Abstract
The MXene Ti3C2Tx was synthesized using hydrofluoric acid and an improved multilayer method in this study. Subsequently, a Bi2O3/Ti3C2Tx composite material was produced through hydrothermal synthesis. This composite boasts a unique layered structure, offering a large surface area that provides numerous contact and reaction sites, facilitating the adsorption of ammonia on its surface. The prepared Bi2O3/Ti3C2Tx-based sensor exhibits excellent sensing performance for ammonia gas, including high responsiveness, good repeatability, and rapid response–recovery time. The sensor’s response to 100 ppm ammonia gas is 61%, which is 11.3 times and 1.6 times the response values of the Ti3C2Tx gas sensor and Bi2O3 gas sensor, with response/recovery times of 61 s/164 s at room temperature, respectively. Additionally, the gas sensitivity mechanism of the Bi2O3/Ti3C2Tx-based sensor was analyzed, and the gas sensing response mechanism was proposed. This study shows that the sensor can effectively enhance the accuracy and precision of ammonia detection at room temperature and has a wide range of application scenarios.
1. Introduction
Ammonia, a colorless gas with a strong, pungent odor at room temperature, finds extensive use in the electronics, food, and chemical industries, as well as in scientific research. When released into the atmosphere, ammonia reacts with nitrogen and sulfur oxides, contributing to smog formation, which reduces visibility and poses severe health risks, particularly to the respiratory and cardiovascular systems [1]. Prolonged exposure to low concentrations of ammonia can cause significant health issues, including burns to the eyes and skin, respiratory damage, and even death [2,3]. Additionally, ammonia is a natural metabolic byproduct in the human body. Elevated levels of ammonia in exhaled breath can indicate liver, kidney, or lung diseases [4,5]. For instance, patients with end-stage renal disease (ESRD) often have an average exhaled ammonia concentration exceeding 4.88 ppm [6]. Therefore, developing gas-sensitive materials with large specific surface areas and high carrier mobility [7,8] for precise ammonia detection at room temperature is crucial for health and safety.
Various materials have been developed for ammonia detection, including metal oxides, conductive polymers, and carbon-based materials [9,10,11,12]. Metal oxides like zinc oxide, copper oxide, and tin oxide are commonly used due to their good response, sensitivity, and selectivity. For example, Bhardwaj et al. tested SnO2, and Meng et al. tested TiO2 for gas sensitivity [2,3]. Although these materials show good gas sensitivity, they require high operating temperatures, making precise room temperature detection challenging. Hence, there is a need to develop high-performance ammonia sensors capable of accurate detection at room temperature.
Metal oxide semiconductors are gaining attention for their excellent bandgap, high electron mobility, and two-dimensional structure and are increasingly used in gas-sensitive material development [13,14]. MXene, a two-dimensional compound composed of transition metal carbides, carbonitrides, or nitrides, has a high specific surface area, abundant active sites, and excellent conductivity, making it promising for gas sensor applications [15,16,17]. Discovered by Naguib et al. in 2011 [18], MXene has since been widely researched and applied in ammonia detection. Lu et al. successfully prepared a MXene/Na2Ti3O7 @PANI-composite-based gas sensor that is highly sensitive (185.44%) to 100 ppm NH3 at 45% RH, and a relative response of 283.8% was obtained at 90% RH along with a low detection limit of 186 ppb [19]. Wang et al. prepared a Ti3C2 MXene multilayer and TiO2 using a novel preparation technique based on the liquid-phase deposition of (NH4)2TiF6. The UOFEs coated with Ti3C2 MXene/TiO2 hybrid films were 12-fold more sensitive to RI with a maximum RI response value of 943–1056%/RIU than bare fibers [20]. Yu et al. successfully obtained superior NH3 sensors with high response and stability by wrapping the SnO2 nanoparticles on Ti3C2Tx MXene nanosheets via a facile hydrothermal method, whose observed response, rapid response, and recovery times were 109, 342 s, and 75% for 500 ppm NH3 gas, respectively [21]. Hou et al. prepared a MXene Ti3C2Tx-TiO2-CuO by one-step in situ oxidation method using Cu(NO3)2•6H2O as a precursor, which exhibited an improved NH3 gas sensing response of 56.9 toward 100 ppm NH3 under ultraviolet (UV) light exposure at room temperature [22]. To enhance sensor performance, heterostructured materials with unique physical and chemical properties have become a research focus in nanomaterials and composite materials [23]. Bi2O3, with its large bandgap and surface area [24], is effective in detecting organic compounds, metal ions, humidity, and gases [25,26,27,28,29]. However, there are no reports on the ammonia detection performance of Bi2O3-modified Ti3C2Tx MXene materials.
In this study, MXene Ti3C2Tx was synthesized using hydrofluoric acid and an improved multilayer method, and Bi2O3/Ti3C2Tx nanocomposites were synthesized via hydrothermal methods. The material’s composition, crystalline structure, and appearance were analyzed using XRD, SEM, and XPS techniques. The results indicate that Bi2O3/Ti3C2Tx nanocomposites exhibit high response values to ammonia gas, good repeatability, and rapid response and recovery times, making them promising for ammonia detection applications in agricultural, industrial, medical, health care, and other fields.
2. Experiments
Ti3C2Tx was prepared by removing the Al layer of the MAX phase (Jilin 11 Technology Co., Ltd., Jilin, China). Bismuth nitrate pentahydrate (AR, 99%) was purchased from McLean Biochemical (Shanghai Technology Co., Ltd., Shanghai, China). HF aqueous solution (AR, ≥40%), Ammonia aqueous (AR), and Sodium Hydroxide (ACS, K ≤ 0.02%, ≥97.0% (T), Falkes) were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Anhydrous ethanol, formaldehyde, acetone, dimethylformamide, methanol, and glacial acetic acid were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
2.1. Synthesis of Multilayer Ti3C2Tx MXene
Using the improved method from Alhabeb et al. [30], the multilayer Ti3C2Tx was prepared. To achieve this, the desired multilayer MXene was produced by etching Ti3AlC2 with HF. A total of 1 g of MAX phase material was slowly (over 10–15 min) added to 20 mL of hydrofluoric acid. The mixture was stirred continuously for 24 h with a magnetic stirrer at 500 r/min and 35 °C. After stirring, the resulting black suspension was collected and centrifuged at 3500 r/min with deionized water. This centrifugation was repeated until the supernatant reached a pH value close to 6. The supernatant was then separated, and the sediment was collected. Then, the sediment was dried under vacuum at 60 °C for 12 h, producing multilayer Ti3C2Tx MXene.
2.2. Synthesis of Bi2O3/Ti3C2Tx Nanocomposites
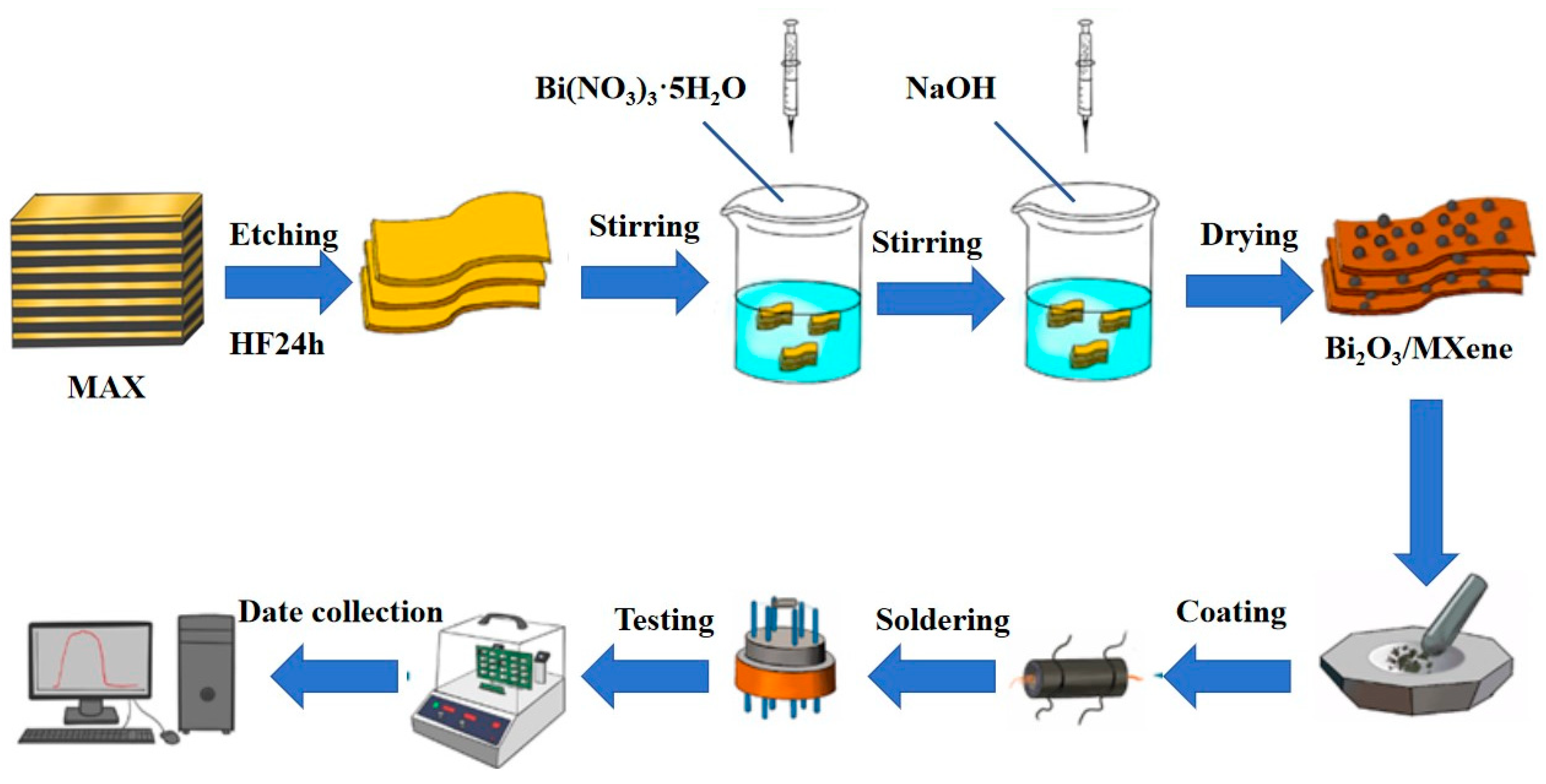
The Bi2O3/Ti3C2Tx nanocomposites were synthesized using a hydrothermal method. 500 mg of Bi(NO3)3•5H2O was accurately measured and mixed with 10 mL of diluted nitric acid solution, ensuring complete dissolution through stirring. A total of 100 mg of multilayer Ti3C2Tx was then gradually added to this solution while maintaining stirring for over 30 min to ensure complete integration. This mixture was subsequently added to a 5 mol/L NaOH solution and stirred at 500 r/min and 80 °C for 30 min. The resulting precipitate was collected by centrifugation and dried under vacuum at 60 °C for 12 h to obtain Bi2O3/Ti3C2Tx nanocomposites. Different loading ratios were prepared using the same method, and the products were labeled BO/M-4, BO/M-5, and BO/M-6 for differentiation. Pure Bi2O3 was prepared using the same method without adding multilayer Ti3C2Tx. Figure 1 illustrates the detailed experimental procedure.
