AI Applications in Adult Stroke Recovery and Rehabilitation: A Scoping Review Using AI
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility and Exclusion Criteria
2.2. Information Sources and Search Strategy
2.3. Data Extraction and Screening
2.4. Data Analysis
2.4.1. Topic Modeling for Identifying Research Themes
2.4.2. Mapping into a Clinical Ontology
2.4.3. Development of Interactive Dashboard
2.5. Evaluation of AI for Upper Limb Rehabilitation
3. Results
3.1. Search Results and Selection
3.2. Overview of Results
3.3. Research Themes on AI Technology Approaches in Adult Stroke Rehabilitation and Recovery
3.3.1. AI-Based Applications in Post-Stroke Impairments
3.3.2. AI-Based Systems in Assisted Intervention
3.3.3. AI-Based Systems in Outcome Prediction and Prognosis
3.3.4. AI-Based Systems in Imaging and Neuroscience
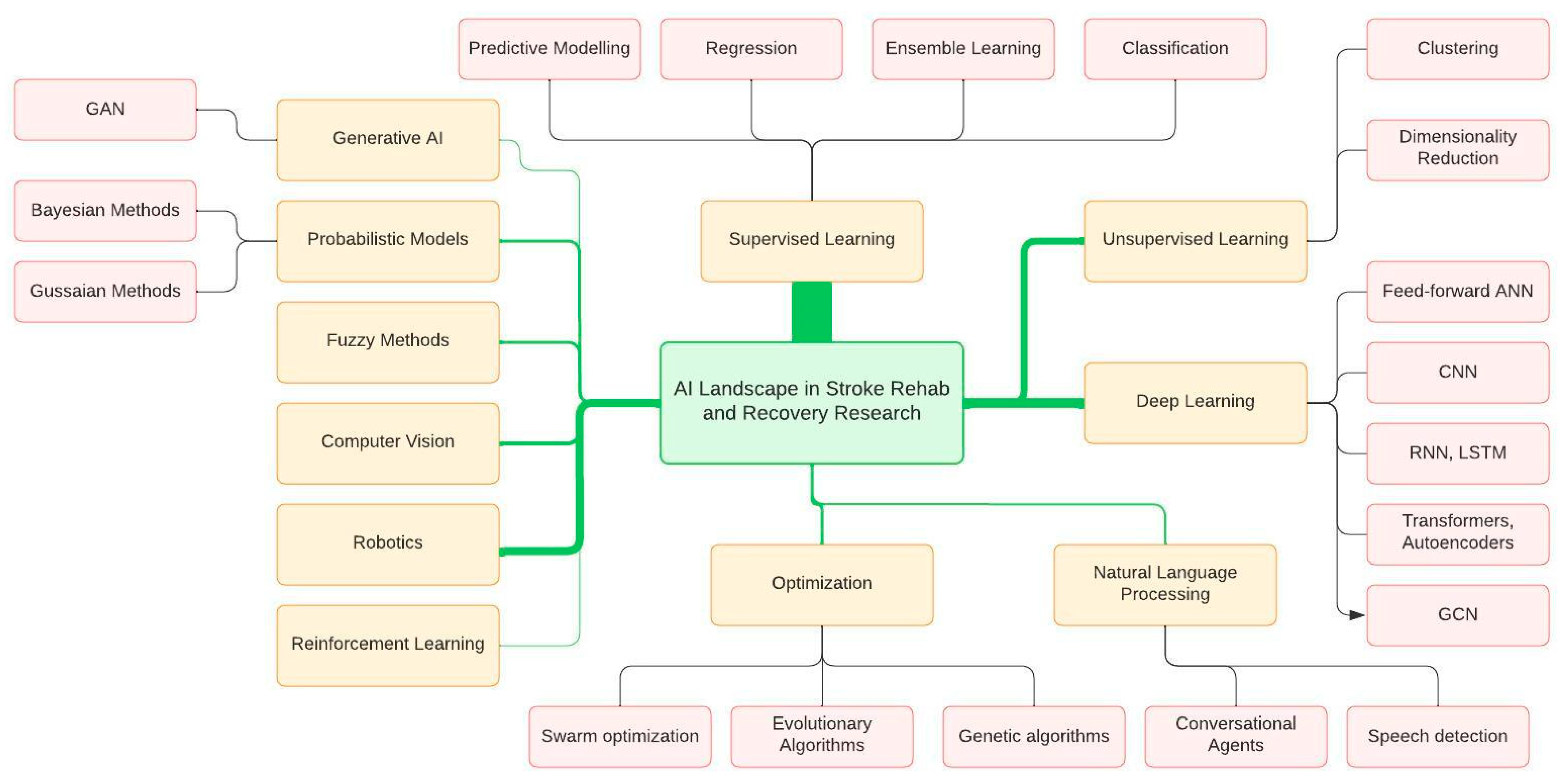
3.4. Commonly Used AI Techniques
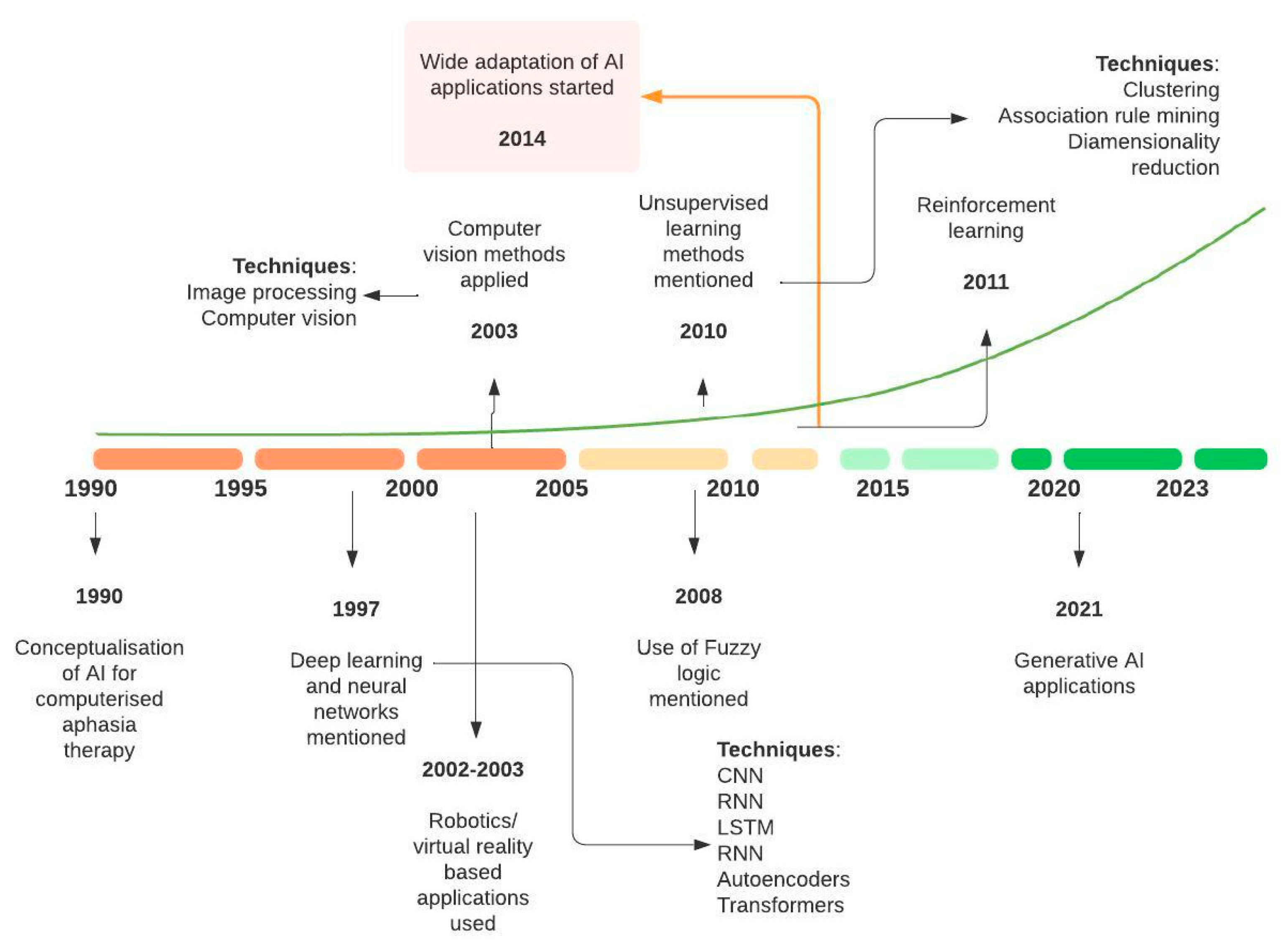
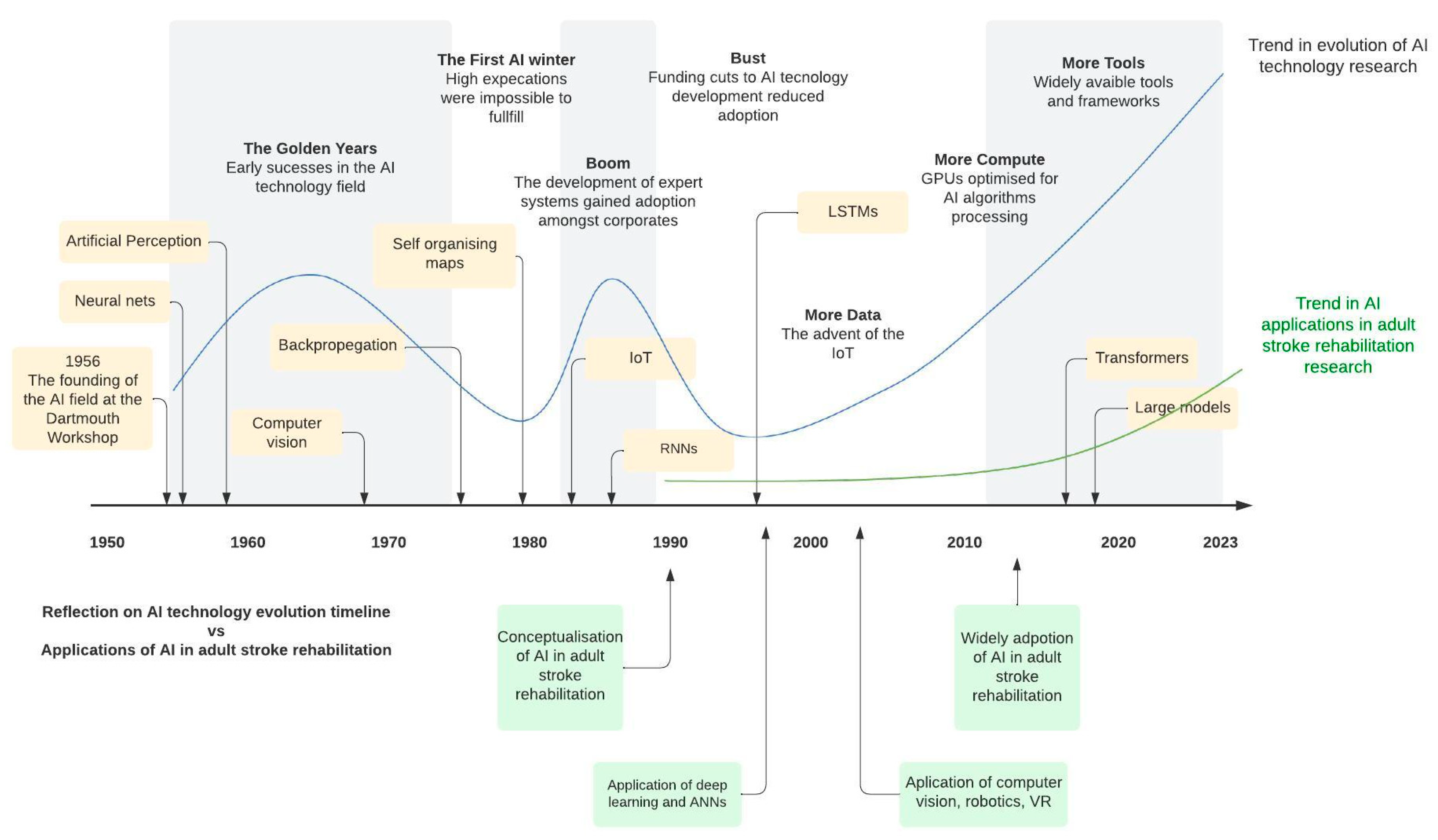
3.5. Time-Based Analysis of AI Terminology and Evolution of Topics in Stroke Rehabilitation and Recovery
4. Discussion
4.1. Progression of AI in Adult Stroke Rehabilitation
4.2. Upper Limb Rehabilitaion
4.3. Lower Limb Rehabilitation
4.4. Cognitive and Speech Rehabilitation
4.5. Limitations and Gaps in Current Methods
4.6. Review Methodology and Its Impact on Findings
4.7. Future Clinical Development Areas and Roadmap
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Artificial Intelligence | Stroke | Rehabilitation | Recovery |
|---|---|---|---|
| Artificial intelligence (AI) Machine learning (ML) Deep learning Computational Intelligence Machine intelligence AI application Supervised learning Unsupervised learning Classification Artificial neural network (ANN) Computer reasoning Computer vision Computer intelligence Cognitive computing Expert systems Natural language processing (NLP) Robotic intelligence Smart machines Synthetic intelligence Intelligent systems Automated intelligence Reinforcement learning Fuzzy logic Decision trees Classification Clustering Regression Bayesian networks Genetic algorithms Sentiment analysis Speech recognition Image processing Data mining Predictive analysis Sentiment analysis | Stroke Cerebrovascular accident (CVA) Cerebral vascular accident Cerebrovascular disorders Ischemic Hemorrhagic Brain attack Brain injury Infarction Neuroscience Adult | Rehabilitation Neurological rehabilitation Neurorehabilitation Therapy Language Skill Movement Activity Sensation Learning Motor Cognition Training Function Task Participation Performance Rehab Telerehabilitation Cognitive Speech Sensory Somatosensory Intervention | Recovery Profile Trajectory Impairment Stroke recovery Post stroke Post-stroke Poststroke Human Relearning Independence Function Quality of life Emotional well-being Engagement Adaptation |
References
- Sirsat, M.S.; Fermé, E.; Câmara, J. Machine Learning for Brain Stroke: A Review. J. Stroke Cerebrovasc. Dis. 2020, 29, 105162. [Google Scholar] [CrossRef]
- Lutz, B.J.; Ellen Young, M.; Cox, K.J.; Martz, C.; Rae Creasy, K. The crisis of stroke: Experiences of patients and their family caregivers. Top. Stroke Rehabil. 2011, 18, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H. Rehabilitation after Stroke. N. Engl. J. Med. 2005, 352, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Luvizutto, G.J.; Silva, G.F.; Nascimento, M.R.; Sousa Santos, K.C.; Appelt, P.A.; de Moura Neto, E.; de Souza, J.T.; Wincker, F.C.; Miranda, L.A.; Hamamoto Filho, P.T.; et al. Use of artificial intelligence as an instrument of evaluation after stroke: A scoping review based on international classification of functioning, disability and health concept: AI applications for stroke evaluation. Top. Stroke Rehabil. 2022, 29, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Adikari, A.; Hernandez, N.; Alahakoon, D.; Rose, M.L.; Pierce, J.E. From concept to practice: A scoping review of the application of AI to aphasia diagnosis and management. Disabil. Rehabil. 2023, 46, 1288–1297. [Google Scholar] [CrossRef]
- Carey, L.M. Stroke Rehabilitation: Insights from Neuroscience and Imaging; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Mahmoud, H.; Aljaldi, F.; El-Fiky, A.; Battecha, K.; Thabet, A.; Alayat, M.; Elkafy, E.; Ebid, A.; Ibrahim, A. Artificial Intelligence machine learning and conventional physical therapy for upper limb outcome in patients with stroke: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4812–4827. [Google Scholar]
- Gittler, M.; Davis, A.M. Guidelines for adult stroke rehabilitation and recovery. Jama 2018, 319, 820–821. [Google Scholar] [CrossRef]
- Rahman, S.; Sarker, S.; Haque, A.N.; Uttsha, M.M.; Islam, M.F.; Deb, S. AI-driven Stroke Rehabilitation Systems and Assessment: A Systematic Review. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 31, 192–207. [Google Scholar] [CrossRef]
- Vanhook, P. The domains of stroke recovery: A synopsis of the literature. J. Neurosci. Nurs. 2009, 41, 6–17. [Google Scholar] [CrossRef]
- Lee, D.; Yoon, S.N. Application of artificial intelligence-based technologies in the healthcare industry: Opportunities and challenges. Int. J. Environ. Res. Public Health 2021, 18, 271. [Google Scholar] [CrossRef]
- Barry, D.T. Adaptation, artificial intelligence, and physical medicine and rehabilitation. PMR 2018, 10, S131–S143. [Google Scholar] [CrossRef] [PubMed]
- El Naamani, K.; Musmar, B.; Gupta, N.; Ikhdour, O.; Abdelrazeq, H.; Ghanem, M.; Wali, M.; El-Hajj, J.; Alhussein, A.; Alhussein, R.; et al. The Artificial Intelligence Revolution in Stroke Care: A Decade of Scientific Evidence in Review. World Neurosurg. 2024, 184, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Adikari, A.; Nawaratne, R.; De Silva, D.; Carey, D.L.; Walsh, A.; Baum, C.; Davis, S.; Donnan, G.A.; Alahakoon, D.; Carey, L.M. Is Mild Really Mild?: Generating Longitudinal Profiles of Stroke Survivor Impairment and Impact Using Unsupervised Machine Learning. Appl. Sci. 2024, 14, 6800. [Google Scholar] [CrossRef]
- Zihni, E.; Madai, V.I.; Livne, M.; Galinovic, I.; Khalil, A.A.; Fiebach, J.B.; Frey, D. Opening the black box of artificial intelligence for clinical decision support: A study predicting stroke outcome. PLoS ONE 2020, 15, e0231166. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Tang, Y.; Pan, C.; Li, G.; Liu, N.; Hu, Y.; Tang, Z. Artificial intelligence: The dawn of a new era for cutting-edge technology based diagnosis and treatment for stroke. Brain Hemorrhages 2020, 1, 1–5. [Google Scholar] [CrossRef]
