A Small Highly Sensitive Glucose Sensor Based on a Glucose Oxidase-Modified U-Shaped Microfiber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
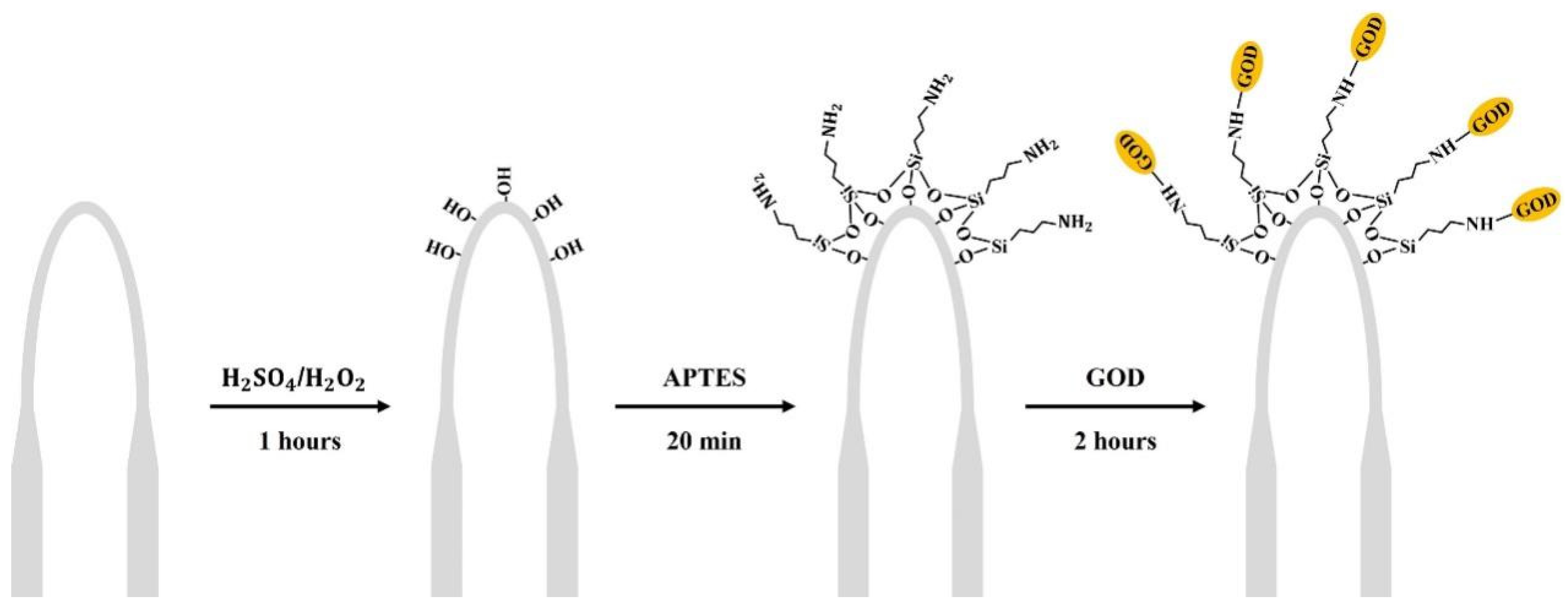
2.2. U-Shaped Microfiber Fabrication and Surface Functionalization
2.3. Characterizations of the Sensor
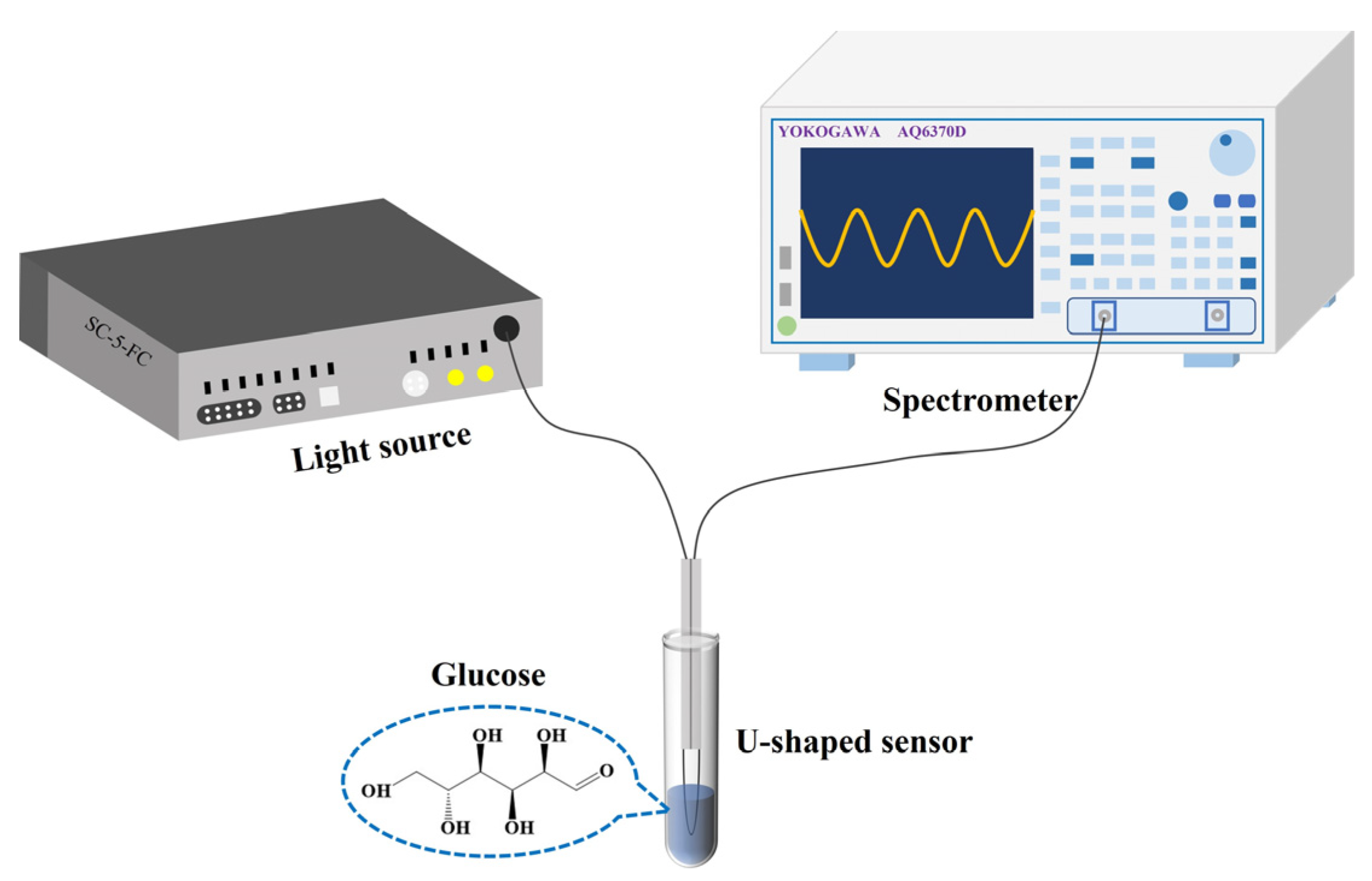
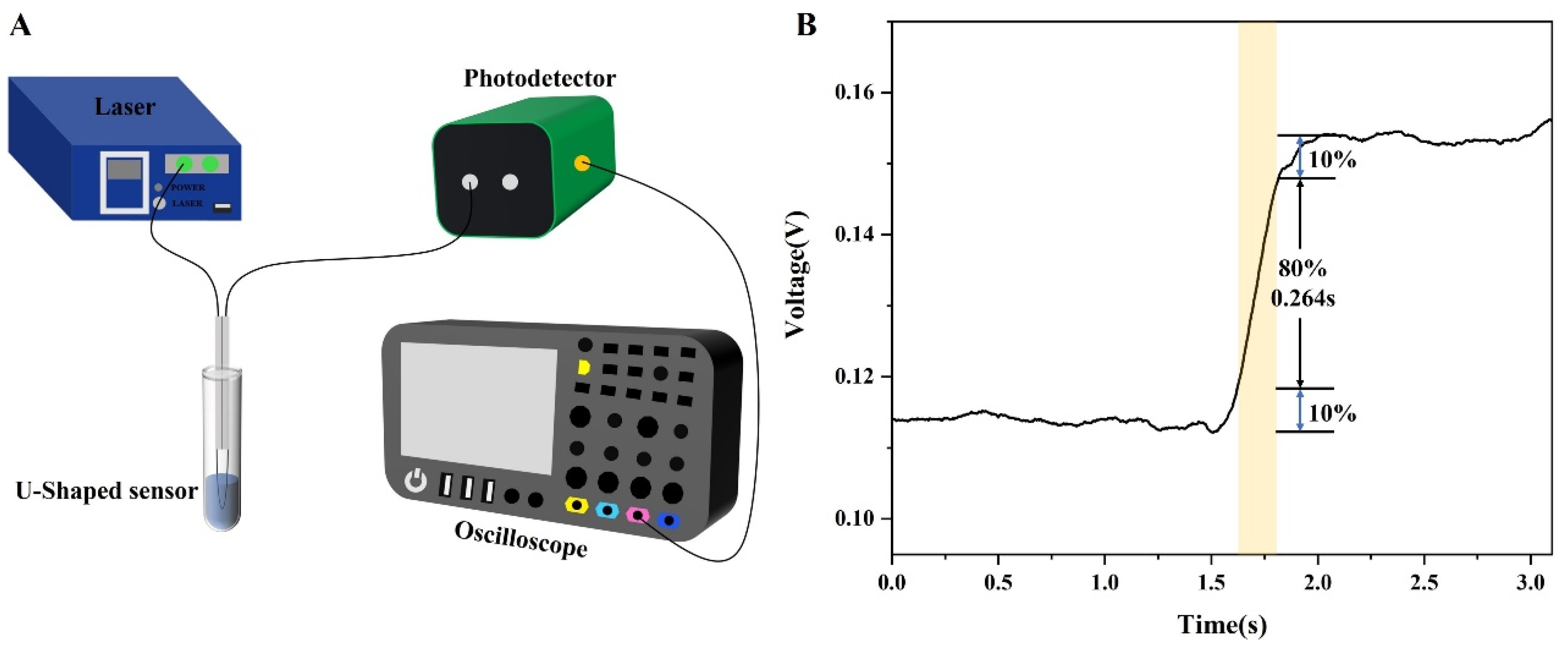
2.4. Experimental System
2.5. Theoretical Analysis
3. Results
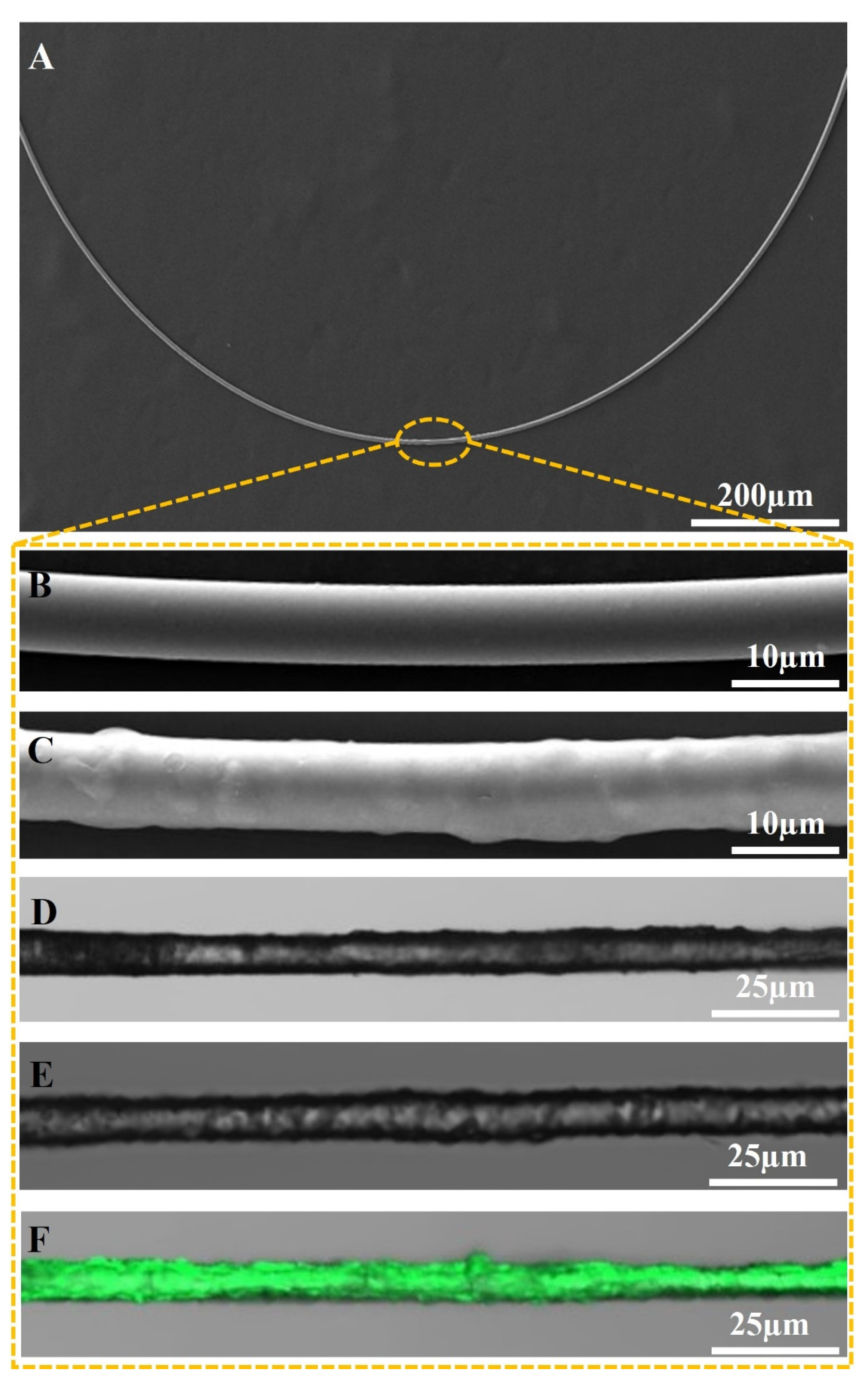
3.1. Morphology Analysis of the Sensor
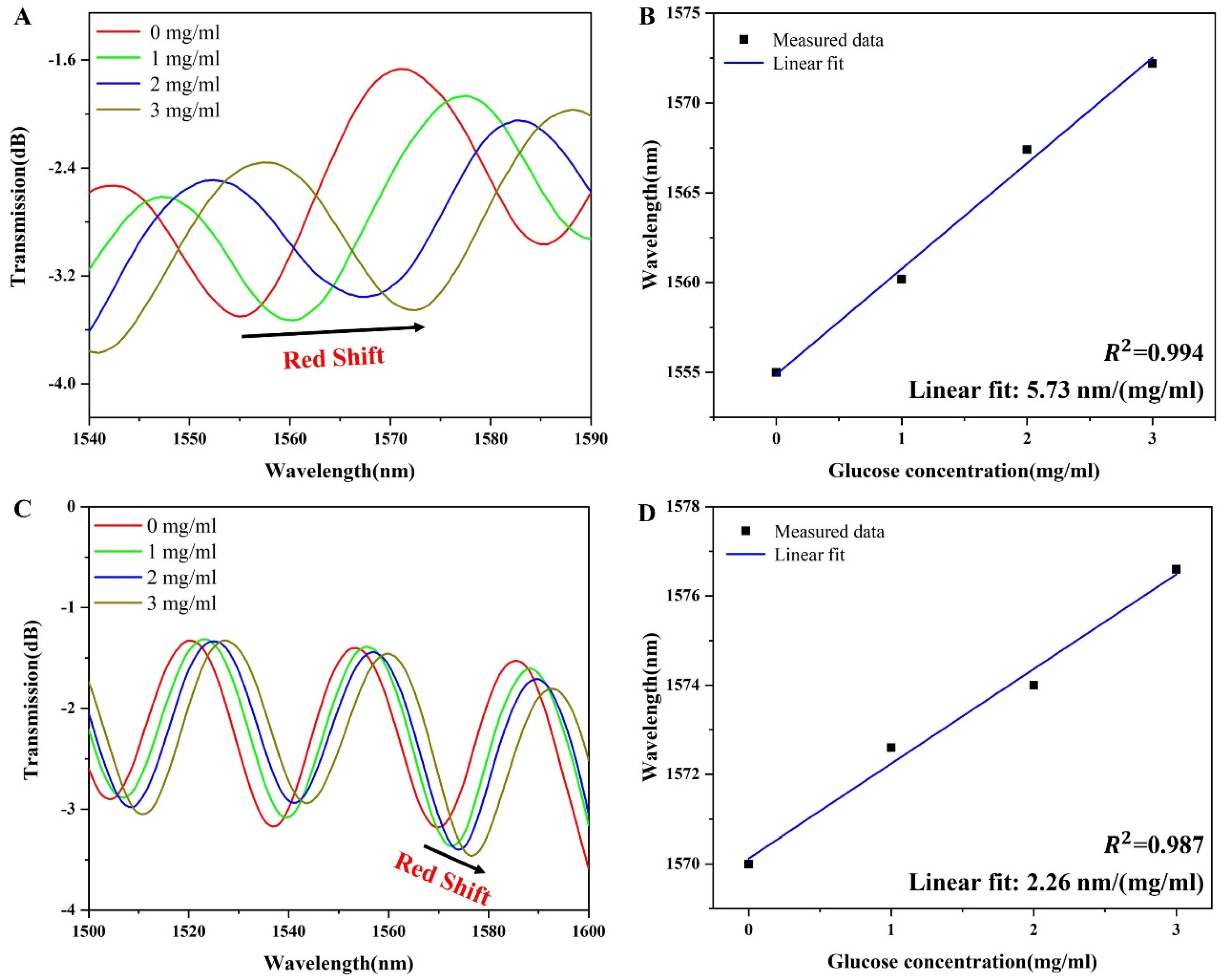
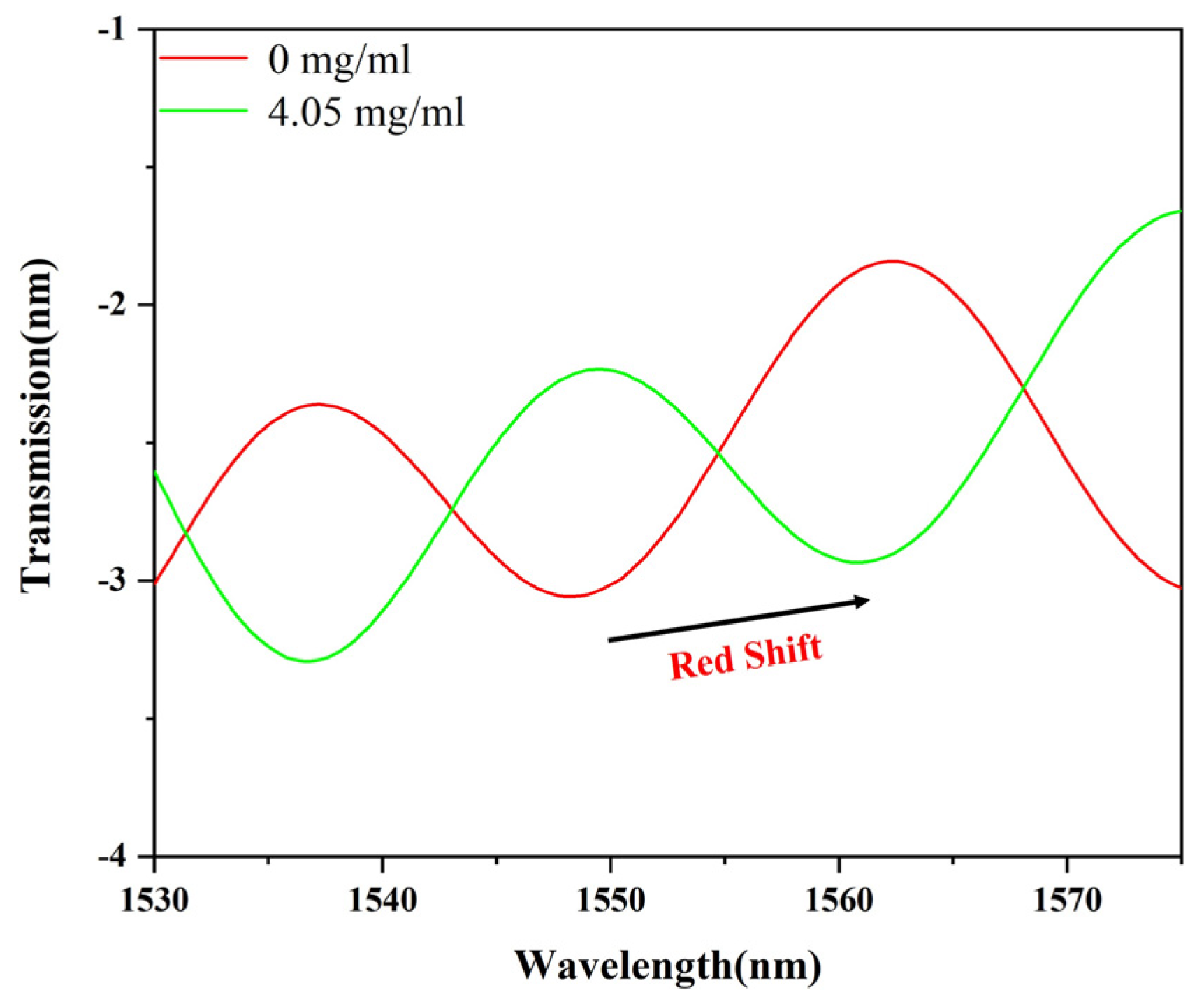
3.2. Sensing Performance of the Sensor
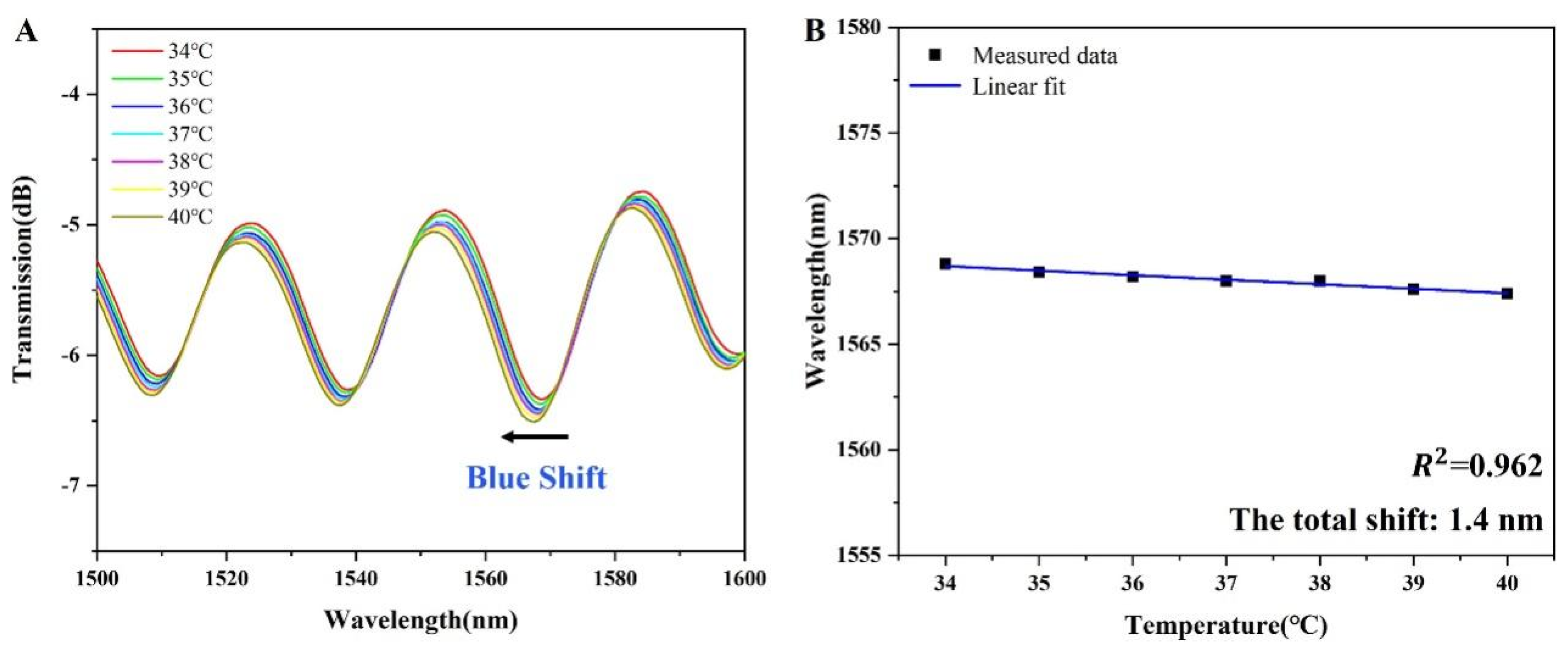
3.3. Stability and Temperature Cross-Sensitivity Analysis of the Sensor
3.4. Specificity and Dynamic Response Analysis of the Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Donnelly, R.F.; Wael, K.D. Wearable hollow microneedle sensing patches for the transdermal electrochemical monitoring of glucose. Talanta 2022, 249, 123695. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Bugga, P.; Madison, L.R.; Henry, A.I.; Blaber, M.G.; Greeneltch, N.G.; Chiang, N.; Mrksich, M.; Schatz, G.C.; Van Duyne, R.P. Bisboronic acids for selective, physiologically relevant direct glucose sensing with surfaceenhanced Raman spectroscopy. J. Am. Chem. Soc. 2016, 138, 13952–13959. [Google Scholar] [CrossRef] [PubMed]
- Quesada-González, D.; Merkoçi, A. Nanomaterial-based devices for point-of-care diagnostic applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef] [PubMed]
