Muscle Oxygen Saturation Dynamics During Upper-Body Resistance Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.3. Bench Press Strength Testing and Protocol
2.4. Muscle Oxygenation Assessment
2.5. Statistical Analysis
3. Results
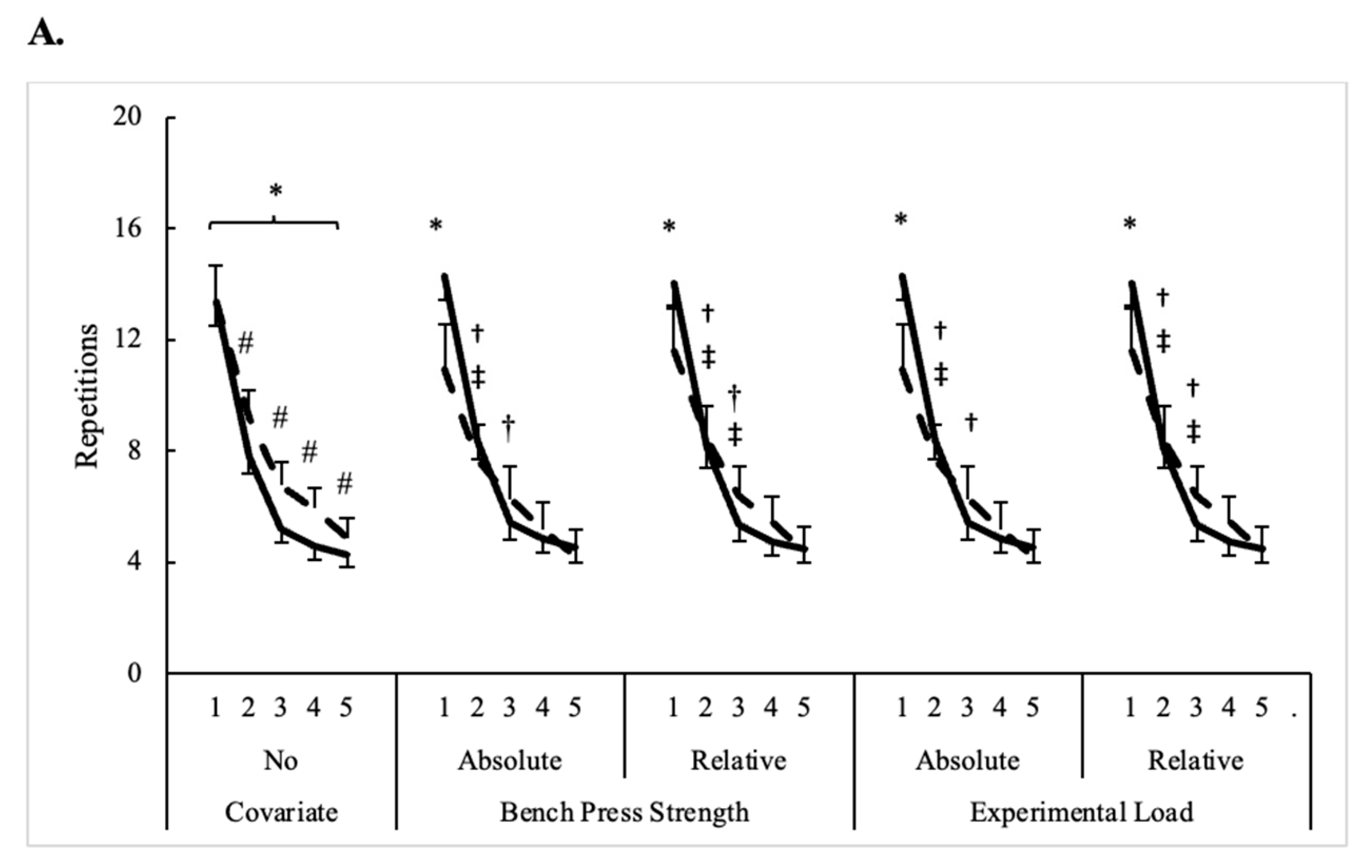
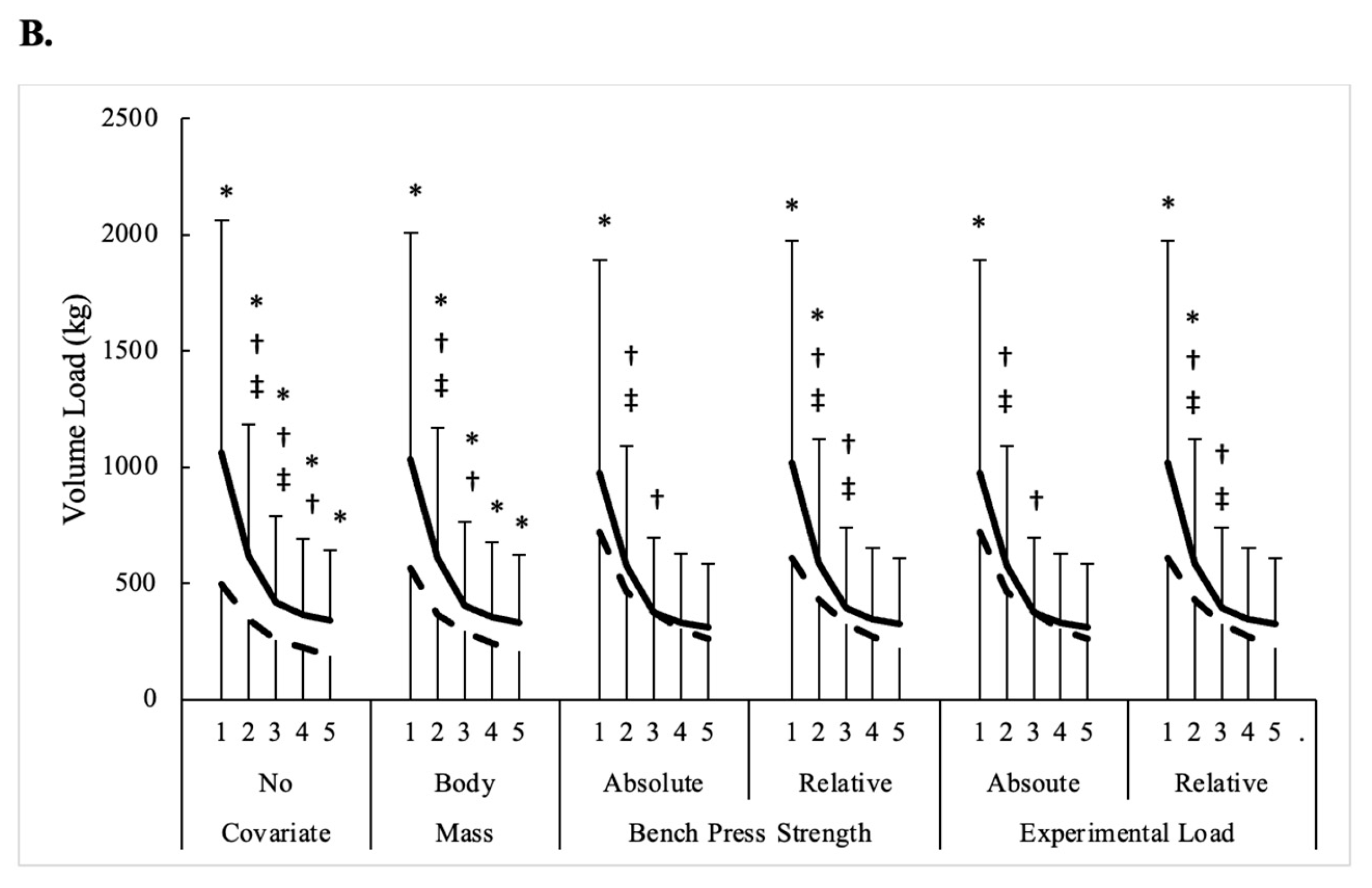
3.1. Bench Press Performance
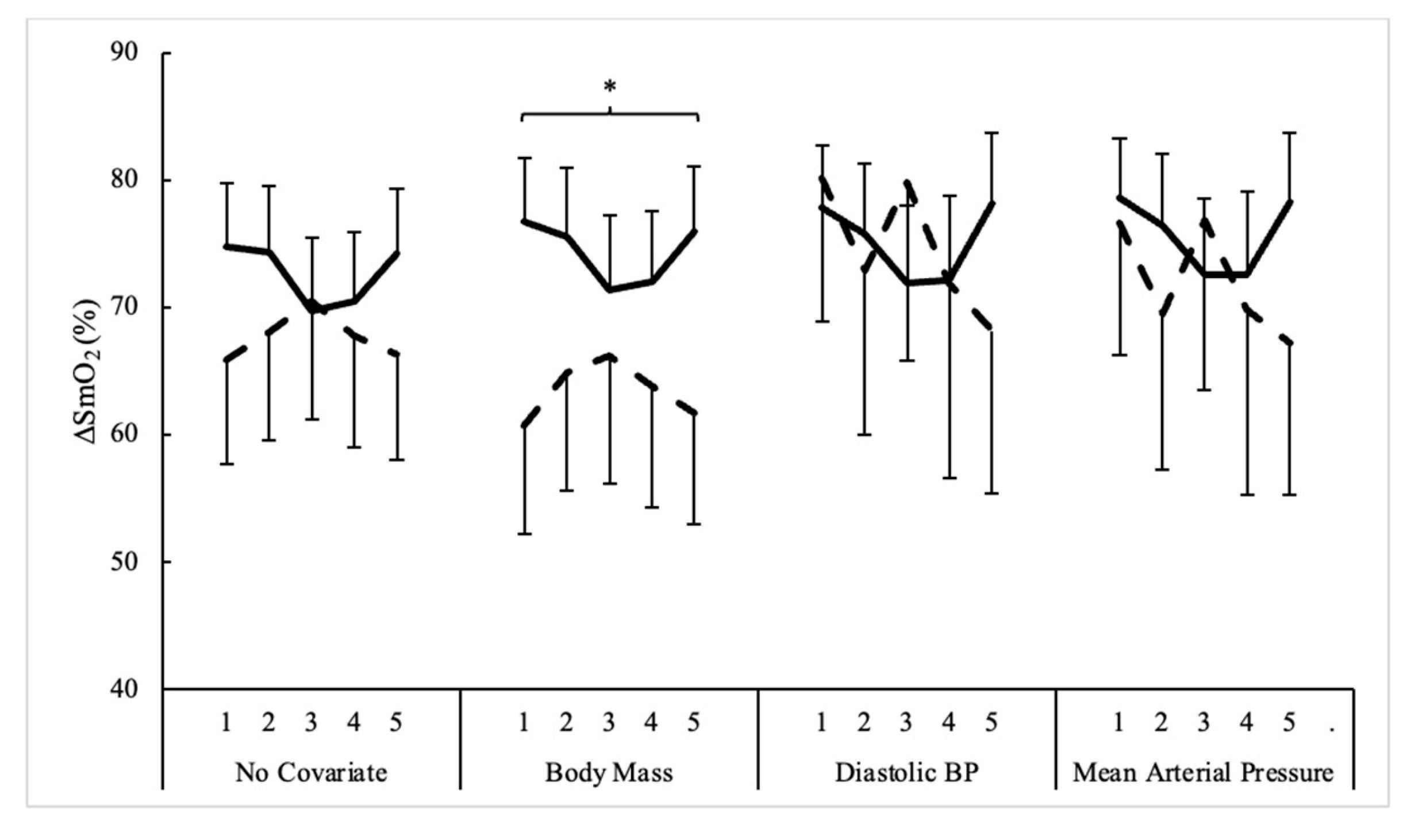
3.2. Muscle Oxygenation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuesta, M.; Yáñez-Sepúlveda, R.; Verdugo-Marchese, H.; Mateluna, C.; Alvear-Ordenes, I. Near-infrared spectroscopy used to assess physiological muscle adaptations in exercise clinical trials: A systematic review. Biology 2022, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Fuentes, C.; Chirosa-Ríos, L.J.; Guisado-Requena, I.M.; Delgado-Floody, P.; Jerez-Mayorga, D. Changes in muscle oxygen saturation measured using wireless near-infrared spectroscopy in resistance training: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 4293. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef] [PubMed]
- Perrey, S.; Ferrari, M. Muscle oximetry in sports science: A systematic review. Sports Med. 2018, 48, 597–616. [Google Scholar] [CrossRef]
- Crum, E.; O’connor, W.; Van Loo, L.; Valckx, M.; Stannard, S. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur. J. Sport Sci. 2017, 17, 1037–1043. [Google Scholar]