- Choo, Y.J.; Chang, M.C. Use of machine learning in stroke rehabilitation: A narrative review. Brain Neurorehabilit. 2022, 15, e26. [Google Scholar] [CrossRef]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. The role of artificial intelligence in future rehabilitation services: A systematic literature review. IEEE Access 2023, 11, 11024–11043. [Google Scholar] [CrossRef]
- Mouridsen, K.; Thurner, P.; Zaharchuk, G. Artificial Intelligence Applications in Stroke. Stroke 2020, 51, 2573–2579. [Google Scholar] [CrossRef]
- Yeo, M.; Kok, H.K.; Kutaiba, N.; Maingard, J.; Thijs, V.; Tahayori, B.; Russell, J.; Jhamb, A.; Chandra, R.V.; Brooks, M.; et al. Artificial intelligence in clinical decision support and outcome prediction–applications in stroke. J. Med. Imaging Radiat. Oncol. 2021, 65, 518–528. [Google Scholar] [CrossRef]
- Liu, K.; Yin, M.; Cai, Z. Research and application advances in rehabilitation assessment of stroke. J. Zhejiang Univ.-Sci. B 2022, 23, 625–641. [Google Scholar] [CrossRef]
- Nizamis, K.; Athanasiou, A.; Almpani, S.; Dimitrousis, C.; Astaras, A. Converging robotic technologies in targeted neural rehabilitation: A review of emerging solutions and challenges. Sensors 2021, 21, 2084. [Google Scholar] [CrossRef]
- Lo, K.; Stephenson, M.; Lockwood, C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients: A systematic review. JBI Evid. Synth. 2017, 15, 3049–3091. [Google Scholar]
- Mohan, D.M.; Khandoker, A.H.; Wasti, S.A.; Ismail Ibrahim Ismail Alali, S.; Jelinek, H.F.; Khalaf, K. Assessment methods of post-stroke gait: A scoping review of technology-driven approaches to gait characterization and analysis. Front. Neurol. 2021, 12, 650024. [Google Scholar] [CrossRef]
- Huo, C.-C.; Zheng, Y.; Lu, W.-W.; Zhang, T.-Y.; Wang, D.-F.; Xu, D.-S.; Li, Z.-Y. Prospects for intelligent rehabilitation techniques to treat motor dysfunction. Neural Regen. Res. 2021, 16, 264–269. [Google Scholar]
- Yang, S.; Li, R.; Li, H.; Xu, K.; Shi, Y.; Wang, Q.; Yang, T.; Sun, X. Exploring the use of brain-computer interfaces in stroke neurorehabilitation. BioMed Res. Int. 2021, 2021, 9967348. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, K.; Kokkotis, C.; Moustakidis, S.; Karakasis, E.; Sakellari, P.; Koutra, C.; Tsiptsios, D.; Karatzetzou, S.; Vadikolias, K.; Aggelousis, N. Machine Learning Algorithms for the Prediction of Language and Cognition Rehabilitation Outcomes of Post-stroke Patients: A Scoping Review. Hum.-Centric Intell. Syst. 2024, 4, 147–160. [Google Scholar] [CrossRef]
- Azevedo, N.; Kehayia, E.; Jarema, G.; Le Dorze, G.; Beaujard, C.; Yvon, M. How artificial intelligence (AI) is used in aphasia rehabilitation: A scoping review. Aphasiology 2024, 38, 305–336. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Chowdhary, K.; Chowdhary, K. Natural language processing. In Fundamentals of Artificial Intelligence; Springer: Berlin/Heidelberg, Germany, 2020; pp. 603–649. [Google Scholar]
- Grootendorst, M. BERTopic: Neural topic modeling with a class-based TF-IDF procedure. arXiv 2022, arXiv220305794. [Google Scholar]
- Soshnikov, D.; Soshnikova, V. Using Text Analytics for Health to Get Meaningful Insights from a Corpus of COVID Scientific Papers. arXiv 2021, arXiv211015453. [Google Scholar]
- Wang, J.; Yu, L.; Wang, J.; Guo, L.; Gu, X.; Fang, Q. Automated Fugl-Meyer Assessment Using SVR Model. In Proceedings of the 2014 IEEE International Symposium on Bioelectronics and Bioinformatics (IEEE ISBB 2014), Chung Li, Taiwan, 11–14 April 2014; pp. 1–4. [Google Scholar]
- Lee, M.H.; Siewiorek, D.P.; Smailagic, A.; Bernardino, A.; Badia, S.B. Enabling AI and robotic coaches for physical rehabilitation therapy: Iterative design and evaluation with therapists and post-stroke survivors. Int. J. Soc. Robot. 2024, 16, 1–22. [Google Scholar] [CrossRef]
- Kim, J.K.; Choo, Y.J.; Chang, M.C. Prediction of motor function in stroke patients using machine learning algorithm: Development of practical models. J. Stroke Cerebrovasc. Dis. 2021, 30, 105856. [Google Scholar] [CrossRef] [PubMed]
- Matic, A.; Mehta, P.; Rehg, J.M.; Osmani, V.; Mayora, O. Monitoring dressing activity failures through RFID and video. Methods Inf. Med. 2012, 51, 45–54. [Google Scholar]
- Zhang, X.; D’Arcy, R.; Menon, C. Scoring upper-extremity motor function from EEG with artificial neural networks: A preliminary study. J. Neural Eng. 2019, 16, 036013. [Google Scholar] [CrossRef]
- Solis-Escalante, T.; De Kam, D.; Weerdesteyn, V. Classification of rhythmic cortical activity elicited by whole-body balance perturbations suggests the cortical representation of direction-specific changes in postural stability. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2566–2574. [Google Scholar] [CrossRef]
- Lee, M.H.; Siewiorek, D.P.; Smailagic, A.; Bernardino, A.; Badia, S.B. Learning to Assess the Quality of Stroke Rehabilitation Exercises. In Proceedings of the 24th International Conference on Intelligent User Interfaces, Marina del Ray, CA, USA, 17–20 March 2019; pp. 218–228. [Google Scholar]
- Lee, W.H.; Lim, M.H.; Seo, H.G.; Seong, M.Y.; Oh, B.-M.; Kim, S. Development of a novel prognostic model to predict 6-month swallowing recovery after ischemic stroke. Stroke 2020, 51, 440–448. [Google Scholar] [CrossRef]
- Hedjazi, N.; Kharboutly, H.; Benali, A.; Dibi, Z. PCA-based selection of distinctive stability criteria and classification of post-stroke pathological postural behaviour. Australas. Phys. Eng. Sci. Med. 2018, 41, 189–199. [Google Scholar] [CrossRef]
- Sadarangani, G.P.; Jiang, X.; Simpson, L.A.; Eng, J.J.; Menon, C. Force myography for monitoring grasping in individuals with stroke with mild to moderate upper-extremity impairments: A preliminary investigation in a controlled environment. Front. Bioeng. Biotechnol. 2017, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Cóias, A.R.; Lee, M.H.; Bernardino, A. A low-cost virtual coach for 2D video-based compensation assessment of upper extremity rehabilitation exercises. J. NeuroEngineering Rehabil. 2022, 19, 83. [Google Scholar] [CrossRef]