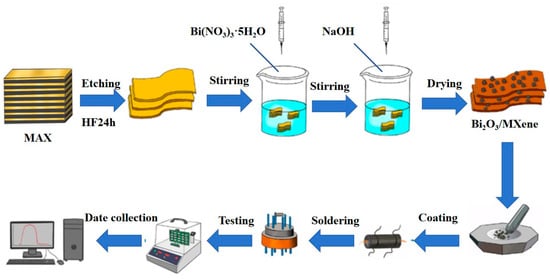
Figure 1.
A flowchart of the experimental procedure.
2.3. Material Characterization
The crystal structures, valency, and microscopic morphology of the as-prepared samples were investigated using X-ray diffraction (XRD, X’Pert PRO, Malvern Panalytical Ltd., Almelo, The Netherlands) with Cu Kα radiation (λ = 1.5442 Å), X-ray photoelectric spectroscopy (XPS, PHI-5300, Perkin Elmer, Waltham, MA, USA) with a monochromatic Al Kα radiation (1486.6 eV), and scanning electron microscope (SEM, Gemini SEM 300, ZEISS, Oberkochen, Germany), respectively. During the analysis process, the XRD detection parameters are set as a current density of 40 mA, a tube voltage of 40 kV, with the scan angle ranging from 5° to 90°, and a scan rate of 10° per minute.
2.4. Fabrication and Measurement of Gas Sensor
A total of 20 mg of Bi2O3/Ti3C2Tx nanocomposites was added to an appropriate amount of absolute ethanol, and the mixture was sonicated for 2 min until the composite material was sufficiently dissolved. Then, it was uniformly coated in thickness to Al2O3 ceramic tubes with Au electrodes. The dried ceramic tube, which had been vacuum dried at 80 °C for 6 h, was welded to the base correspondingly. After that, the prepared sensor was aged for 6 h using a WS-30B gas-sensitive element test system (Zhengzhou Wensen Electronic Technology Co., Ltd., Zhengzhou, China) to ensure its stability and reliability. As shown in Figure 1, the gas-sensitive performance experiments of the aged sensor were performed at room temperature (25 °C) by WS-30B. Additionally, pure Bi2O3-based and Ti3C2Tx-based sensors were tested using the same methodology for comparison, respectively.
The gas-sensitive response of the sensors to common hazardous gases, including NH3, C2H5OH, CH3OH, DMF, CH3COOH, HCHO, and CH3COCH3, was tested, respectively, at 25 °C. After obtaining a stable baseline, the tested gases were injected into the WS-30B chamber with a microsyringe, and the evaporation was accelerated by heating. After a period of adsorption, a stable baseline was obtained again. As a typical N-type semiconductor, the response value (Rs) of the Bi2O3/Ti3C2Tx-based sensor to the detected gas can be defined by Equation (1):
where Ra and Rg denote the resistance of the sensor in pure air versus in a detected gas environment, respectively.
Rs = |Ra − Rg|/Ra × 100%
3. Results
3.1. Characterization Results
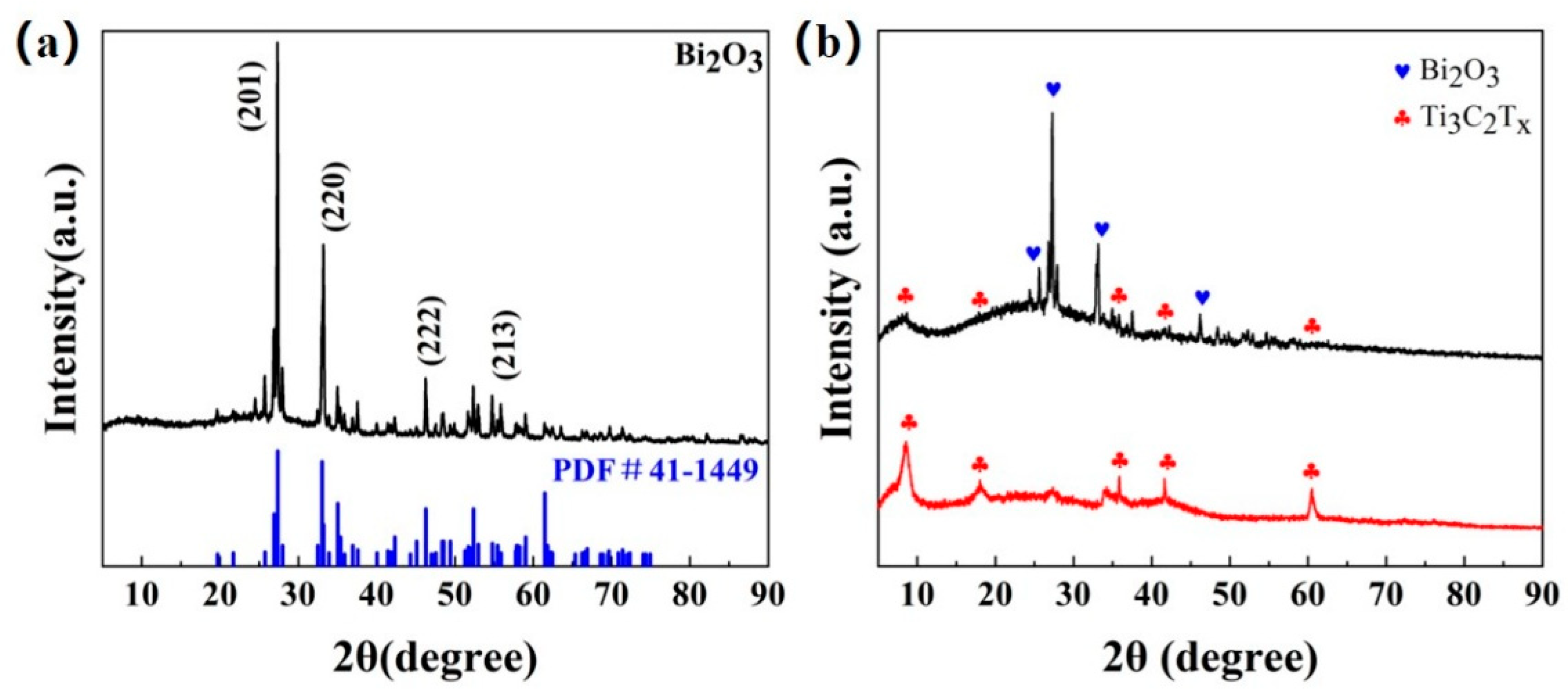
The XRD patterns of Bi2O3, Ti3C2Tx MXene, and Bi2O3/Ti3C2Tx are shown in Figure 2a and Figure 2b, respectively. It can be observed that the diffraction peaks of the Bi2O3-based sample almost coincide with the (201), (220), (400), (222), and (213) facets of the standard XRD pattern of Bi2O3 (PDF card 41-1449), which indicates that body-centered cubic Bi2O3-based materials have been successfully prepared in this paper. For the XRD pattern of Ti3C2Tx MXene, the diffraction peaks at 9.0°, 18.6°, 27.5°, and 60.7° on the (002), (004), (006), and (110) facets were clearly observed, as shown in Figure 2b, which was consistent with the results observed by Kuang et al. [31]. Moreover, the characteristic peaks of Bi2O3 and Ti3C2Tx MXene can be observed in the XRD pattern of Bi2O3/Ti3C2Tx nanocomposites, which indicates that all of the materials have been successfully prepared.
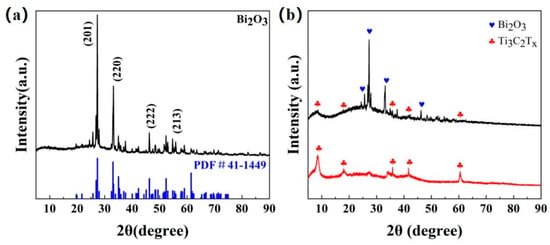
Figure 2.
(a) XRD patterns of Bi2O3. (b) XRD patterns of Ti3C2Tx MXene and Bi2O3/Ti3C2Tx nanocomposites.