- Spiering, B.A.; Clark, B.C.; Schoenfeld, B.J.; Foulis, S.A.; Pasiakos, S.M. Maximizing Strength: The Stimuli and Mediators of Strength Gains and Their Application to Training and Rehabilitation. J. Strength Cond. Res. 2022, 10, 1519. [Google Scholar]
- McCully, K.; Iotti, S.; Kendrick, K.; Wang, Z.; Posner, J.; Leigh, J., Jr.; Chance, B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J. Appl. Physiol. 1994, 77, 5–10. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Im, J.; Rundell, K.W.; Kang, J.; Nioka, S.; Speiring, B.A.; Kime, R.; Chance, B. Effect of muscle oxygenation during resistance exercise on anabolic hormone response. Med. Sci. Sports Exerc. 2003, 35, 1929–1934. [Google Scholar] [CrossRef]
- Gómez-Carmona, C.D.; Bastida-Castillo, A.; Rojas-Valverde, D.; de la Cruz Sánchez, E.; García-Rubio, J.; Ibáñez, S.J.; Pino-Ortega, J. Lower-limb dynamics of muscle oxygen saturation during the back-squat exercise: Effects of training load and effort level. J. Strength Cond. Res. 2020, 34, 1227–1236. [Google Scholar] [CrossRef]
- Azuma, K.; Homma, S.; Kagaya, A. Oxygen supply-consumption balance in the thigh muscles during exhausting knee-extension exercise. J. Biomed. Opt. 2000, 5, 97–101. [Google Scholar] [CrossRef]
- Davis, P.R.; Yakel, J.P.; Anderson, D.J. Muscle oxygen demands of the vastus lateralis in back and front squats. Int. J. Exerc. Sci. 2020, 13, 734. [Google Scholar] [PubMed]