- Crocher, V.; Sahbani, A.; Robertson, J.; Roby-Brami, A.; Morel, G. Constraining upper limb synergies of hemiparetic patients using a robotic exoskeleton in the perspective of neuro-rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 247–257. [Google Scholar] [CrossRef]
- Jung, H.-T.; Daneault, J.-F.; Lee, H.; Kim, K.; Kim, B.; Park, S.; Ryu, T.; Kim, Y.; Lee, S.I. Remote assessment of cognitive impairment level based on serious mobile game performance: An initial proof of concept. IEEE J. Biomed. Health Inform. 2019, 23, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Ballester, B.R.; Leiva Bañuelos, N.; Duarte Oller, E.; Verschure, P.F. Adaptive conjunctive cognitive training (ACCT) in virtual reality for chronic stroke patients: A randomized controlled pilot trial. J. Neuroengineering Rehabil. 2020, 17, 42. [Google Scholar] [CrossRef]
- Rivas, J.J.; del Carmen Lara, M.; Castrejón, L.; Hernández-Franco, J.; Orihuela-Espina, F.; Palafox, L.; Williams, A.; Bianchi-Berthouze, N.; Sucar, L.E. Multi-Label and Multimodal Classifier for Affective States Recognition in Virtual Rehabilitation. IEEE Trans. Affect. Comput. 2022, 13, 1183–1194. [Google Scholar] [CrossRef]
- Schicketmueller, A.; Rose, G.; Hofmann, M. Feasibility of a sensor-based gait event detection algorithm for triggering functional electrical stimulation during robot-assisted gait training. Sensors 2019, 19, 4804. [Google Scholar] [CrossRef]
- Dobkin, B.H.; Xu, X.; Batalin, M.; Thomas, S.; Kaiser, W. Reliability and validity of bilateral ankle accelerometer algorithms for activity recognition and walking speed after stroke. Stroke 2011, 42, 2246–2250. [Google Scholar] [CrossRef]
- Derungs, A.; Schuster-Amft, C.; Amft, O. Longitudinal walking analysis in hemiparetic patients using wearable motion sensors: Is there convergence between body sides? Front. Bioeng. Biotechnol. 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Bian, G.-B.; Hou, Z.-G.; Zhao, J.; Su, G.; Zhou, H.; Peng, L.; Wang, W. Simultaneous recognition and assessment of post-stroke hemiparetic gait by fusing kinematic, kinetic, and electrophysiological data. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 856–864. [Google Scholar] [CrossRef]
- Shimada, Y.; Ando, S.; Matsunaga, T.; Misawa, A.; Aizawa, T.; Shirahata, T.; Itoi, E. Clinical application of acceleration sensor to detect the swing phase of stroke gait in functional electrical stimulation. Tohoku J. Exp. Med. 2005, 207, 197–202. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Honda, K.; Owaki, D.; Izumi, S.-I. Classification of ankle joint stiffness during walking to determine the use of ankle foot orthosis after stroke. Brain Sci. 2021, 11, 1512. [Google Scholar] [CrossRef]
- Yang, N.; An, Q.; Kogami, H.; Yamakawa, H.; Tamura, Y.; Takahashi, K.; Kinomoto, M.; Yamasaki, H.; Itkonen, M.; Shibata-Alnajjar, F.; et al. Temporal features of muscle synergies in sit-to-stand motion reflect the motor impairment of post-stroke patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 2118–2127. [Google Scholar] [CrossRef]
- Moore, J.; Stuart, S.; McMeekin, P.; Walker, R.; Celik, Y.; Pointon, M.; Godfrey, A. Enhancing free-living fall risk assessment: Contextualizing mobility based IMU data. Sensors 2023, 23, 891. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Tong, K.; Zhu, H. Support vector machine for classification of walking conditions of persons after stroke with dropped foot. Hum. Mov. Sci. 2009, 28, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Fulk, G.D.; Sazonov, E. Using sensors to measure activity in people with stroke. Top. Stroke Rehabil. 2011, 18, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, K.; Wit, A.; Krawczyk, M.; Zaborski, J.; Gajewski, J. Associations between gait patterns, brain lesion factors and functional recovery in stroke patients. Gait Posture 2012, 35, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.; Novak, D.; Omlin, X.; Pulfer, M.; Perreault, E.; Zimmerli, L.; Mihelj, M.; Riener, R. Real-time closed-loop control of cognitive load in neurological patients during robot-assisted gait training. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 453–464. [Google Scholar] [CrossRef]
- Capela, N.A.; Lemaire, E.D.; Baddour, N. Feature selection for wearable smartphone-based human activity recognition with able bodied, elderly, and stroke patients. PLoS ONE 2015, 10, e0124414. [Google Scholar] [CrossRef]
- Oubre, B.; Daneault, J.-F.; Jung, H.-T.; Whritenour, K.; Miranda, J.G.V.; Park, J.; Ryu, T.; Kim, Y.; Lee, S.I. Estimating upper-limb impairment level in stroke survivors using wearable inertial sensors and a minimally-burdensome motor task. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 601–611. [Google Scholar] [CrossRef]
- Bai, J.; Song, A. Development of a novel home based multi-scene upper limb rehabilitation training and evaluation system for post-stroke patients. IEEE Access 2019, 7, 9667–9677. [Google Scholar] [CrossRef]
- Chae, S.H.; Kim, Y.; Lee, K.; Park, H. Development and clinical evaluation of a web-based upper limb home rehabilitation system using a smartwatch and machine learning model for chronic stroke survivors: Prospective comparative study. JMIR MHealth UHealth 2020, 8, e17216. [Google Scholar] [CrossRef]
- Miao, S.; Shen, C.; Feng, X.; Zhu, Q.; Shorfuzzaman, M.; Lv, Z. Upper limb rehabilitation system for stroke survivors based on multi-modal sensors and machine learning. IEEE Access 2021, 9, 30283–30291. [Google Scholar] [CrossRef]
- Bochniewicz, E.M.; Emmer, G.; McLeod, A.; Barth, J.; Dromerick, A.W.; Lum, P. Measuring functional arm movement after stroke using a single wrist-worn sensor and machine learning. J. Stroke Cerebrovasc. Dis. 2017, 26, 2880–2887. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Ryser, A.; Veerbeek, J.M.; Verheyden, G.; Vogt, J.E.; Luft, A.R.; Awai Easthope, C. Classification of functional and non-functional arm use by inertial measurement units in individuals with upper limb impairment after stroke. Front. Physiol. 2022, 13, 952757. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Cranny, A.; Gupta, N.; Maharatna, K.; Achner, J.; Klemke, J.; Jöbges, M.; Ortmann, S. Recognizing upper limb movements with wrist worn inertial sensors using k-means clustering classification. Hum. Mov. Sci. 2015, 40, 59–76. [Google Scholar] [CrossRef]