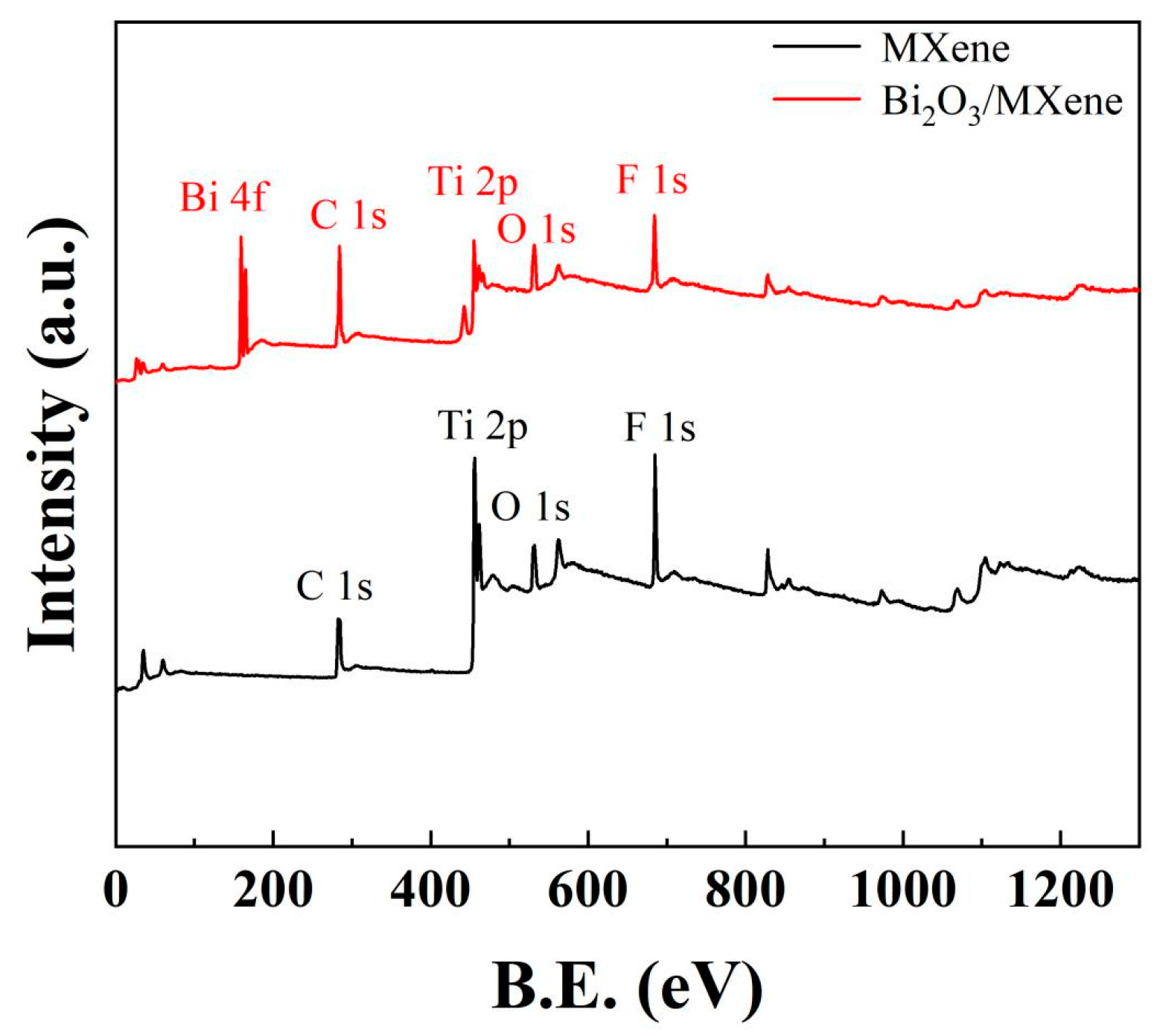
Figure 3 represents the full survey XPS spectrum of Ti3C2Tx MXene and Bi2O3/Ti3C2Tx nanocomposites. It can be identified multiple elements in the full survey XPS spectrum of Ti3C2Tx MXene, such as C 1s, Ti 2p, O 1s, and F 1s. Meanwhile, the elemental mapping of Al in the MAX phase is not found, indicating that the present paper has successfully etched multilayer Ti3C2Tx MXene. Furthermore, the presence of elements O and F also indicates that the multilayer MXene surface was covered by -O and -F functional groups. The elemental Bi in Bi2O3 was also observed for C1s, Ti 2p, O 1s, and F 1s contained in multilayer Ti3C2Tx MXene shown in Bi2O3/Ti3C2Tx full pattern, especially the peak intensity of O element increases significantly because of the introduction of O element in Bi2O3. All of these proved that the Bi2O3/Ti3C2Tx nanocomposites have been successfully synthesized.
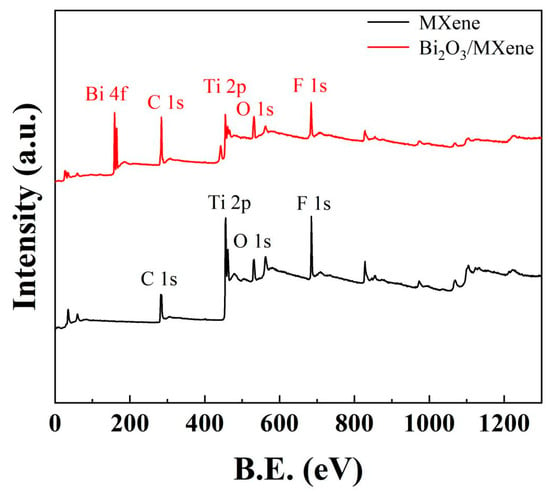
Figure 3.
Full survey XPS spectrum of Ti3C2Tx MXene and Bi2O3/Ti3C2Tx nanocomposites.
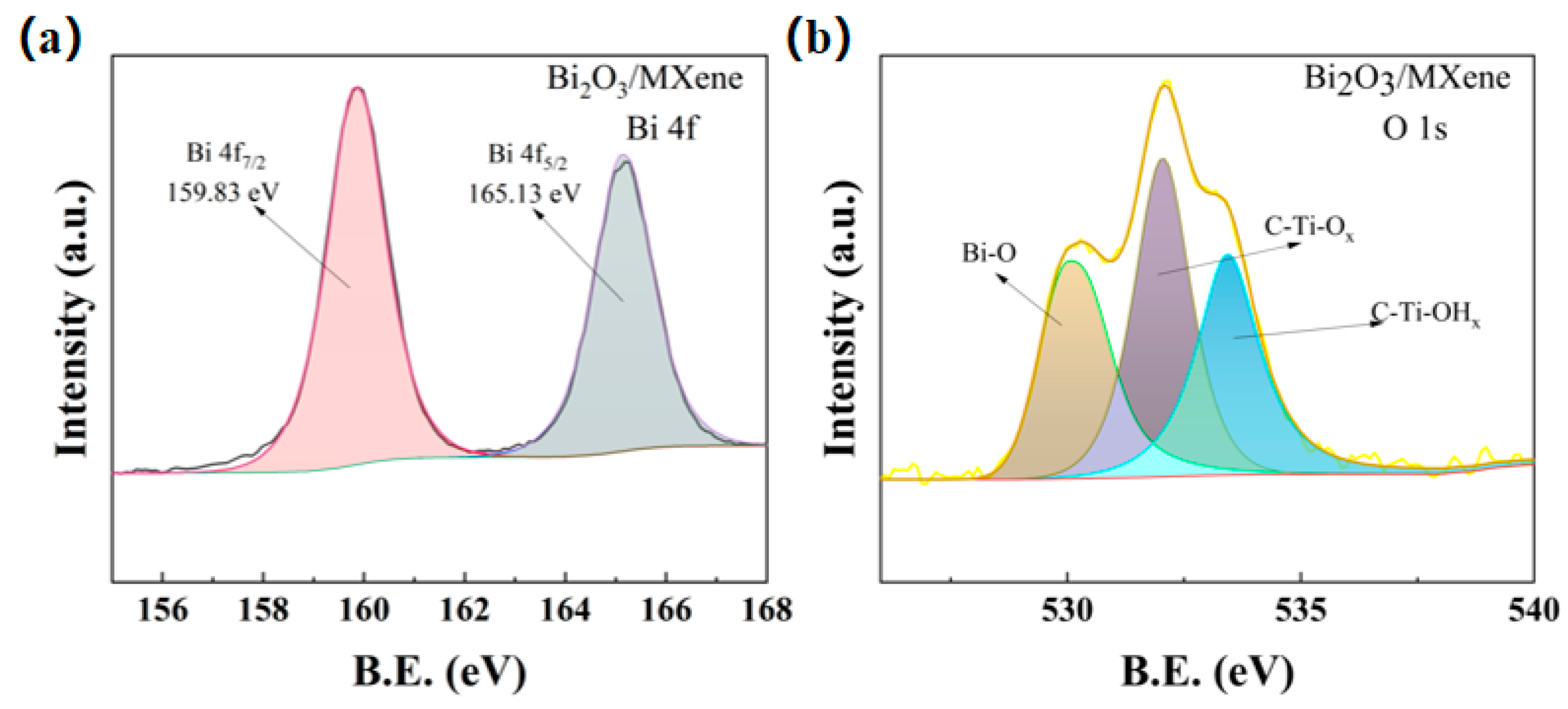
The C-Ti-OHx can be observed from the XPS spectra of O1s in Figure 4a, which indicated that besides -O and -F functional groups, -OH functional groups also existed in the multilayer MXene. The XPS spectra of the 4f orbitals of Bi elements are shown in Figure 4b, and the spectral positions of the characteristic peaks of 159.83 eV and 165.13 eV for Bi 4f7/2 and Bi 4f5/2 indicate that the electronic binding energy difference between them is 5.3 eV, which are approximately equal to the positions of the spectral peaks of Bi3+ 3d in the standard XPS spectrograms. It demonstrates that the elemental Bi exists in the form of Bi3+ in the Bi2O3/Ti3C2Tx nanocomposites.
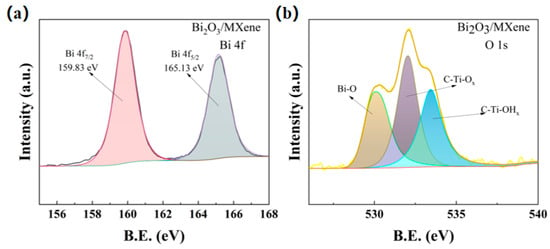
Figure 4.
(a) XPS spectra of O 1s and (b) XPS spectra of Bi 4f for Bi2O3/Ti3C2Tx nanocomposites.