- Timón, R.; Ponce-González, J.G.; González-Montesinos, J.L.; Olcina, G.; Pérez-Pérez, A.; Castro-Piñero, J. Inertial flywheel resistance training and muscle oxygen saturation. J. Sports Med. Phys. Fit. 2017, 58, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Wells, A.J.; Gordon, J.A.; Arroyo, E.; Varanoske, A.N.; Coker, N.A.; Fukuda, D.H.; Stout, J.R.; Hoffman, J.R. Differences in muscle oxygenation between young and middle-aged recreationally active men during high-volume resistance exercise. Kinesiology 2019, 51, 3–11. [Google Scholar] [CrossRef]
- Dipla, K.; Triantafyllou, A.; Koletsos, N.; Papadopoulos, S.; Sachpekidis, V.; Vrabas, I.S.; Gkaliagkousi, E.; Zafeiridis, A.; Douma, S. Impaired muscle oxygenation and elevated exercise blood pressure in hypertensive patients: Links with vascular stiffness. Hypertension 2017, 70, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R. Norms for Fitness, Performance, and Health; Human Kinetics: Champaign, IL, USA, 2006. [Google Scholar]
- Brzycki, M. Strength testing—Predicting a one-rep max from reps-to-fatigue. J. Phys. Educ. Recreat. Danc. 1993, 64, 88–90. [Google Scholar] [CrossRef]
- McManus, C.J.; Collison, J.; Cooper, C.E. Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J. Biomed. Opt. 2018, 23, 015007. [Google Scholar] [CrossRef]
- Feldmann, A.; Schmitz, R.; Erlacher, D. Near-infrared spectroscopy-derived muscle oxygen saturation on a 0% to 100% scale: Reliability and validity of the Moxy Monitor. J. Biomed. Opt. 2019, 24, 115001. [Google Scholar] [CrossRef]