- Gorle, D.B.; Ponnada, S.; Kiai, M.S.; Nair, K.K.; Nowduri, A.; Swart, H.C.; Ang, E.H.; Nanda, K.K. Review on recent progress in metal-organic framework-based materials for fabricating electrochemical glucose sensors. J. Mater. Chem. B 2021, 9, 7927–7954. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Liu, P.; Yu, D.G.; Ge, R. Electrospun nanofiber-based glucose sensors for glucose detection. Front. Chem. 2022, 10, 944428. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Yu, J.; Lei, Y.T.; Lu, S.J.; Wang, C.H.; Liu, D.X.; Guo, Q.C. Synthesis and characterization of nickel oxide hollow spheres–reduced graphene oxide–nafion composite and its biosensing for glucose. Sensors Actuat. B. Chem. 2015, 208, 90–98. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.; Chu, S.; Qian, S.; Wang, F.; Masson, J.F.; Han, X.; Peng, W. Fiber-optic surface plasmon resonance glucose sensor enhanced with phenylboronic acid modified Au nanoparticles. Biosens. Bioelectron. 2018, 117, 637–643. [Google Scholar] [CrossRef]
- Zheng, W.L.; Zhang, Y.N.; Li, L.K.; Li, X.G.; Zhao, Y. A plug-and-play optical fiber SPR sensor for simultaneous measurement of glucose and cholesterol concentrations. Biosens. Bioelectron. 2022, 198, 113798. [Google Scholar] [CrossRef]
- Tang, S.; Zou, M.; Zhao, C.; Jiang, Y.; Chen, R.; Xu, Z.; Yang, C.; Wang, X.; Dong, B.; Wang, Y.; et al. Fabry-Perot Interferometer Based on a Fiber-Tip Fixed-Supported Bridge for Fast Glucose Concentration Measurement. Biosensors 2022, 12, 391. [Google Scholar] [CrossRef]
- Fang, Y.L.; Wang, C.T.; Chiang, C.C. A Small U-Shaped Bending-Induced Interference Optical Fiber Sensor for the Measurement of Glucose Solutions. Sensors 2016, 16, 1460. [Google Scholar] [CrossRef]