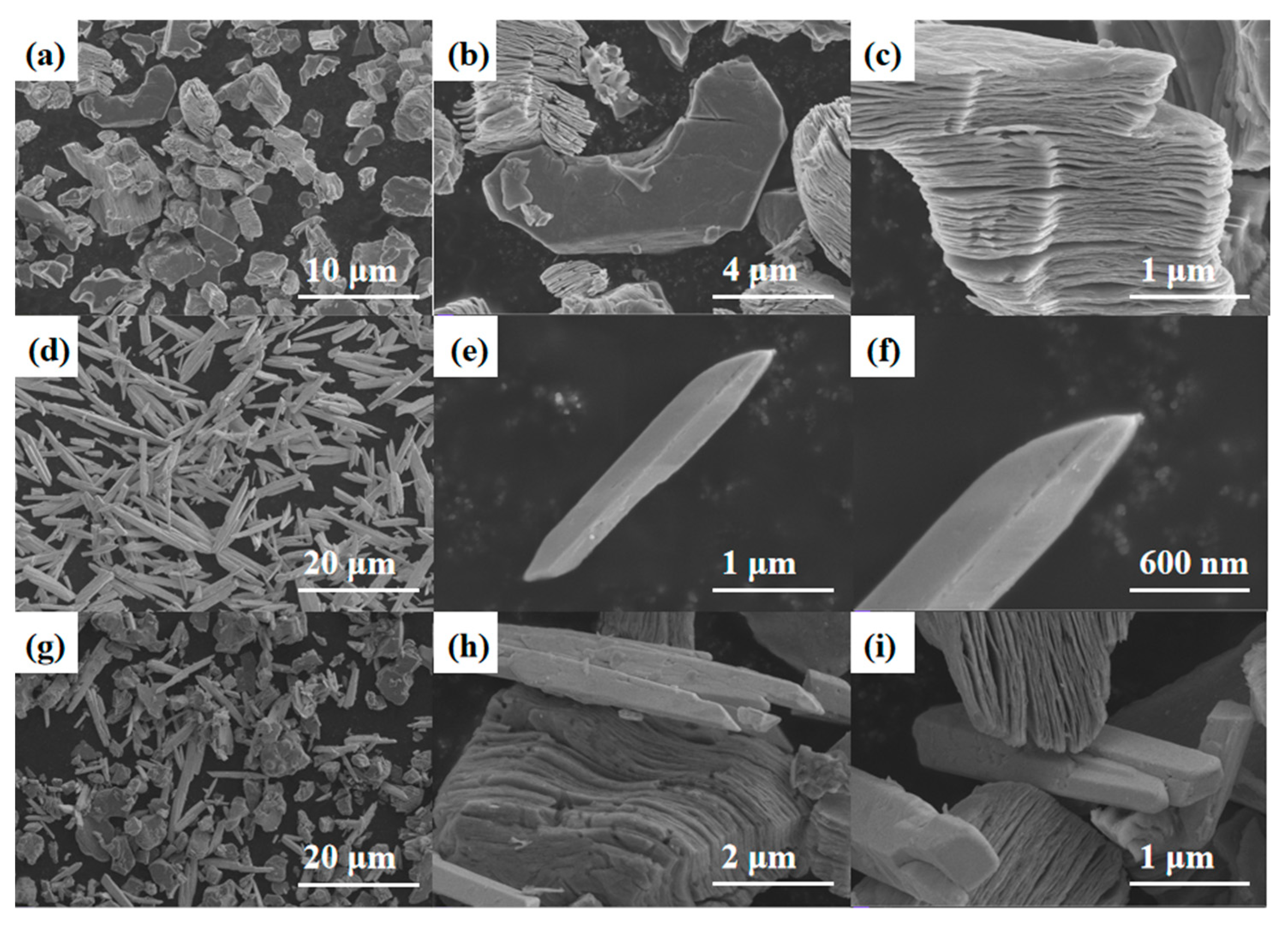
Figure 5a–c show the SEM image of Ti3C2Tx MXene, where it can be observed that the MXene exhibits a multilayer structure similar to an accordion, and the surface of individual granular-like structures is smooth and free of impurities. SEM images of Bi2O3 are shown in Figure 5d–f, from which it can be observed that pure Bi2O3 exhibits a uniformly distributed needle structure. The SEM image of Bi2O3/Ti3C2Tx nanocomposites is shown in Figure 5g–i, from which it can be observed that Ti3C2Tx multilayer MXene and Bi2O3 are evenly distributed. The successfully prepared composite material has a uniformly distributed nanostructure, which makes it an excellent potential for NH3-sensitive sensing.
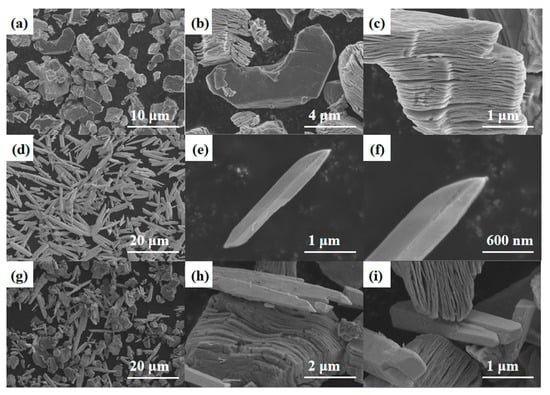
Figure 5.
(a–c) SEM images of Ti3C2Tx MXene. (d–f) Pure Bi2O3 needle. (g–i) SEM images of Bi2O3/Ti3C2Tx nanocomposites.
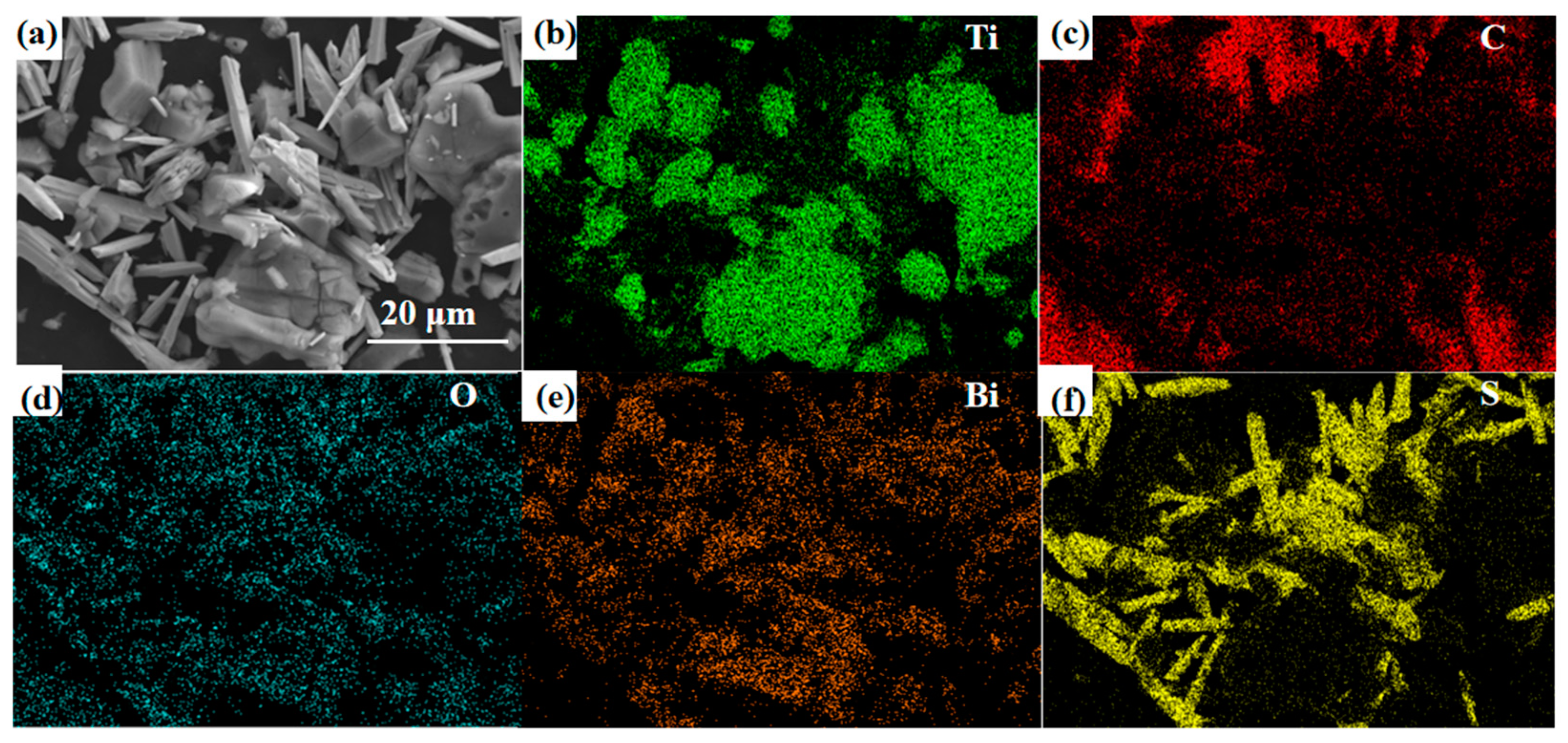
As shown in Figure 6, the element distribution state was tested using an energy spectrometer integrated with the SEM. The Ti, C, O, F, and Bi elements can be observed in the composite material. In this context, the oxygen (O) element is partly derived from the surface -O and -OH functional groups, while the fluorine (F) element originates from the -F functional group on the surface of MXene. These findings align with previous XPS analysis results, mutually verifying the conclusions. The correspondence between the bismuth (Bi) and oxygen (O) elements further confirms the successful synthesis of Bi2O3.
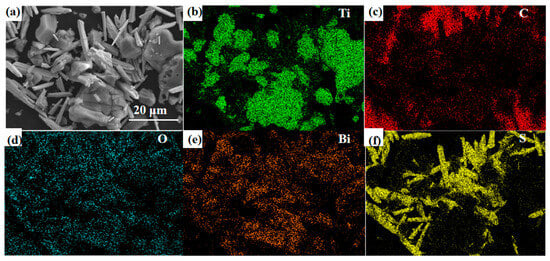
Figure 6.
(a) SEM images and (b–f) EDS mappings of Bi2O3/Ti3C2Tx nanocomposites.