- Friedman, N.; Rowe, J.B.; Reinkensmeyer, D.J.; Bachman, M. The manumeter: A wearable device for monitoring daily use of the wrist and fingers. IEEE J. Biomed. Health Inform. 2014, 18, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, X.; Zhu, X.; Gao, X.; Chen, X.; Chen, X. A regression-based framework for quantitative assessment of muscle spasticity using combined EMG and inertial data from wearable sensors. Front. Neurosci. 2019, 13, 398. [Google Scholar] [CrossRef]
- Wang, C.; Peng, L.; Hou, Z.-G.; Li, J.; Zhang, T.; Zhao, J. Quantitative assessment of upper-limb motor function for post-stroke rehabilitation based on motor synergy analysis and multi-modality fusion. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 943–952. [Google Scholar] [CrossRef]
- Yang, H.; Guan, C.; Chua, K.S.G.; Wang, C.C.; Soon, P.K.; Tang, C.K.Y.; Ang, K.K. Detection of motor imagery of swallow EEG signals based on the dual-tree complex wavelet transform and adaptive model selection. J. Neural Eng. 2014, 11, 035016. [Google Scholar] [CrossRef]
- Lu, Z.; Tong, K.; Zhang, X.; Li, S.; Zhou, P. Myoelectric pattern recognition for controlling a robotic hand: A feasibility study in stroke. IEEE Trans. Biomed. Eng. 2018, 66, 365–372. [Google Scholar] [CrossRef]
- Yang, G.; Deng, J.; Pang, G.; Zhang, H.; Li, J.; Deng, B.; Pang, Z.; Xu, J.; Jiang, M.; Liljeberg, P.; et al. An IoT-enabled stroke rehabilitation system based on smart wearable armband and machine learning. IEEE J. Transl. Eng. Health Med. 2018, 6, 2100510. [Google Scholar] [CrossRef]
- Cai, S.; Chen, Y.; Huang, S.; Wu, Y.; Zheng, H.; Li, X.; Xie, L. SVM-based classification of sEMG signals for upper-limb self-rehabilitation training. Front. Neurorobotics 2019, 13, 31. [Google Scholar] [CrossRef]
- Ngeo, J.; Tamei, T.; Shibata, T.; Orlando, M.F.; Behera, L.; Saxena, A.; Dutta, A. Control of an Optimal Finger Exoskeleton Based on Continuous Joint Angle Estimation from EMG Signals. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 338–341. [Google Scholar]
- Meng, L.; Zhang, T.; Zhao, X.; Wang, D.; Xu, R.; Yang, A.; Ming, D. A quantitative lower limb function assessment method based on fusion of surface EMG and inertial data in stroke patients during cycling task. Biomed. Signal Process. Control 2023, 85, 104880. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.-J. Prediction of myoelectric biomarkers in post-stroke gait. Sensors 2021, 21, 5334. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.M.D.P.M.; Weraniyagoda, W.A.S.A.; Rajapaksha, R.T.M.; Wijesekara, P.A.D.S.N.; Sudheera, K.L.K.; Chong, P.H.J. Automatic assessment of aphasic speech sensed by audio sensors for classification into aphasia severity levels to recommend speech therapies. Sensors 2022, 22, 6966. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, L.; Hillis, A.E.; Wilmskoetter, J.; Hickok, G.; Basilakos, A.; Munsell, B.; Rorden, C.; Fridriksson, J. Neural structures supporting spontaneous and assisted (entrained) speech fluency. Brain 2019, 142, 3951–3962. [Google Scholar] [CrossRef]
- ElGohary, S.H.; Lithy, A.; Khamis, S.; Ali, A.; Alaa el-din, A.; Abd El-Azim, H. Interactive Virtual Rehabilitation for Aphasic Arabic-Speaking Patients. Adv. Sci. Technol. Eng. Syst. J. 2020, 5, 1225–1232. [Google Scholar] [CrossRef]
- Moses, D.A.; Metzger, S.L.; Liu, J.R.; Anumanchipalli, G.K.; Makin, J.G.; Sun, P.F.; Chartier, J.; Dougherty, M.E.; Liu, P.M.; Abrams, G.M.; et al. Neuroprosthesis for decoding speech in a paralyzed person with anarthria. N. Engl. J. Med. 2021, 385, 217–227. [Google Scholar] [CrossRef]
- Mahmoud, S.S.; Kumar, A.; Tang, Y.; Li, Y.; Gu, X.; Fu, J.; Fang, Q. An efficient deep learning based method for speech assessment of mandarin-speaking aphasic patients. IEEE J. Biomed. Health Inform. 2020, 24, 3191–3202. [Google Scholar] [CrossRef]
- Ye, W.; Jiang, Z.; Li, Q.; Liu, Y.; Mou, Z. A hybrid model for pathological voice recognition of post-stroke dysarthria by using 1DCNN and double-LSTM networks. Appl. Acoust. 2022, 197, 108934. [Google Scholar] [CrossRef]
- Yourganov, G.; Smith, K.G.; Fridriksson, J.; Rorden, C. Predicting aphasia type from brain damage measured with structural MRI. Cortex 2015, 73, 203–215. [Google Scholar] [CrossRef]
- Cervera, M.A.; Soekadar, S.R.; Ushiba, J.; Millán, J.d.R.; Liu, M.; Birbaumer, N.; Garipelli, G. Brain-computer interfaces for post-stroke motor rehabilitation: A meta-analysis. Ann. Clin. Transl. Neurol. 2018, 5, 651–663. [Google Scholar] [CrossRef]
- Ang, K.K.; Guan, C. EEG-Based Strategies to Detect Motor Imagery for Control and Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 392–401. [Google Scholar] [CrossRef]
- Carino-Escobar, R.I.; Carrillo-Mora, P.; Valdés-Cristerna, R.; Rodriguez-Barragan, M.A.; Hernandez-Arenas, C.; Quinzaños-Fresnedo, J.; Galicia-Alvarado, M.A.; Cantillo-Negrete, J. Longitudinal Analysis of Stroke Patients’ Brain Rhythms during an Intervention with a Brain-Computer Interface. Neural Plast. 2019, 2019, 7084618. [Google Scholar] [CrossRef] [PubMed]
- Leamy, D.J.; Kocijan, J.; Domijan, K.; Duffin, J.; Roche, R.A.; Commins, S.; Collins, R.; Ward, T.E. An exploration of EEG features during recovery following stroke—Implications for BCI-mediated neurorehabilitation therapy. J. NeuroEngineering Rehabil. 2014, 11, 9. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.-J. Quantitative Evaluation of Task-Induced Neurological Outcome after Stroke. Brain Sci. 2021, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Al-Qazzaz, N.K.; Ali, S.H.B.M.; Ahmad, S.A.; Islam, M.S.; Escudero, J. Discrimination of stroke-related mild cognitive impairment and vascular dementia using EEG signal analysis. Med. Biol. Eng. Comput. 2018, 56, 137–157. [Google Scholar] [CrossRef]
- Mak, J.; Kocanaogullari, D.; Huang, X.; Kersey, J.; Shih, M.; Grattan, E.S.; Skidmore, E.R.; Wittenberg, G.F.; Ostadabbas, S.; Akcakaya, M. Detection of Stroke-Induced Visual Neglect and Target Response Prediction Using Augmented Reality and Electroencephalography. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Chen, S.; Zhang, X.; Jin, J.; Xu, R.; Daly, I.; Jia, J.; Wang, X.; Cichocki, A.; Jung, T.-P. BCI-Based Rehabilitation on the Stroke in Sequela Stage. Neural Plast. 2020, 2020, 8882764. [Google Scholar] [CrossRef]