- Gong, P.; Li, X.; Zhou, X.; Zhang, Y.; Chen, N.; Wang, S.; Zhang, S.; Zhao, Y. Optical fiber sensors for glucose concentration measurement: A review. Opt. Laser Technol. 2021, 139, 106981. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent advancements in optical biosensors for cancer detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef]
- Li, B.; Yan, X.; Zhang, X.; Wang, F.; Li, S.; Suzuki, T.; Ohishi, Y.; Cheng, T. No-core optical fiber sensor based on surface plasmon resonance for glucose solution concentration and temperature measurement. Opt. Express 2021, 29, 12930. [Google Scholar] [CrossRef] [PubMed]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef]
- Lukas, H.; Xu, C.; Yu, Y.; Gao, W. Emerging Telemedicine Tools for Remote COVID-19 Diagnosis, Monitoring, and Management. ACS Nano 2020, 14, 16180–16193. [Google Scholar] [CrossRef]
- Phan, L.M.T.; Vo, T.A.T.; Hoang, T.X.; Selvam, S.P.; Pham, H.L.; Kim, J.Y.; Cho, S. Trending Technology of Glucose Monitoring during COVID-19 Pandemic: Challenges in Personalized Healthcare. Adv. Mater. Technol. 2021, 6, 2100020. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.J.Y.; Ng, S.M.; Stoddart, P.R.; Chua, H.S. Theoretical Model and Design Considerations of U-Shaped Fiber Optic Sensors: A Review. IEEE Sens. J. 2020, 20, 14578–14589. [Google Scholar] [CrossRef]
- Danny, C.G.; Raj, M.D.; Sai, V.V.R. Investigating the refractive index sensitivity of U-bent fiber optic sensors using ray optics. J. Light. Technol. 2020, 38, 1580–1588. [Google Scholar] [CrossRef]
- Dong, J.; Sang, M.; Wang, S.; Xu, T.; Yu, X.; Liu, T. Ultrasensitive Label-Free Biosensor Based on the Graphene-Oxide-Coated-U-Bent Long-Period Fiber Grating Inscribed in a Two-Mode Fiber. J. Light. Technol. 2021, 39, 4013–4019. [Google Scholar] [CrossRef]