3.2. Gas Sensing Performance
3.2.1. Comparison of Gas Sensitivity Performance of Sensors with Different Ratios to Ammonia
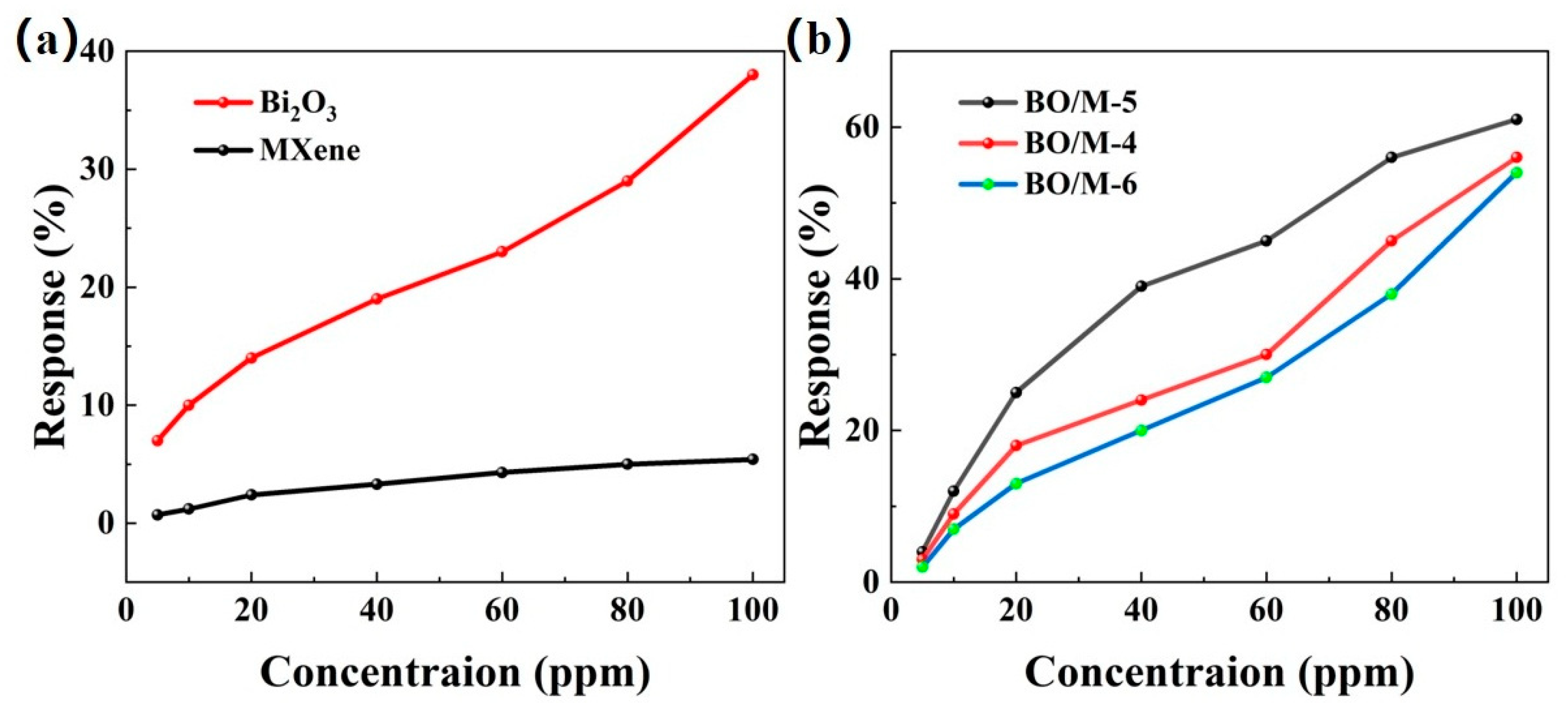
To evaluate the gas sensing performance of the prepared composite material sensors, a comparative experiment was conducted with pure Bi2O3 and Ti3C2Tx gas sensors to clarify their performance advantages. The response curves of different loading ratios to various concentrations of ammonia were tested at room temperature, and their response values were calculated. As shown in Figure 7, all the prepared gas sensors exhibited increasing response values with the increase in ammonia concentration. Figure 7a shows that the response values of pure Ti3C2Tx and pure Bi2O3 sensors from 5 ppm to 100 ppm ammonia were relatively lower than those of the Bi2O3 and Ti3C2Tx doped sensors, with pure Ti3C2Tx and Bi2O3 sensors showing responses of 5.4% and 38% at room temperature, respectively. Figure 7b shows that among the three different loading ratio sensors, the response value in the same testing environment was in the order of BO/M-5 > BO/M-4 > BO/M-6, with BO/M-5 (5:1) showing the highest response value, reaching 61% at room temperature. The BO/M-5 gas sensor’s response value was 11.3 times higher than that of Ti3C2Tx and 1.6 times higher than Bi2O3, demonstrating the effectiveness of doping. Hence, the BO/M-5 gas sensor is used for subsequent testing.
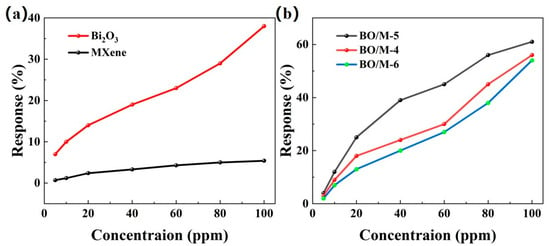
Figure 7.
The response of pure Ti3C2Tx, Bi2O3 sensors (a) and sensors BO/M-4, BO/M-5, and BO/M-6 with different composite ratios (b) to 100 ppm of ammonia at room temperature.
3.2.2. Selectivity Test of BO/M-5 Gas Sensor
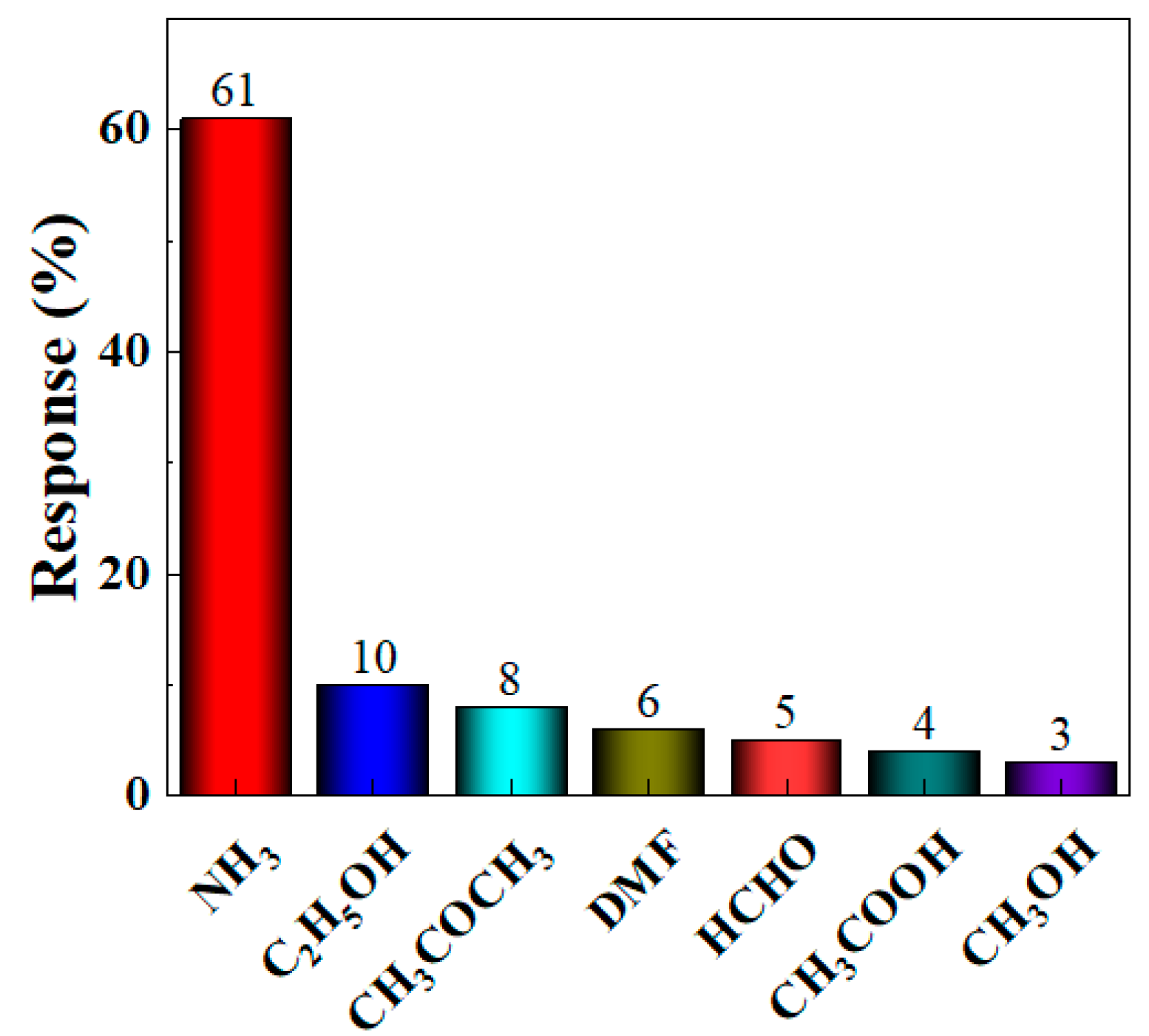
Based on the optimal loading ratio, further in-depth research on the gas sensitivity characteristics of the BO/M-5 gas sensor with the optimal loading ratio is established. Selectivity measures the sensor’s ability to distinguish between different interfering gases at a specific concentration. To test the interference resistance of the BO/M-5 gas sensor, gases such as 100 ppm ammonia, acetone, ethanol, DMF, formaldehyde, acetic acid, and methanol were tested at room temperature, and their response values were calculated. Figure 8 shows that the BO/M-5 gas sensor had a significantly higher response to 100 ppm ammonia compared to other gases, demonstrating its excellent selectivity and sensitivity to ammonia (see Figure S1 in Supplementary Materials for detail). The sensor’s response to ammonia reached 61%, while responses to other gases were all below 10%. The response of the BO/M-5 sensor to ammonia was 6.1–20 times higher than to other gases, indicating excellent selectivity for ammonia.
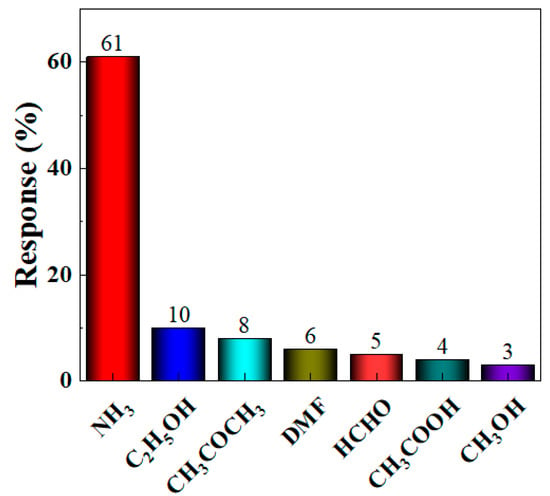
Figure 8.
The response of the BO/M-5 gas sensor to detect 100 ppm of various gases.