- Abdallah, I.B.; Bouteraa, Y. An Optimized Stimulation Control System for Upper Limb Exoskeleton Robot-Assisted Rehabilitation Using a Fuzzy Logic-Based Pain Detection Approach. Sensors 2024, 24, 1047. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, J.; Deng, J.; Dewald, J.P.A. EEG-based classification for elbow versus shoulder torque intentions involving stroke subjects. Comput. Biol. Med. 2009, 39, 443–452. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Hirata, M.; Saitoh, Y.; Goto, T.; Kishima, H.; Fukuma, R.; Yokoi, H.; Kamitani, Y.; Yoshimine, T. Real-time control of a prosthetic hand using human electrocorticography signals: Technical note. J. Neurosurg. 2011, 114, 1715–1722. [Google Scholar] [CrossRef]
- Cantillo-Negrete, J.; Carino-Escobar, R.I.; Carrillo-Mora, P.; Elias-Vinas, D.; Gutierrez-Martinez, J. Motor Imagery-Based Brain-Computer Interface Coupled to a Robotic Hand Orthosis Aimed for Neurorehabilitation of Stroke Patients. J. Healthc. Eng. 2018, 2018, 1624637. [Google Scholar] [CrossRef] [PubMed]
- Bong, S.Z.; Wan, K.; Murugappan, M.; Ibrahim, N.M.; Rajamanickam, Y.; Mohamad, K. Implementation of wavelet packet transform and non linear analysis for emotion classification in stroke patient using brain signals. Biomed. Signal Process. Control 2017, 36, 102–112. [Google Scholar] [CrossRef]
- Oña, E.; Cano-de La Cuerda, R.; Sánchez-Herrera, P.; Balaguer, C.; Jardón, A. A review of robotics in neurorehabilitation: Towards an automated process for upper limb. J. Healthc. Eng. 2018, 2018, 9758939. [Google Scholar] [CrossRef] [PubMed]
- Zollo, L.; Rossini, L.; Bravi, M.; Magrone, G.; Sterzi, S.; Guglielmelli, E. Quantitative evaluation of upper-limb motor control in robot-aided rehabilitation. Med. Biol. Eng. Comput. 2011, 49, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Kitago, T.; Goldsmith, J.; Harran, M.; Kane, L.; Berard, J.; Huang, S.; Ryan, S.L.; Mazzoni, P.; Krakauer, J.W.; Huang, V.S. Robotic therapy for chronic stroke: General recovery of impairment or improved task-specific skill? J. Neurophysiol. 2015, 114, 1885–1894. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Liu, C.-W.; Lai, C.-H.; Kang, J.-H.; Tseng, S.-H.; Su, E.C.-Y. Prediction of robotic neurorehabilitation functional ambulatory outcome in patients with neurological disorders. J. NeuroEngineering Rehabil. 2021, 18, 174. [Google Scholar] [CrossRef]
- Kyrarini, M.; Lygerakis, F.; Rajavenkatanarayanan, A.; Sevastopoulos, C.; Nambiappan, H.R.; Chaitanya, K.K.; Babu, A.R.; Mathew, J.; Makedon, F. A Survey of Robots in Healthcare. Technologies 2021, 9, 8. [Google Scholar] [CrossRef]
- Lyu, M.; Chen, W.-H.; Ding, X.; Wang, J. Knee exoskeleton enhanced with artificial intelligence to provide assistance-as-needed. Rev. Sci. Instrum. 2019, 90, 094101. [Google Scholar] [CrossRef]
- Rea, M.; Rana, M.; Lugato, N.; Terekhin, P.; Gizzi, L.; Brötz, D.; Fallgatter, A.; Birbaumer, N.; Sitaram, R.; Caria, A. Lower Limb Movement Preparation in Chronic Stroke: A Pilot Study Toward an fNIRS-BCI for Gait Rehabilitation. Neurorehabil. Neural Repair 2014, 28, 564–575. [Google Scholar] [CrossRef]
- Chung, C.-R.; Su, M.-C.; Lee, S.-H.; Wu, E.H.-K.; Tang, L.-H.; Yeh, S.-C. An Intelligent Motor Assessment Method Utilizing a Bi-Lateral Virtual-Reality Task for Stroke Rehabilitation on Upper Extremity. IEEE J. Transl. Eng. Health Med. 2022, 10, 2100811. [Google Scholar] [CrossRef]
- Avola, D.; Cinque, L.; Foresti, G.L.; Marini, M.R. An interactive and low-cost full body rehabilitation framework based on 3D immersive serious games. J. Biomed. Inform. 2019, 89, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Maskeliūnas, R.; Damaševičius, R.; Blažauskas, T.; Canbulut, C.; Adomavičienė, A.; Griškevičius, J. BiomacVR: A Virtual Reality-Based System for Precise Human Posture and Motion Analysis in Rehabilitation Exercises Using Depth Sensors. Electronics 2023, 12, 339. [Google Scholar] [CrossRef]
- Chatterjee, K.; Buchanan, A.; Cottrell, K.; Hughes, S.; Day, T.W.; John, N.W. Immersive Virtual Reality for the Cognitive Rehabilitation of Stroke Survivors. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Liu, J. Wearable Sensing Based Virtual Reality Rehabilitation Scheme for Upper Limb Training. In Intelligent Robotics and Applications; Liu, H., Yin, Z., Liu, L., Jiang, L., Gu, G., Wu, X., Ren, W., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2022; Volume 13457, pp. 24–36. ISBN 978-3-031-13834-8. [Google Scholar]
- Zainal, N.; Al-Hadi, I.A.A.-Q.; Ghaleb, S.M.; Hussain, H.; Ismail, W.; Aldailamy, A.Y. Predicting MIRA Patients’ Performance Using Virtual Rehabilitation Programme by Decision Tree Modeling. In Recent Advances in Intelligent Systems and Smart Applications; Al-Emran, M., Shaalan, K., Hassanien, A.E., Eds.; Studies in Systems, Decision and Control; Springer International Publishing: Cham, Switzerland, 2021; Volume 295, pp. 451–462. ISBN 978-3-030-47410-2. [Google Scholar]
- Zhi, Y.X.; Lukasik, M.; Li, M.H.; Dolatabadi, E.; Wang, R.H.; Taati, B. Automatic detection of compensation during robotic stroke rehabilitation therapy. IEEE J. Transl. Eng. Health Med. 2017, 6, 2100107. [Google Scholar] [CrossRef]
- Oña, E.D.; Jardón, A.; Balaguer, C. The Automated Box and Blocks Test an Autonomous Assessment Method of Gross Manual Dexterity in Stroke Rehabilitation. In Towards Autonomous Robotic Systems; Gao, Y., Fallah, S., Jin, Y., Lekakou, C., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2017; Volume 10454, pp. 101–114. ISBN 978-3-319-64106-5. [Google Scholar]
- Weiss Cohen, M.; Regazzoni, D. Hand rehabilitation assessment system using leap motion controller. AI Soc. 2020, 35, 581–594. [Google Scholar] [CrossRef]
- Sucar, L.E.; Luis, R.; Leder, R.; Hernández, J.; Sánchez, I. Gesture Therapy: A Vision-Based System for Upper Extremity Stroke Rehabilitation. In Proceedings of the 2010 Annual international conference of the IEEE engineering in medicine and biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3690–3693. [Google Scholar]
- Han, Y.; Varadarajan, A.; Kim, T.; Zheng, G.; Kitani, K.; Kelliher, A.; Rikakis, T.; Park, Y.-L. Smart Skin: Vision-Based Soft Pressure Sensing System for In-Home Hand Rehabilitation. Soft Robot. 2022, 9, 473–485. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Feng, Y.; Li, W.; Niu, J.; Gan, Z. Measurement Method of Human Lower Limb Joint Range of Motion Through Human-Machine Interaction Based on Machine Vision. Front. Neurorobotics 2021, 15, 753924. [Google Scholar] [CrossRef] [PubMed]