- Tang, Z.; Gomez, D.; He, C.; Korposh, S.; Morgan, S.P.; Correia, R.; Hayes-Gill, B.; Setchfield, K.; Liu, L. A U-Shape Fibre-Optic pH Sensor Based on Hydrogen Bonding of Ethyl Cellulose with a Sol-Gel Matrix. J. Light. Technol. 2021, 39, 1557–1564. [Google Scholar] [CrossRef]
- Wu, Q.L.; Zhao, Y.; Zhang, Y.N.; Yang, Y. High sensitive applied load measurement using optical fiber tapered-loop probe with SPR effect. Opt. Laser Technol. 2019, 114, 95–102. [Google Scholar] [CrossRef]
- Gupta, B.; Sharma, N.K. Fabrication and characterization of U-shaped fiber-optic pH probes. Sensors Actuat. B. Chem. 2002, 82, 89–93. [Google Scholar] [CrossRef]
- Wen, H.Y.; Hsu, H.C.; Tsai, Y.T.; Feng, W.K.; Lin, C.L.; Chiang, C.C. U-Shaped Optical Fiber Probes Coated with Electrically Doped GQDs for Humidity Measurements. Polymers 2021, 13, 2696. [Google Scholar] [CrossRef]
- Zhu, H.T.; Zhan, L.W.; Dai, Q.; Xu, B.; Chen, Y.; Lu, Y.Q.; Xu, F. Self-Assembled Wavy Optical Microfiber for Stretchable Wearable Sensor. Adv. Opt. Mater. 2021, 9, 2002206. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, Y.; Chen, M.Q.; Xia, F.; Tong, R.J. U-shaped microfiber coupler coated with polyvinyl alcohol film for highly sensitive humidity detection. Sensors Actuat. A Phys. 2019, 285, 628–636. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, H.; Xia, H.; Luo, H.; Xie, K. U-shaped microfiber sensor coated with PVA nanofibers for the simultaneous measurement of humidity and temperature. Sensors Actuat. B. Chem. 2023, 378, 133203. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, Y.; Chen, M.Q.; Tong, R.J. Humidity sensor based on unsymmetrical U-shaped microfiber with apolyvinyl alcohol overlay. Sensors Actuat. B. Chem. 2018, 263, 312–318. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Y.; Hu, X.G.; Chen, M.Q. Humidity sensor based on unsymmetrical U-shaped twisted microfiber coupler with wide detection range. Sensors Actuat. B. Chem. 2019, 290, 406–413. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Liu, J.; Yuan, J.; Chan, H.P.; Wu, T.; Wang, M.Y.; Wan, S.P.; He, X.D.; Wu, Q. U-shape panda polarization-maintaining microfiber sensor coated with graphene oxide for relative humidity measurement. J. Light. Technol. 2021, 39, 6308–6314. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.N.; Zheng, W.; Lv, R.; Zhao, Y. Dual-channel in-fiber SPR sensor for simultaneous and highly sensitive measurement of salinity and temperature. Opt. Lett. 2023, 48, 952–955. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Zhang, X.; Wang, S.; Xia, Z.; Guo, X.; Zhao, Y.; Wang, P.; Wang, X.H. Microstructured Optical Fiber-Enhanced Light-Matter Interaction Enables Highly Sensitive Exosome-Based Liquid Biopsy of Breast Cancer. Anal. Chem. 2023, 95, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Brice, I.; Grundsteins, K.; Atvars, A.; Alnis, J.; Viter, R.; Ramanavicius, A. Whispering gallery mode resonator and glucose oxidase based glucose biosensor. Sensors Actuat. B. Chem. 2020, 318, 128004. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, M.; Lu, L.; Lou, X.; Dong, M.; Zhu, L. Metal-organic framework/enzyme coated optical fibers as waveguide-based biosensors. Sensors Actuat. B. Chem. 2019, 288, 12–19. [Google Scholar] [CrossRef]