3.2.3. Gas Sensitivity Performance of BO/M-5 Gas Sensor at Different Humidities
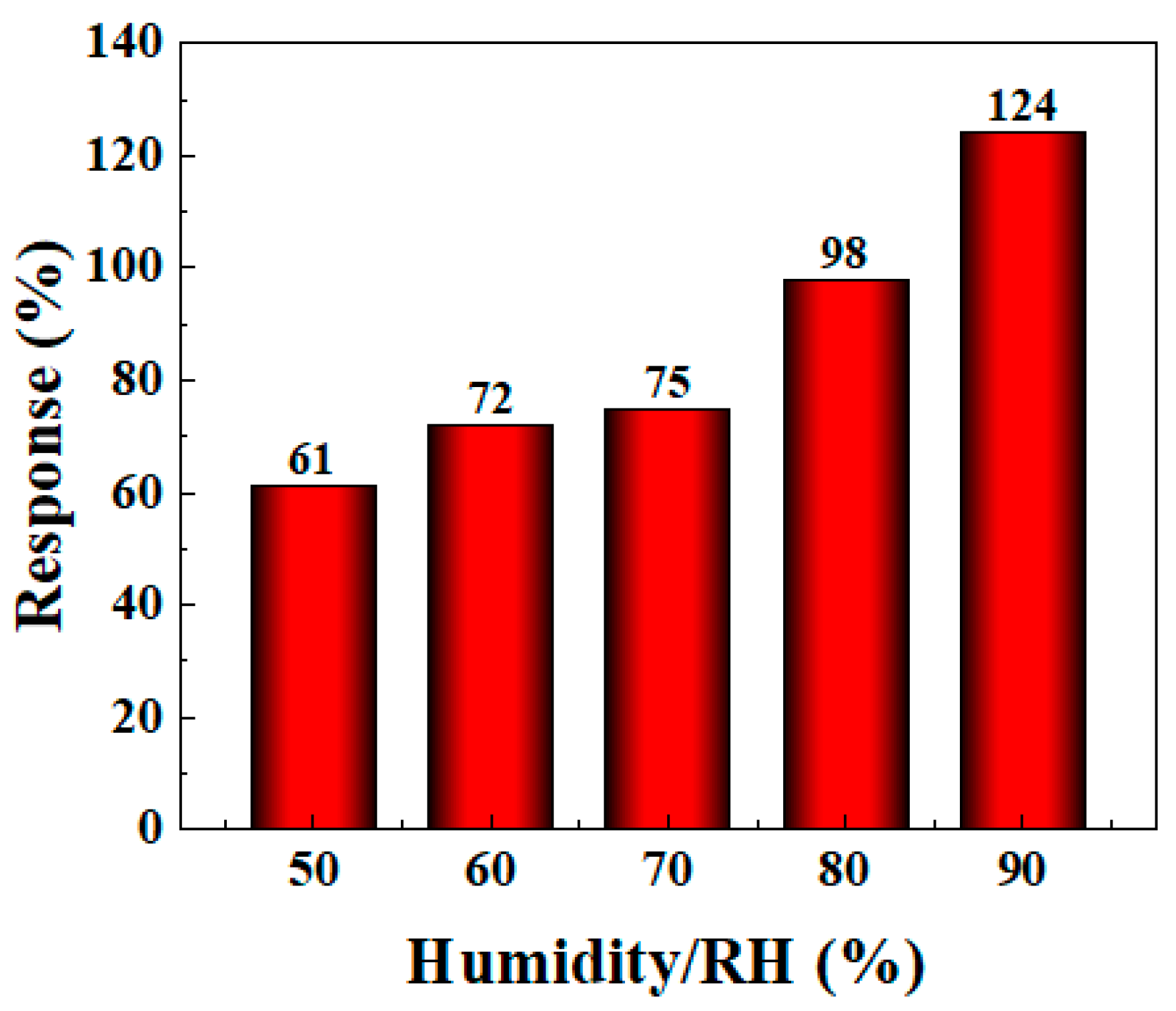
The gas sensitivity performance of most gas sensors can be affected by different humidity conditions, and the detection environment often varies in practical applications. Therefore, it is very important to further study the sensor’s gas sensitivity performance under different humidity conditions. In the experiment, to simulate the effect of different humidity environments on the gas sensor’s performance, deionized water was added to the evaporation stage of the WS-30B gas sensor testing instrument and rapidly evaporated using a heating device to increase the humidity level in the test chamber. An external humidity meter was used to monitor the humidity in the closed chamber to achieve the target humidity. As shown in Figure 9, the gas sensitivity of the BO/M-5 sensor improved with increasing humidity (see Figure S2 in Supplementary Materials for detail). The response value reached 124% at 90% humidity, proving that the sensor can adapt to complex humidity environments and even exhibit enhanced gas sensitivity in high-humidity conditions, which is beneficial for ammonia detection in subsequent applications.
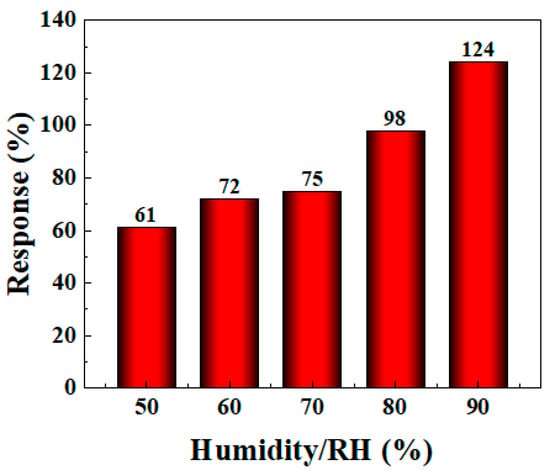
Figure 9.
The response of BO/M-5 gas sensor to 100 ppm of ammonia at different levels of humidity.
3.2.4. Response Recovery Performance of the BO/M-5 Gas Sensor
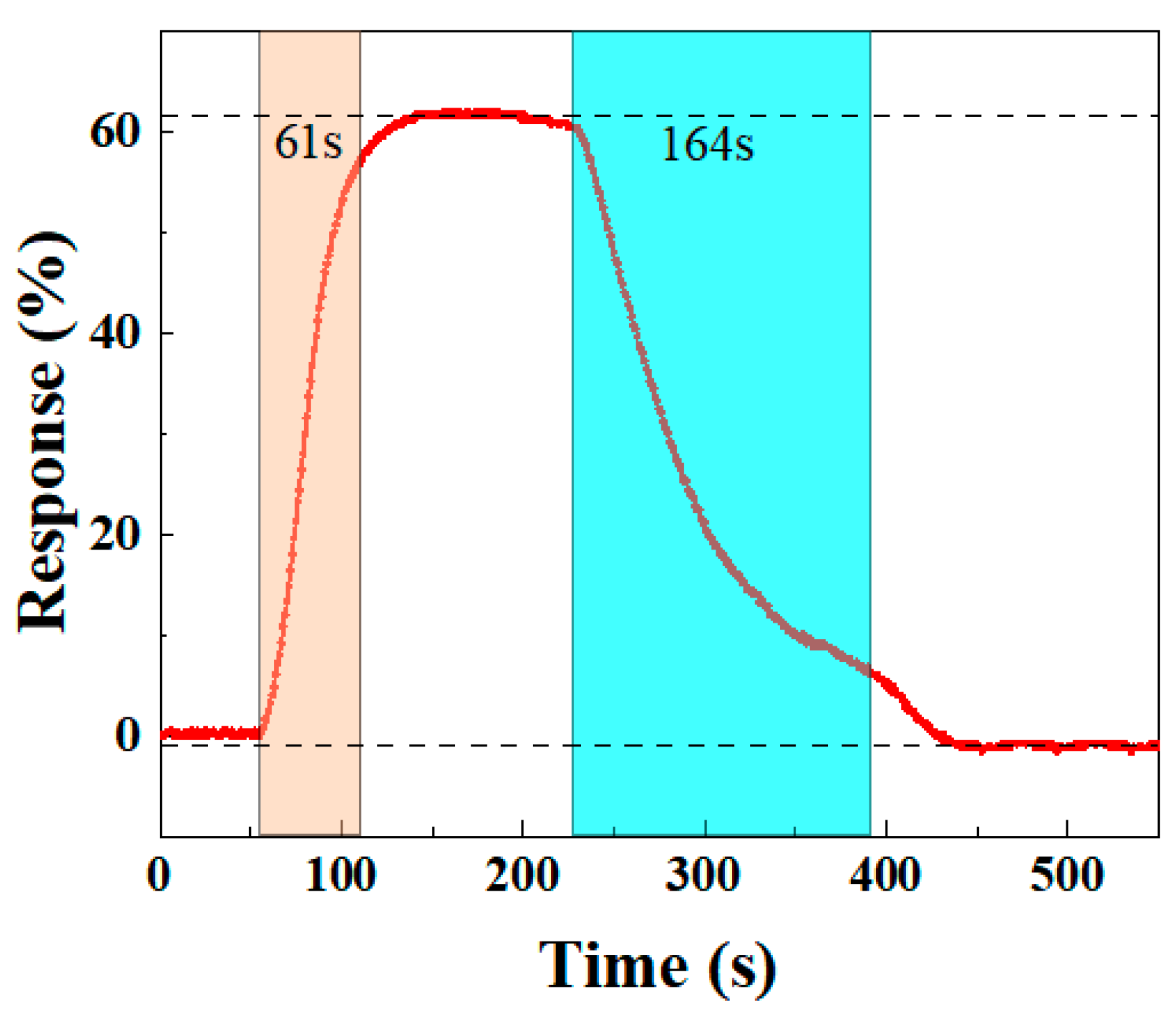
An ideal gas sensor should have rapid response and recovery times to quickly detect the target gas and allow sufficient time for further processing. Thus, response and recovery times are crucial for evaluating sensor performance. Based on the definitions of Equation (1), the BO/M-5 sensor’s response time to 100 ppm ammonia is 61 s, while the recovery time is 164 s, as shown in Figure 10. The sensor’s resistance quickly drops upon NH3 injection for 55 s (red area in Figure 10), and after NH3 is removed at 230 s (blue area in Figure 10), the resistance starts to increase until it returns to its original state. These results show that the sensor performs well in both response and recovery times, supporting its potential for further applications.
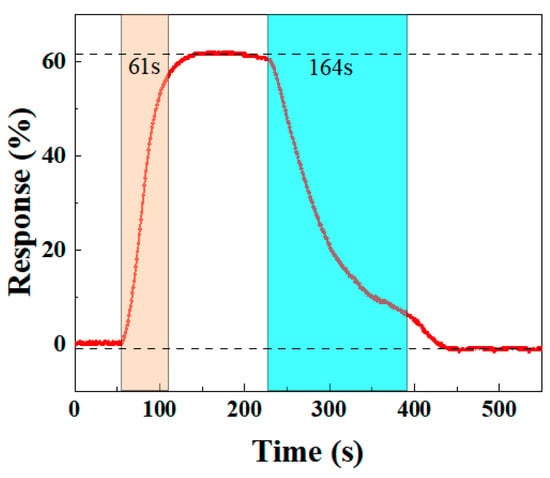
Figure 10.
The response and recovery curves for the BO/M-5 gas sensor to 100 ppm ammonia.