- Panwar, M.; Biswas, D.; Bajaj, H.; Jobges, M.; Turk, R.; Maharatna, K.; Acharyya, A. Rehab-Net: Deep Learning Framework for Arm Movement Classification Using Wearable Sensors for Stroke Rehabilitation. IEEE Trans. Biomed. Eng. 2019, 66, 3026–3037. [Google Scholar] [CrossRef]
- Bijalwan, V.; Semwal, V.B.; Singh, G.; Mandal, T.K. HDL-PSR: Modelling Spatio-Temporal Features Using Hybrid Deep Learning Approach for Post-Stroke Rehabilitation. Neural Process. Lett. 2023, 55, 279–298. [Google Scholar] [CrossRef]
- Lonini, L.; Moon, Y.; Embry, K.; Cotton, R.J.; McKenzie, K.; Jenz, S.; Jayaraman, A. Video-Based Pose Estimation for Gait Analysis in Stroke Survivors during Clinical Assessments: A Proof-of-Concept Study. Digit. Biomark. 2022, 6, 9–18. [Google Scholar] [CrossRef]
- Rose, L.; Bazzocchi, M.C.F.; Nejat, G. End-to-End Deep Reinforcement Learning for Exoskeleton Control. In Proceedings of the 2020 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Toronto, ON, Canada, 11 October 2020; pp. 4294–4301. [Google Scholar]
- Wang, P.; Zhang, Q.; Li, L.; Ru, F.; Li, D.; Jin, Y. Deep Learning-Based Gesture Recognition for Control of Mobile Body-Weight Support Platform. In Proceedings of the 2018 13th IEEE Conference on Industrial Electronics and Applications (ICIEA), Wuhan, China, 31 May–2 June 2018; pp. 1803–1808. [Google Scholar]
- Iosa, M.; Paolucci, S.; Antonucci, G.; Ciancarelli, I.; Morone, G. Application of an Artificial Neural Network to Identify the Factors Influencing Neurorehabilitation Outcomes of Patients with Ischemic Stroke Treated with Thrombolysis. Biomolecules 2023, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Campagnini, S.; Arienti, C.; Patrini, M.; Liuzzi, P.; Mannini, A.; Carrozza, M.C. Machine learning methods for functional recovery prediction and prognosis in post-stroke rehabilitation: A systematic review. J. NeuroEngineering Rehabil. 2022, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Hughes, R.; Hester, T.; Stein, J.; Akay, M.; Dy, J.G.; Bonato, P. A Novel Approach to Monitor Rehabilitation Outcomes in Stroke Survivors Using Wearable Technology. Proc. IEEE 2010, 98, 450–461. [Google Scholar] [CrossRef]
- Kurtz, P.; Peres, I.T.; Soares, M.; Salluh, J.I.F.; Bozza, F.A. Hospital Length of Stay and 30-Day Mortality Prediction in Stroke: A Machine Learning Analysis of 17,000 ICU Admissions in Brazil. Neurocrit. Care 2022, 37, 313–321. [Google Scholar] [CrossRef]
- Fast, L.; Temuulen, U.; Villringer, K.; Kufner, A.; Ali, H.F.; Siebert, E.; Huo, S.; Piper, S.K.; Sperber, P.S.; Liman, T.; et al. Machine learning-based prediction of clinical outcomes after first-ever ischemic stroke. Front. Neurol. 2023, 14, 1114360. [Google Scholar] [CrossRef]
- Oczkowski, W.J.; Barreca, S. Neural network modeling accurately predicts the functional outcome of stroke survivors with moderate disabilities. Arch. Phys. Med. Rehabil. 1997, 78, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Chang, H.; Nam, H.S. A Bayesian Network Model for Predicting Post-stroke Outcomes With Available Risk Factors. Front. Neurol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Nishi, H.; Oishi, N.; Ishii, A.; Ono, I.; Ogura, T.; Sunohara, T.; Chihara, H.; Fukumitsu, R.; Okawa, M.; Yamana, N.; et al. Predicting Clinical Outcomes of Large Vessel Occlusion Before Mechanical Thrombectomy Using Machine Learning. Stroke 2019, 50, 2379–2388. [Google Scholar] [CrossRef]
- Monteiro, M.; Fonseca, A.C.; Freitas, A.T.; Pinho E Melo, T.; Francisco, A.P.; Ferro, J.M.; Oliveira, A.L. Using Machine Learning to Improve the Prediction of Functional Outcome in Ischemic Stroke Patients. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 15, 1953–1959. [Google Scholar] [CrossRef]
- Asadi, H.; Dowling, R.; Yan, B.; Mitchell, P. Machine Learning for Outcome Prediction of Acute Ischemic Stroke Post Intra-Arterial Therapy. PLoS ONE 2014, 9, e88225. [Google Scholar] [CrossRef]
- Tozlu, C.; Edwards, D.; Boes, A.; Labar, D.; Tsagaris, K.Z.; Silverstein, J.; Pepper Lane, H.; Sabuncu, M.R.; Liu, C.; Kuceyeski, A. Machine Learning Methods Predict Individual Upper-Limb Motor Impairment Following Therapy in Chronic Stroke. Neurorehabil. Neural Repair 2020, 34, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Chen, C.-H.; Tseng, Y.-J.; Tsai, Y.-T.; Chang, C.-Y.; Wang, H.-Y.; Chen, C.-K. Predicting post-stroke activities of daily living through a machine learning-based approach on initiating rehabilitation. Int. J. Med. Inf. 2018, 111, 159–164. [Google Scholar] [CrossRef]
- Martinez, H.B.; Cisek, K.; García-Rudolph, A.; Kelleher, J.D.; Hines, A. Understanding and Predicting Cognitive Improvement of Young Adults in Ischemic Stroke Rehabilitation Therapy. Front. Neurol. 2022, 13, 886477. [Google Scholar] [CrossRef]
- Choo, Y.J.; Kim, J.K.; Kim, J.H.; Chang, M.C.; Park, D. Machine learning analysis to predict the need for ankle foot orthosis in patients with stroke. Sci. Rep. 2021, 11, 8499. [Google Scholar] [CrossRef]
- Imura, T.; Inoue, Y.; Tanaka, R.; Matsuba, J.; Umayahara, Y. Clinical Features for Identifying the Possibility of Toileting Independence after Convalescent Inpatient Rehabilitation in Severe Stroke Patients: A Decision Tree Analysis Based on a Nationwide Japan Rehabilitation Database. J. Stroke Cerebrovasc. Dis. 2021, 30, 105483. [Google Scholar] [CrossRef]
- Gandolfi, M.; Boscolo Galazzo, I.; Gasparin Pavan, R.; Cruciani, F.; Vale, N.; Picelli, A.; Storti, S.F.; Smania, N.; Menegaz, G. eXplainable AI Allows Predicting Upper Limb Rehabilitation Outcomes in Sub-Acute Stroke Patients. IEEE J. Biomed. Health Inform. 2023, 27, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Lin, K.; Li, Y.; Lin, C.-J. Predicting patient-reported outcome of activities of daily living in stroke rehabilitation: A machine learning study. J. NeuroEngineering Rehabil. 2023, 20, 25. [Google Scholar] [CrossRef]
- Sale, P.; Ferriero, G.; Ciabattoni, L.; Cortese, A.M.; Ferracuti, F.; Romeo, L.; Piccione, F.; Masiero, S. Predicting Motor and Cognitive Improvement Through Machine Learning Algorithm in Human Subject that Underwent a Rehabilitation Treatment in the Early Stage of Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 2962–2972. [Google Scholar] [CrossRef] [PubMed]