- Chen, K.C.; Li, Y.L.; Wu, C.W.; Chiang, C.C. Glucose Sensor Using U-Shaped Optical Fiber Probe with Gold Nanoparticles and Glucose Oxidase. Sensors 2018, 18, 1217. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.S.; Duerkop, A.; Wolfbeis, O.S. Optical methods for sensing glucose. Chem. Soc. Rev. 2011, 40, 4805–4839. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Tiwari, U.; Kumar, P.; Mishra, V.; Jain, S.C.; Singh, N.; Kapur, P.; Bharadwaj, L.M. Immobilization of enzyme on long period grating fibers for sensitive glucose detection. Biosens. Bioelectron. 2012, 33, 190–195. [Google Scholar] [CrossRef]
- Yu, S.; Ding, L.; Lin, H.; Wu, W.; Huang, J. A novel optical fiber glucose biosensor based on carbon quantum dots-glucose oxidase/cellulose acetate complex sensitive film. Biosens. Bioelectron. 2019, 146, 111760. [Google Scholar] [CrossRef]
- Xu, B.; Huang, J.; Ding, L.; Cai, J. Graphene oxide-functionalized long period fiber grating for ultrafast labelfree glucose biosensor. Mat. Sci. Eng. C Mater. 2020, 107, 110329. [Google Scholar] [CrossRef]
- Zhang, J.; Mai, X.; Hong, X.; Chen, Y.; Li, X. Optical fiber SPR biosensor with a solid-phase enzymatic reaction device for glucose detection. Sensors Actuat. B. Chem. 2022, 366, 131984. [Google Scholar] [CrossRef]
- Ran, Y.; Long, J.; Xu, Z.; Yin, Y.; Hu, D.; Long, X.; Zhang, Y.; Liang, L.; Liang, H.; Guan, B.O. Harmonic optical microfiber Bragg grating immunosensor for the accelerative test of cardiac biomarker (cTn-I). Biosens. Bioelectron. 2022, 179, 113081. [Google Scholar] [CrossRef]