3.2.5. Repeatability and Long-Term Stability of BO/M-5 Gas Sensor
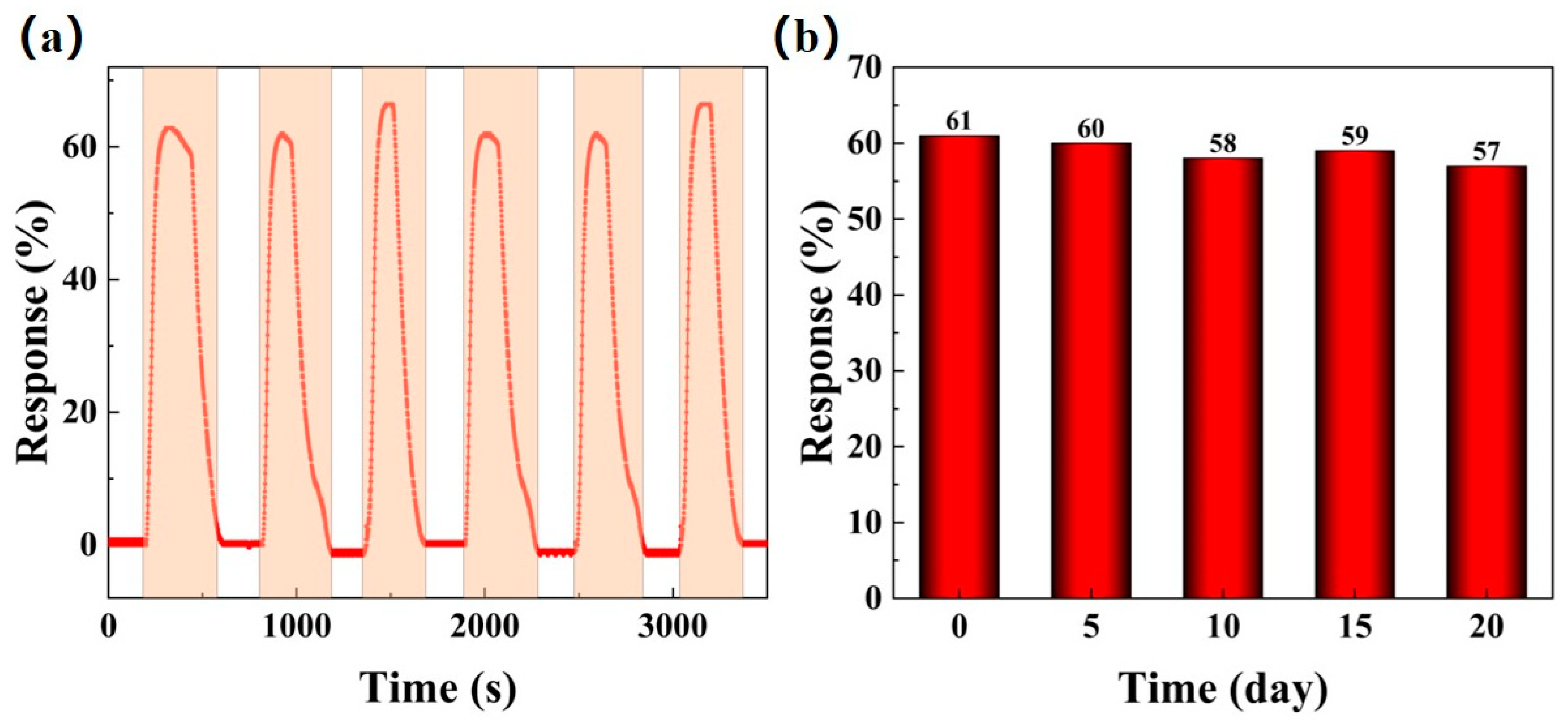
For long-term use, gas sensors must exhibit good repeatability and stability to ensure consistent performance. Figure 11 investigates the repeatability and long-term stability of the BO/M-5 sensor with 100 ppm ammonia under natural conditions. When exposed repeatedly to 100 ppm ammonia at room temperature, the sensor showed consistent response values with minimal variation, as shown by the red line in Figure 11a. Additionally, the BO/M-5 sensor demonstrated excellent long-term stability in practical applications, with response values remaining stable over a 20-day period with tests conducted every five days, as shown in Figure 11b.
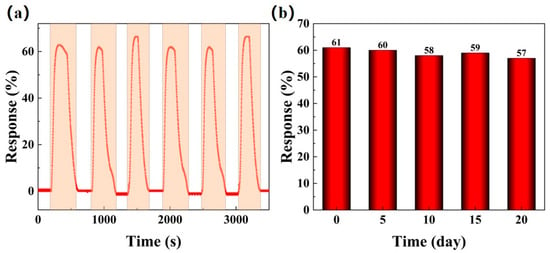
Figure 11.
The repeatability (a) and long-term stability (b) of BO/M-5 to 100 ppm ammonia at room temperature under natural conditions.
4. Discussion
The gas sensing mechanism of the sensor for ammonia was analyzed based on the sensing characteristics of the prepared materials. The resistance changes during the adsorption and desorption processes of the composite material indicate that the sensor acts as an N-type semiconductor with reduced resistance when ammonia molecules adsorb onto its surface. At room temperature, oxygen adsorbs on the composite material surface as O2- [32]. When the sensor is exposed to ammonia, a redox reaction occurs, as shown in Equation (2):
4NH3 + 5O2− → 4NO + 6H2O + 5e−
This reaction generates electrons, which further combine with hole carriers in Ti3C2Tx, leading to an increase in resistance. Once the sensor is removed from NH3, the resistance gradually recovers to its initial or partially recovered state, as detailed in Equations (3) and (4):
2NH3 + 3O− → N2 + 3H2O + 3e−
NH3 + OH− → NH2 + H2O + e−
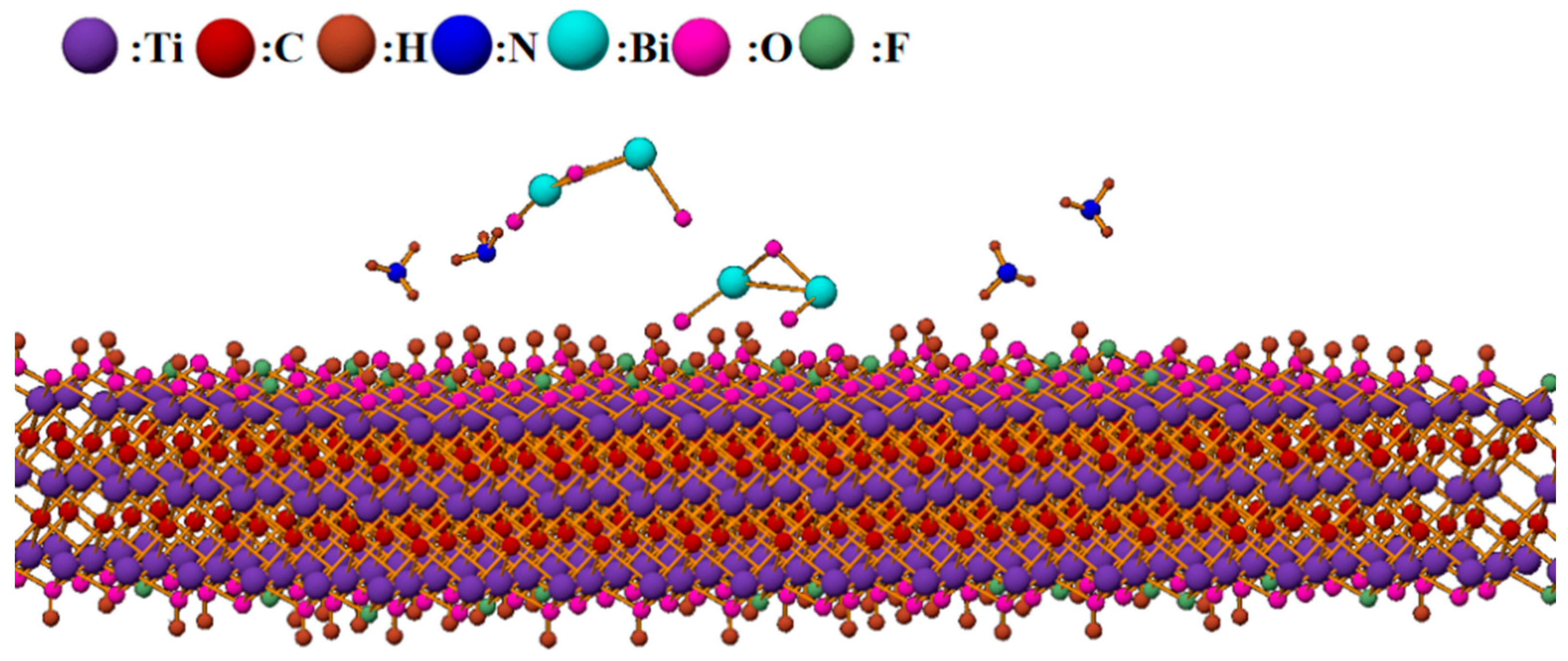
Furthermore, MXene materials possess high electronic mobility, which facilitates rapid carrier transport and enhances sensing performance, providing strong support for better sensing characteristics [5]. The large surface area of MXene provides more contact points and reaction sites for ammonia adsorption on the composite material surface. The surface of MXene also contains numerous functional groups and structural defects that play significant roles in adsorption and desorption. The gas sensing mechanism model of Bi2O3/Ti3C2Tx nanocomposites is shown in Figure 12.
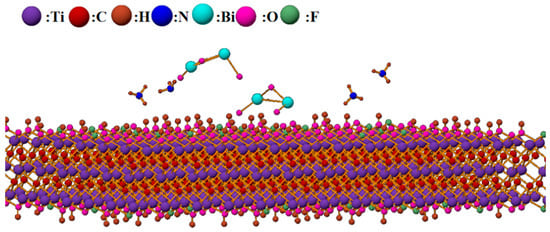
Figure 12.
Schematic diagram of the reaction between Bi2O3/Ti3C2Tx nanocomposites and ammonia.
5. Conclusions
In summary, Bi2O3/Ti3C2Tx nanocomposites were successfully prepared using the hydrothermal method. The materials were characterized using XRD, XPS, SEM, and EDS, and their gas-sensing properties were analyzed using WS-30B. XRD results confirmed that Bi2O3 matched the standard reference, and the XRD pattern of the prepared multilayer MXene was consistent with the literature data. Characteristic peaks for MXene and Bi2O3 were observed in the composite material. XPS analysis of composition and elemental valence states further confirmed the successful synthesis of the materials. The gas sensing performance of the prepared sensor was investigated with WS-30B. The Bi2O3/Ti3C2Tx sensor achieved a response value of 61% to 100 ppm ammonia at room temperature, outperforming Ti3C2Tx and Bi2O3 sensors by factors of 11.3 and 1.6, respectively. The sensor also demonstrated excellent repeatability and rapid response and recovery times (61 s/164 s). Moreover, the gas sensing mechanism of the Bi2O3/Ti3C2Tx sensor was also explored.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s24206514/s1, Figure S1. The response of BO/M-5 sensor to detect 100 ppm of acetone, ethanol, DMF, formaldehyde, acetic acid and methanol gas. Figure S2. The response of BO/M-5 gas sensor to 100 ppm of ammonia at relative humidity 60% (a), 70% (b), 80% (c) and 90% (d).