- Rondina, J.M.; Park, C.; Ward, N.S. Brain regions important for recovery after severe post-stroke upper limb paresis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 737–743. [Google Scholar] [CrossRef]
- Rafiei, M.H.; Kelly, K.M.; Borstad, A.L.; Adeli, H.; Gauthier, L.V. Predicting Improved Daily Use of the More Affected Arm Poststroke Following Constraint-Induced Movement Therapy. Phys. Ther. 2019, 99, 1667–1678. [Google Scholar] [CrossRef]
- Halloran, S.; Tang, L.; Guan, Y.; Shi, J.Q.; Eyre, J. Remote Monitoring of Stroke Patients’ Rehabilitation Using Wearable Accelerometers. In Proceedings of the 23rd International Symposium on Wearable Computers, London, UK, 9 September 2019; pp. 72–77. [Google Scholar]
- Rehme, A.K.; Volz, L.J.; Feis, D.-L.; Bomilcar-Focke, I.; Liebig, T.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Identifying Neuroimaging Markers of Motor Disability in Acute Stroke by Machine Learning Techniques. Cereb. Cortex 2015, 25, 3046–3056. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, S.; Darainy, M.; Thiel, A.; Ostry, D.J. A Single Session of Robot-Controlled Proprioceptive Training Modulates Functional Connectivity of Sensory Motor Networks and Improves Reaching Accuracy in Chronic Stroke. Neurorehabil. Neural Repair 2019, 33, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.J.; Park, C.-H.; Girard, G.; Beanato, E.; Egger, P.; Evangelista, G.G.; Lee, J.; Wessel, M.J.; Morishita, T.; Koch, G.; et al. The structural connectome and motor recovery after stroke: Predicting natural recovery. Brain 2021, 144, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhai, X.; Cheng, D.; Pan, Y.; Dou, W. EEG Microstate-Specific Functional Connectivity and Stroke-Related Alterations in Brain Dynamics. Front. Neurosci. 2022, 16, 848737. [Google Scholar] [CrossRef] [PubMed]
- Del Gaizo, J.; Fridriksson, J.; Yourganov, G.; Hillis, A.E.; Hickok, G.; Misic, B.; Rorden, C.; Bonilha, L. Mapping Language Networks Using the Structural and Dynamic Brain Connectomes. eNeuro 2017, 4, ENEURO.0204-17.2017. [Google Scholar] [CrossRef]
- Mohanty, R.; Sinha, A.M.; Remsik, A.B.; Dodd, K.C.; Young, B.M.; Jacobson, T.; McMillan, M.; Thoma, J.; Advani, H.; Nair, V.A.; et al. Machine Learning Classification to Identify the Stage of Brain-Computer Interface Therapy for Stroke Rehabilitation Using Functional Connectivity. Front. Neurosci. 2018, 12, 353. [Google Scholar] [CrossRef]
- Liu, C.-F.; Hsu, J.; Xu, X.; Ramachandran, S.; Wang, V.; Miller, M.I.; Hillis, A.E.; Faria, A.V.; The STIR and VISTA Imaging investigators. Deep learning-based detection and segmentation of diffusion abnormalities in acute ischemic stroke. Commun. Med. 2021, 1, 61. [Google Scholar] [CrossRef]
- Stib, M.T.; Vasquez, J.; Dong, M.P.; Kim, Y.H.; Subzwari, S.S.; Triedman, H.J.; Wang, A.; Wang, H.-L.C.; Yao, A.D.; Jayaraman, M.; et al. Detecting Large Vessel Occlusion at Multiphase CT Angiography by Using a Deep Convolutional Neural Network. Radiology 2020, 297, 640–649. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, E.-J.; Kim, Y.-H.; Woo, J.C.; Ryu, O.-W.; Kwon, M.; Kwon, S.U.; Kim, J.S.; Kang, D.-W. Deep Learning Approach Using Diffusion-Weighted Imaging to Estimate the Severity of Aphasia in Stroke Patients. J. Stroke 2022, 24, 108–117. [Google Scholar] [CrossRef]
- Chauhan, S.; Vig, L.; De Filippo De Grazia, M.; Corbetta, M.; Ahmad, S.; Zorzi, M. A Comparison of Shallow and Deep Learning Methods for Predicting Cognitive Performance of Stroke Patients From MRI Lesion Images. Front. Neuroinformatics 2019, 13, 53. [Google Scholar] [CrossRef]
- Hilbert, A.; Ramos, L.A.; Van Os, H.J.A.; Olabarriaga, S.D.; Tolhuisen, M.L.; Wermer, M.J.H.; Barros, R.S.; Van Der Schaaf, I.; Dippel, D.; Roos, Y.B.W.E.M.; et al. Data-efficient deep learning of radiological image data for outcome prediction after endovascular treatment of patients with acute ischemic stroke. Comput. Biol. Med. 2019, 115, 103516. [Google Scholar] [CrossRef] [PubMed]
- Hatami, N.; Cho, T.-H.; Mechtouff, L.; Eker, O.F.; Rousseau, D.; Frindel, C. CNN-LSTM Based Multimodal MRI and Clinical Data Fusion for Predicting Functional Outcome in Stroke Patients. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, Scotland, UK, 11 July 2022; pp. 3430–3434. [Google Scholar]
- Boukhennoufa, I.; Zhai, X.; Utti, V.; Jackson, J.; McDonald-Maier, K.D. Wearable sensors and machine learning in post-stroke rehabilitation assessment: A systematic review. Biomed. Signal Process. Control 2022, 71, 103197. [Google Scholar] [CrossRef]
- Lee, S.I.; Adans-Dester, C.P.; OBrien, A.T.; Vergara-Diaz, G.P.; Black-Schaffer, R.; Zafonte, R.; Dy, J.G.; Bonato, P. Predicting and Monitoring Upper-Limb Rehabilitation Outcomes Using Clinical and Wearable Sensor Data in Brain Injury Survivors. IEEE Trans. Biomed. Eng. 2021, 68, 1871–1881. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Zhu, X.; Cao, X.; Yi, C.; Chen, Y.; Jia, J.; Lu, X. FER-PCVT: Facial Expression Recognition with Patch-Convolutional Vision Transformer for Stroke Patients. Brain Sci. 2022, 12, 1626. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; Chen, X.; Ying, J.; Wang, X.; Wang, H. Wearable Supernumerary Robotic Limb System Using a Hybrid Control Approach Based on Motor Imagery and Object Detection. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1298–1309. [Google Scholar] [CrossRef]
- Der Lee, J.; Chang, T.C.; Ting Yang, S.; Hsien Huang, C.; Hsieh, F.H.; Yi Wu, C. Prediction of quality of life after stroke rehabilitation. Neuropsychiatry 2016, 6, 369–375. [Google Scholar] [CrossRef]
- Epalte, K.; Tomsone, S.; Vētra, A.; Bērziņa, G. Patient experience using digital therapy “Vigo” for stroke patient recovery: A qualitative descriptive study. Disabil. Rehabil. Assist. Technol. 2023, 18, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Rose, L.; Bazzocchi, M.C.F.; Nejat, G. A model-free deep reinforcement learning approach for control of exoskeleton gait patterns. Robotica 2022, 40, 2189–2214. [Google Scholar] [CrossRef]
- Maskeliunas, R.; Damasevicius, R.; Paulauskas, A.; Ceravolo, M.G.; Charalambous, M.; Kambanaros, M.; Pampoulou, E.; Barbabella, F.; Poli, A.; Carvalho, C.V. Deep Reinforcement Learning-Based iTrain Serious Game for Caregivers Dealing with Post-Stroke Patients. Information 2022, 13, 564. [Google Scholar] [CrossRef]