- Luo, B.; Yan, Z.; Sun, Z.; Li, J.; Zhang, L. Novel glucose sensor based on enzyme-immobilized 81° tilted fiber grating. Opt. Express 2014, 22, 30571. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, X.; Gong, D.; Liu, F.; Hu, W.; Cai, W.; Huang, J.; Yang, M. Investigation for terminal reflection optical fiber SPR glucose sensor and glucose sensitive membrane with immobilized GODs. Opt. Express 2017, 25, 3884–3898. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Han, B.; E, S.; Sun, Y.; Li, X.; Cai, Y.; Zhang, Y.N. Highly-sensitive and reflective glucose sensor based on optical fiber surface plasmon resonance. Microchem. J. 2020, 157, 105010. [Google Scholar] [CrossRef]
- Sun, D.; Sun, L.P.; Guo, T.; Guan, B.O. Label-Free Thrombin Detection Using a Tapered Fiber-Optic Interferometric Aptasensor. J. Light. Technol. 2019, 37, 2756–2761. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Xu, S.; Sun, D.; Fu, Y. Relative humidity sensor based on U-shaped microfiber interferometer coated with MoS2 films. Mater. Lett. 2021, 301, 130245. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, P.; Liang, H.; Xiao, A.; Zeng, S.; Guan, B.O. Nucleic acid hybridization on a plasmonic nanointerface of optical microfiber enables ultrahigh-sensitive detection and potential photothermal therapy. Biosens. Bioelectron. 2020, 156, 112147. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, C.; Yu, R.; Hu, Z.; Xiao, L. Graphitic Carbon Nitride for Enhancing Humidity Sensing of Microfibers. J. Light. Technol. 2021, 39, 3896–3902. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.; Gan, L.; Liu, Q.; Yan, Z.; Liu, D.; Sun, Q. Immobilized optical fiber microprobe for selective and high sensitiveglucose detection. Sensors Actuat. B. Chem. 2018, 255, 3004–3010. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, K.; Wang, C.; Sun, Q.; Yin, G.; Tai, Z.; Wilson, K.; Zhao, J.; Zhang, L. Label-free glucose biosensor based on enzymatic grapheneoxide-functionalized tilted fiber grating. Sensors Actuat. B. Chem. 2018, 254, 1033–1039. [Google Scholar] [CrossRef]