Author Contributions
Conceptualization, Z.Z. and B.Z.; methodology, B.Z.; software, Z.L.; validation, Z.L.; formal analysis, Z.L.; investigation, Z.Z.; resources, Z.C.; data curation, B.Z.; writing—original draft preparation, B.Z.; writing—review and editing, Z.Z.; visualization, Z.L.; supervision, S.K.; project administration, Z.Z.; funding acquisition, Z.Z. and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Technology R&D Program Joint Fund of Henan Provincial Department of Science and Technology (No. 232103810077), the fund of Henan Key Laboratory of Superhard Abrasives and Grinding Equipment, Henan University of Technology (No. JDKFJJ2022003), the Key Research and Development Program of Henan Province (No. 221111220300), the Youth Backbone Teacher Training Program of Henan University of Technology (No. 21420154), and the Natural Science Project of Zhengzhou Science and Technology Bureau (21ZZXTCX12).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to express our sincere gratitude to all those who contributed to this research project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, M.; He, M.; Hu, Q.; Wu, Q.; Sun, G.; Xie, L.; Zhang, Z.; Zhu, Z.; Zhou, A. Ti3C2Tx MXene-Based Sensors with High Selectivity for NH3 Detection at Room Temperature. ACS Sens. 2019, 4, 2763–2770. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kumar, A.; Sim, U.; Im, H.; Song, S. Synergistic enhancement in the sensing performance of a mixed-potential NH3 sensor using SnO2@CuFe2O4 sensing electrode. Sens. Actuators B Chem. 2020, 308, 127748. [Google Scholar] [CrossRef]
- Meng, W.; Dai, L.; Meng, W.; Zhou, H.; Li, Y.; He, Z.; Wang, L. Mixed-potential type NH3 sensor based on TiO2 sensing electrode with a phase transformation effect. Sens. Actuators B Chem. 2017, 240, 962–970. [Google Scholar] [CrossRef]
- Li, H.; Lee, C.; Kim, D.; Lee, J. Flexible room-temperature NH3 sensor for ultrasensitive, selective, and humidity-independent gas detection. ACS Appl. Mater. Interfaces 2018, 10, 27858–27867. [Google Scholar] [CrossRef]
- Kim, S.; Koh, H.; Ren, C.; Kwon, O.; Maleski, K.; Cho, S.; Anasori, B.; Kim, C.; Choi, Y.; Kim, J.; et al. Metallic Ti3C2Tx MXene gas sensors with ultrahigh signal-to-noise ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef]
- Naderi, H.; Hajati, S.; Ghaedi, M.; Dashtian, K.; Sabzehmeidani, M. Sensitive, selective and rapid ammonia-sensing by gold nanoparticle-sensitized V2O5/CuWO4 heterojunctions for exhaled breath analysis. Appl. Surf. Sci. 2020, 501, 144270. [Google Scholar] [CrossRef]
- Vardhan, D.; Devi, C.; Nagaraju, P.; Muralikrishna, P.; Kumar, B.; Upender, G. Room temperature sensing of ammonia and formaldehyde gases through novel anisotype heterojunction of p-Co3O4/n-Gd0.1Ce0.9O2-δ as highly responsive and stable sensors. Mater. Chem. Phys. 2024, 313, 128694. [Google Scholar] [CrossRef]
- Atkare, S.; Kaushik, S.; Jagtap, S.; Rout, C.S. Room-temperature chemiresistive ammonia sensors based on 2D MXenes and their hybrids: Recent developments and future prospects. Dalton Trans. 2023, 52, 13831–13851. [Google Scholar] [CrossRef]
- Wu, R.; Guo, S.; Li, Y.; Qi, M.; Ge, B.; Song, J. Improving the sensing performance of rambutan-like W18O49 based gas sensor for n-butanol by Ni doping. Sens. Actuators B Chem. 2024, 410, 135671. [Google Scholar] [CrossRef]
- Brophy, R.; Junker, B.; Fakhri, E.; Arnason, H.; Svavarsson, H.; Weimar, U.; Bârsan, N.; Manolescu, A. Ultra Responsive NO2 silicon nanowires gas sensor. Sens. Actuators B Chem. 2024, 410, 135648. [Google Scholar] [CrossRef]
- Hjiri, M.; Algessair, S.; Dhahri, R.; Albargi, H.; Ben Mansour, N.; Assadi, A.; Neri, G. Ammonia gas sensors based on undoped and Ca-doped ZnO nanoparticles. RSC Adv. 2024, 14, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, T.; Chen, T.; He, W.; Chen, M.; Cao, D. The naked-eye NH3 sensor based on fluorinated graphene. Sens. Actuators B Chem. 2019, 281, 789–794. [Google Scholar] [CrossRef]
- Lorencova, L.; Bertok, T.; Filip, J.; Jerigova, M.; Velic, D.; Kasak, P.; Mahmoud, K.; Tkac, J. Highly stable Ti3C2Tx (MXene)/Pt nanoparticles-modified glassy carbon electrode for H2O2 and small molecules sensing applications. Sens. Actuators B Chem. 2018, 263, 360–368. [Google Scholar] [CrossRef]
- Qin, Q.; Olimov, D.; Yin, L. Semiconductor-Type Gas Sensors Based on γ-Fe2O3 Nanoparticles and Its Derivatives in Conjunction with SnO2 and Graphene. Chemosensors 2022, 10, 267. [Google Scholar] [CrossRef]
- Ma, Y.; Yue, Y.; Zhang, H.; Cheng, F.; Zhao, W.; Rao, J.; Luo, S.; Wang, J.; Jiang, X.; Liu, Z.; et al. 3D synergistical Mxene/reduced grapheme oxide aerogel for a piezoresistive sensor. ACS Nano 2018, 12, 3209–3216. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, C.; Wang, F.; Yang, L.; Han, A.; Gao, J. The applicantion progress of two-dimensional Mxene material in the field of gas sensor. J. Funct. Mater. 2022, 53, 3058–3065. [Google Scholar] [CrossRef]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, C.; Zou, Y.; Xu, F.; Sun, L.; Xiang, C. Room-temperature humidity-resistant highly sensitive ammonia sensor based on a porous MXene/Na2Ti3O7 @polyaniline composite. Sens. Actuators B Chem. 2024, 405, 135323. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, L.; Kanda, H. Ti3C2 MXene-TiO2 hybrid-modified U-bend fiberoptic sensor for improved refractive index sensitivity and ammonia detection. Sens. Actuators B Chem. 2023, 393, 134136. [Google Scholar] [CrossRef]
- Yu, H.; Dai, L.; Liu, Y.; Zhou, Y.; Fan, P.; Luo, J.; Zhong, A. Ti3C2Tx MXene-SnO2 nanocomposite for superior room temperature ammonia gas sensor. J. Alloys Compd. 2023, 962, 171170. [Google Scholar] [CrossRef]
- Hou, M.; Jiang, G.; Guo, S.; Gao, J.; Shen, Z.; Wang, Z.; Ye, X.; Yang, L.; Du, Q.; Yi, J.; et al. Mxene Ti3C2Tx derived lamellar Ti3C2Tx-TiO2-CuO heterojunction: Significantly improved ammonia sensor performance. Arab. J. Chem. 2023, 16, 104808. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, D. A piezoelectric six-DOF vibration energy harvester based on parallel mechanism: Dynamic modeling, simulation, and experiment. Smart Mater. Struct. 2017, 26, 035022. [Google Scholar] [CrossRef]
- You, J.; Wang, L.; Xi, F.; Shen, J. Decoupling algorithm and maximum operation frequency of a novel parallel type six-axis accelerometer. IEEE Sens. J. 2020, 20, 12637–12651. [Google Scholar] [CrossRef]
- Hassanein, S.; Ali, R. Investigation of the Morphological, Optical, and D.C Electrical Characteristics of Synthesized (Bi2O3/ZnO) Nanocomposites, as Well as Their Potential Use in Hydrogen Sulfide Gas Sensor. Trans. Electr. Electron. Mater. 2023, 24, 205–216. [Google Scholar] [CrossRef]
- Wang, C.; Niu, Q.; Liu, D.; Dong, X.; You, T. Electrochemical sensor based on Bi/Bi2O3 doped porous carbon composite derived from Bi-MOFs for Pb2+ sensitive detection. Talanta 2023, 258, 124281. [Google Scholar] [CrossRef]
- Chang, C.; Xue, Q.; Wang, R.; Liu, Z.; Liu, Y.; He, L.; Liu, F.; Xie, H. Development of a novel sensor based on Bi2O3 and carbonized UIO-66-NH2 nanocomposite for efficient detection of Pb(II) ion in water environment. Appl. Surf. Sci. 2023, 616, 156510. [Google Scholar] [CrossRef]
- Hieu, N.; Van Phuoc, C.; Hung, N.; Anh, C.; Phan, A.; Nah, J.; Jeong, J.; Huy, P.; Kim, D. A highly stable humidity sensor based on a new Bi2O3/CNT hybrid nanostructure. Sens. Actuators A Phys. 2023, 351, 114141. [Google Scholar] [CrossRef]
- Pandeeswari, R.; Sonia, T.; Balamurugan, D.; Jeyaprakash, B. Highly Selective Dimethylamine Vapour Sensors Based on Spray Deposited β-Bi2O3 Nanospheres at Low Temperature. Sens. Imaging 2022, 23, 1. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Kuang, D.; Guo, X.; Zhu, Z.; Ding, Y.; Sun, X.; Wu, Z.; Zhang, L.; Zhou, Y.; He, Y. Enhanced room temperature ammonia response of 2D-Ti3C2Tx MXene decorated with Ni(OH)2 nanoparticles. Ceram. Int. 2021, 47, 19471–19480. [Google Scholar] [CrossRef]
- Chaudhary, N.; Singh, A.; Aswal, D.; Debnath, A.; Samanta, S.; Koiry, S.; Sharma, S.; Shah, K.; Acharya, S.; Muthe, K.P.; et al. Electron beam modified zinc phthalocyanine thin films for radiation dosimeter application. Synth. Met. 2017, 231, 143–152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).