- Tucan, P.; Gherman, B.; Major, K.; Vaida, C.; Major, Z.; Plitea, N.; Carbone, G.; Pisla, D. Fuzzy Logic-Based Risk Assessment of a Parallel Robot for Elbow and Wrist Rehabilitation. Int. J. Environ. Res. Public Health 2020, 17, 654. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Chen, J.; Chen, X.; Liang, J.; Du, M. Neural network based modeling and control of elbow joint motion under functional electrical stimulation. Neurocomputing 2019, 340, 171–179. [Google Scholar] [CrossRef]
- Andrade, K.O.; Joaquim, R.C.; Caurin, G.A.P.; Crocomo, M.K. Evolutionary Algorithms for a Better Gaming Experience in Rehabilitation Robotics. Comput. Entertain. 2018, 16, 4. [Google Scholar] [CrossRef]
- Xu, F.; Dong, G.; Li, J.; Yang, Q.; Wang, L.; Zhao, Y.; Yan, Y.; Zhao, J.; Pang, S.; Guo, D.; et al. Deep Convolution Generative Adversarial Network-Based Electroencephalogram Data Augmentation for Post-Stroke Rehabilitation with Motor Imagery. Int. J. Neural Syst. 2022, 32, 2250039. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Golan, D.; Hanna, S.; Ramachandran, M. Artificial intelligence, machine learning and the evolution of healthcare: A bright future or cause for concern? Bone Jt. Res. 2018, 7, 223–225. [Google Scholar] [CrossRef]
- Dumitrascu, O.M.; Demaerschalk, B.M. Telestroke. Curr. Cardiol. Rep. 2017, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Cho, S.; Baek, D.; Bang, H.; Paik, N. Upper extremity functional evaluation by fugl-meyer assessment scoring using depth-sensing camera in hemiplegic stroke patients. PLoS ONE 2016, 11, e0158640. [Google Scholar] [CrossRef]
- Shull, P.B.; Jiang, S.; Zhu, Y.; Zhu, X. Hand gesture recognition and finger angle estimation via wrist-worn modified barometric pressure sensing. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Várkuti, B.; Guan, C.; Pan, Y.; Phua, K.S.; Ang, K.K.; Kuah, C.W.K.; Chua, K.S.G.; Ang, B.T.; Birbaumer, N.; Sitaram, R. Resting state changes in functional connectivity correlate with movement recovery for bci and robot-assisted upper-extremity training after stroke. Neurorehabil. Neural Repair 2012, 27, 53–62. [Google Scholar] [CrossRef]
- Kashi, S.; Polak, R.F.; Lerner, B.; Rokach, L.; Levy-Tzedek, S. A machine-learning model for automatic detection of movement compensations in stroke patients. IEEE Trans. Emerg. Top. Comput. 2020, 9, 1234–1247. [Google Scholar] [CrossRef]
- Vélez-Guerrero, M.A.; Callejas-Cuervo, M.; Mazzoleni, S. Artificial intelligence-based wearable robotic exoskeletons for upper limb rehabilitation: A review. Sensors 2021, 21, 2146. [Google Scholar] [CrossRef]
- Sarajchi, M.; Sirlantzis, K. Pediatric Robotic Lower-Limb Exoskeleton: An Innovative Design and Kinematic Analysis. IEEE Access 2023, 11, 115219–115230. [Google Scholar] [CrossRef]
- Alshami, A.; Elsayed, M.; Ali, E.; Eltoukhy, A.E.E.; Zayed, T. Harnessing the Power of ChatGPT for Automating Systematic Review Process: Methodology, Case Study, Limitations, and Future Directions. Systems 2023, 11, 351. [Google Scholar] [CrossRef]
- Thirunavukarasu, A.J.; Ting, D.S.J.; Elangovan, K.; Gutierrez, L.; Tan, T.F.; Ting, D.S.W. Large Language Models in Medicine. Nat. Med. 2023, 29, 1930–1940. [Google Scholar] [CrossRef] [PubMed]



| Base Search Terms (MeSH) |
|---|
| (“stroke”[MeSH Terms]) AND ((“artificial intelligence”[MeSH Terms]) OR (“machine learning”[MeSH Terms])) AND ((“stroke rehabilitation”[MeSH Terms]) OR (“neurological rehabilitation”[MeSH Terms]) OR (“neurosciences”[MeSH Terms]) OR (“Recovery of Function”[MeSH Terms])) AND (“adult”[MeSH Terms]) |
| Expanded Search Terms |
| (“Artificial Intelligence” OR “Machine learning” OR “Deep Learning” OR “Computational Intelligence” OR “Machine Intelligence” OR “AI application*” OR “supervised learning” OR “unsupervised learning” OR “Artificial Neural Network*” OR “Computer Reasoning” OR “Computer Vision*” OR “Expert System*” OR “Natural Language Processing” OR “NLP”) AND (“Cerebral Vascular Accident” OR “Cerebrovascular Accident” OR “CVA” OR “Stroke” OR “Cerebrovascular Disorders” OR “Ischemic” OR “Brain attack” OR “Infarction” OR “Hemorrhagic” OR “Brain injury”) AND (“Rehabilitation” OR “Stroke rehabilitation” OR “neurological rehabilitation” OR “neurorehabilitation” OR “neuroscience” OR “Therapy” OR “Stroke Recovery” OR “Recovery” OR “Post Stroke” OR “Post-Stroke” OR “Poststroke” OR “profile” OR “profiling stroke” OR “trajectory recovery”) AND (“Adult*”) |
| Research Themes and Topics | n | % |
|---|---|---|
| Theme 1: Impairment | ||
| Functional impairment/capacity | 115 | 16 |
| Gait and mobility | 83 | 12 |
| Electromyography (EMG)/Motor impairment | 44 | 6 |
| Upper limb function | 28 | 4 |
| Speech | 23 | 3 |
| Theme 2: Assisted Intervention | ||
| Brain–Computer Interface (BCI) | 100 | 14 |
| Rehabilitation/Robot-assisted therapy | 61 | 9 |
| Virtual reality (VR) | 33 | 5 |
| Computer vision | 23 | 3 |
| Deep learning-based systems | 21 | 3 |
| Theme 3: Prediction | ||
| Outcome prediction | 50 | 7 |
| Data analysis for monitoring and service prediction | 39 | 6 |
| Motor function recovery | 14 | 2 |
| Theme 4: Imaging and Neuroscience | ||
| Functional connectivity | 40 | 6 |
| Medical imaging | 30 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senadheera, I.; Hettiarachchi, P.; Haslam, B.; Nawaratne, R.; Sheehan, J.; Lockwood, K.J.; Alahakoon, D.; Carey, L.M. AI Applications in Adult Stroke Recovery and Rehabilitation: A Scoping Review Using AI. Sensors 2024, 24, 6585. https://doi.org/10.3390/s24206585
Senadheera I, Hettiarachchi P, Haslam B, Nawaratne R, Sheehan J, Lockwood KJ, Alahakoon D, Carey LM. AI Applications in Adult Stroke Recovery and Rehabilitation: A Scoping Review Using AI. Sensors. 2024; 24(20):6585. https://doi.org/10.3390/s24206585
Chicago/Turabian StyleSenadheera, Isuru, Prasad Hettiarachchi, Brendon Haslam, Rashmika Nawaratne, Jacinta Sheehan, Kylee J. Lockwood, Damminda Alahakoon, and Leeanne M. Carey. 2024. "AI Applications in Adult Stroke Recovery and Rehabilitation: A Scoping Review Using AI" Sensors 24, no. 20: 6585. https://doi.org/10.3390/s24206585
APA StyleSenadheera, I., Hettiarachchi, P., Haslam, B., Nawaratne, R., Sheehan, J., Lockwood, K. J., Alahakoon, D., & Carey, L. M. (2024). AI Applications in Adult Stroke Recovery and Rehabilitation: A Scoping Review Using AI. Sensors, 24(20), 6585. https://doi.org/10.3390/s24206585