- Wu, S.; Tan, Q.; Forsberg, E.; Hu, S.; He, S. In-situ dual-channel surface plasmon resonance fiber sensor for temperature-compensated detection of glucose concentration. Opt. Express 2020, 28, 21046. [Google Scholar] [CrossRef]


| Sample | Sensing Element | Glucose Sensitivity (nm/(mg/mL)) | Measurement Range (mg/mL) | LOD | Ref. |
|---|---|---|---|---|---|
| 1 | Microfiber with GOD | 1.74 | 0–3 | / | [48] |
| 2 | Fiber SPR with PS/GOD | 0.31 | 0–4 | / | [39] |
| 3 | TFG with GO/GOD | 1.34 | 0–1.5 | / | [49] |
| 4 | Side-hole fiber SPR with GOD | 34.20 | 0–0.3 | 16.24 μM | [50] |
| 5 | U-shaped microfiber with GOD | 5.73 | 0–3 | 0.17 mg/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Jiang, H.; Xie, K.; Xia, H. A Small Highly Sensitive Glucose Sensor Based on a Glucose Oxidase-Modified U-Shaped Microfiber. Sensors 2024, 24, 684. https://doi.org/10.3390/s24020684
Chen T, Jiang H, Xie K, Xia H. A Small Highly Sensitive Glucose Sensor Based on a Glucose Oxidase-Modified U-Shaped Microfiber. Sensors. 2024; 24(2):684. https://doi.org/10.3390/s24020684
Chicago/Turabian StyleChen, Tingkuo, Haiming Jiang, Kang Xie, and Hongyan Xia. 2024. "A Small Highly Sensitive Glucose Sensor Based on a Glucose Oxidase-Modified U-Shaped Microfiber" Sensors 24, no. 2: 684. https://doi.org/10.3390/s24020684
APA StyleChen, T., Jiang, H., Xie, K., & Xia, H. (2024). A Small Highly Sensitive Glucose Sensor Based on a Glucose Oxidase-Modified U-Shaped Microfiber. Sensors, 24(2), 684. https://doi.org/10.3390/s24020684





