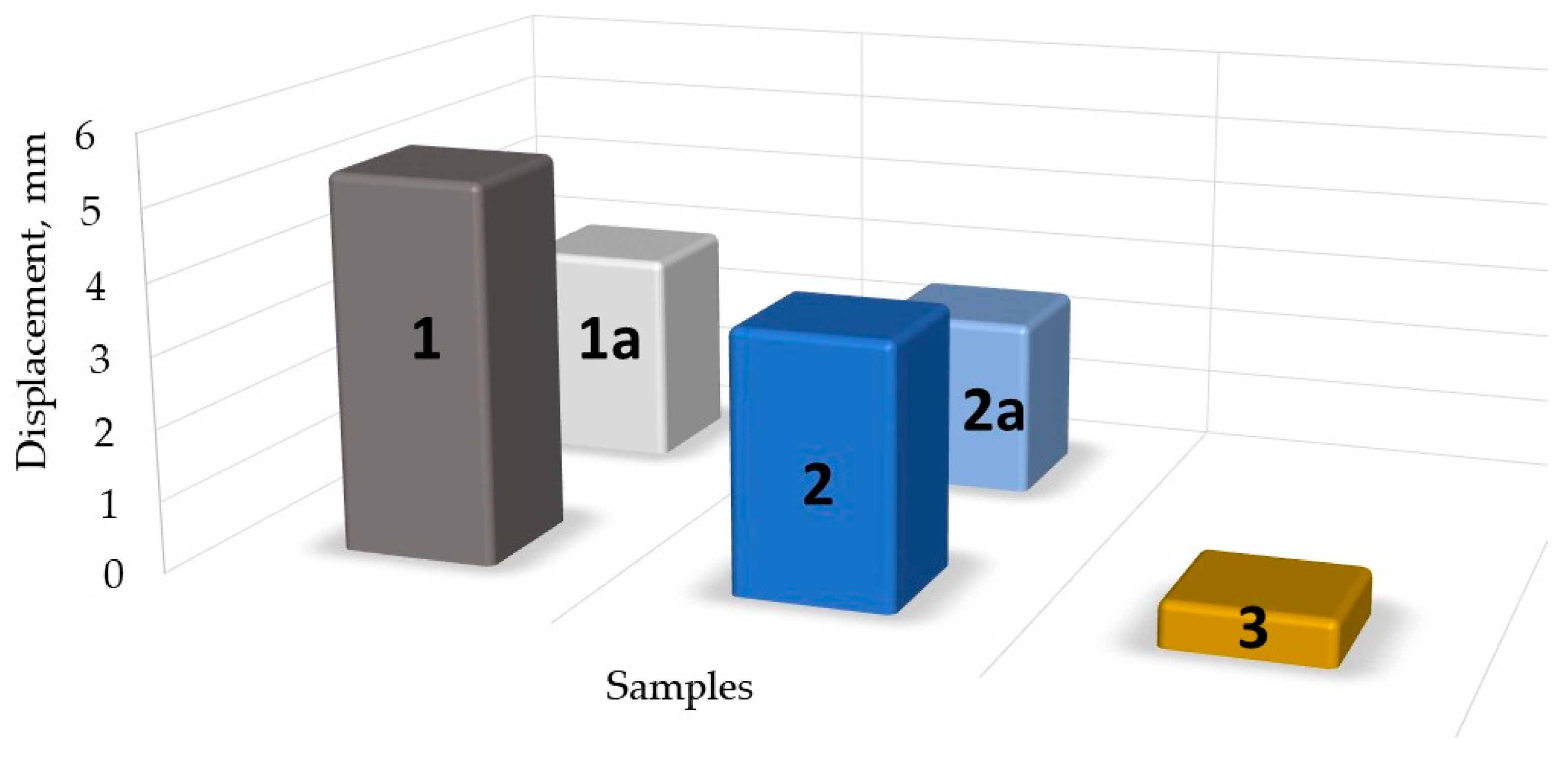
| Sample Info: Composition, (Figure Number) | Conductivity, mS | GF | Sensitivity, MΩ/N |
|---|---|---|---|
| 30 mL Crg + 0.5 mL GLc + 1 g Fe2O3 (Figure 6a,b) | 0.002 to 0.08, | 1.43 | 0.134 |
| 30 mL Crg + 0.5 mL Glc + 1 g Fe2O3 aged for 5 months (Figure 7a,b) | 0.104 | 0.67 | 0.104 |
| 30 mL Crg + 0.5 mL Glc + 0.05 g Fe2O3 (Figure 8a,b) | 2.10 to 0.64 | 10.472 | 0.221 |
| 30 mL Crg + 0.5 mL Glc+ 0.05 g Fe2O3, aged for 5 months (Figure 9a,b) | 2.6 to 2.1 | 3.12 | 0.225 |
| 30 mL Crg + 3 mL Glc + 3 g Fe2O3 (Figure 10a,b) | 0.0012 to 0.092 | 4.16 | 0.123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žaimis, U.; Petronienė, J.J.; Dzedzickis, A.; Bučinskas, V. Stretch Sensor: Development of Biodegradable Film. Sensors 2024, 24, 683. https://doi.org/10.3390/s24020683
Žaimis U, Petronienė JJ, Dzedzickis A, Bučinskas V. Stretch Sensor: Development of Biodegradable Film. Sensors. 2024; 24(2):683. https://doi.org/10.3390/s24020683
Chicago/Turabian StyleŽaimis, Uldis, Jūratė Jolanta Petronienė, Andrius Dzedzickis, and Vytautas Bučinskas. 2024. "Stretch Sensor: Development of Biodegradable Film" Sensors 24, no. 2: 683. https://doi.org/10.3390/s24020683
APA StyleŽaimis, U., Petronienė, J. J., Dzedzickis, A., & Bučinskas, V. (2024). Stretch Sensor: Development of Biodegradable Film. Sensors, 24(2), 683. https://doi.org/10.3390/s24020683