- Haynes, J.T.; Townsend, J.R.; Aziz, M.A.; Jones, M.D.; Littlefield, L.A.; Ruiz, M.D.; Johnson, K.D.; Gonzalez, A.M. Impact of Red Spinach Extract Supplementation on Bench Press Performance, Muscle Oxygenation, and Cognitive Function in Resistance-Trained Males. Sports 2021, 9, 77. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Farney, T.M.; Trepanowski, J.F.; McCarthy, C.G.; Canale, R.E.; Schilling, B.K. Comparison of pre-workout nitric oxide stimulating dietary supplements on skeletal muscle oxygen saturation, blood nitrate/nitrite, lipid peroxidation, and upper body exercise performance in resistance trained men. J. Int. Soc. Sports Nutr. 2010, 7, 16. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Farney, T.M.; McCarthy, C.G.; Schilling, B.K.; Craig, S.A.; Bloomer, R.J. The effects of chronic betaine supplementation on exercise performance, skeletal muscle oxygen saturation and associated biochemical parameters in resistance trained men. J. Strength Cond. Res. 2011, 25, 3461–3471. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Hughes, V.A.; Lutz, K.J.; Evans, W.J. A cross-sectional study of muscle strength and mass in 45-to 78-yr-old men and women. J. Appl. Physiol. 1991, 71, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.J. The influence of aging and sex on skeletal muscle mass and strength. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Cureton, K.; Collins, M. Sex difference in muscular strength in equally-trained men and women. Ergonomics 1987, 30, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.J.; Sung, J.; Silber, H.A.; Fleg, J.L.; Kelemen, M.D.; Turner, K.L.; Bacher, A.C.; Dobrosielski, D.A.; DeRegis, J.R.; Shapiro, E.P. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am. J. Hypertens. 2004, 17, 314–320. [Google Scholar] [CrossRef]
- Bailey, S.J.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. The nitrate-nitrite-nitric oxide pathway: Its role in human exercise physiology. Eur. J. Sport Sci. 2012, 12, 309–320. [Google Scholar] [CrossRef]
| Men (n = 44) | Women (n = 17) | All Participants (n = 61) | |
|---|---|---|---|
| Age (y) | 21.8 ± 2.6 | 20.2 ± 1.8 | 22.1 ± 0.4 |
| Height (cm) | 179 ± 6 | 166 ± 9 | 176 ± 1 |
| Body mass (kg) | 89.3 ± 15.7 | 70.3 ± 14.8 | 81.4 ± 1.7 |
| Resistance Training Experience (y) | 5.8 ± 3.1 | 3.9 ± 1.8 | 5.5 ± 0.5 |
| Resting Blood Pressure (mmHg) | |||
| Systolic | 123 ± 9 | 115 ± 11 | 122 ± 2 |
| Diastolic | 66 ± 7 | 73 ± 7 | 67 ± 1 |
| Mean Arterial | 85 ± 7 | 87 ± 7 | 85 ± 1 |
| Resting Heart Rate (bpm) | |||
| Average | 70 ± 11 | 65 ± 7 | 69 ± 2 |
| Reserve | 128 ± 11 | 134 ± 7 | 129 ± 2 |
| Bench Press Strength (kg) | |||
| Absolute | 108.4 ± 25.6 | 51.5 ± 16.3 | 96.5 ± 4.2 |
| Relative (per kg of body mass) | 1.22 ± 0.25 | 0.75 ± 0.24 | 1.18 ± 0.04 |
| Experimental Load (kg) | |||
| Absolute | 81.3 ± 19.2 | 38.6 ± 12.2 | 72.4 ± 3.1 |
| Relative (per kg of body mass) | 0.92 ± 0.18 | 0.56 ± 0.18 | 0.88 ± 0.03 |
| Set 1 | Set 2 | Set 3 | Set 4 | Set 5 | Across All Sets | |
|---|---|---|---|---|---|---|
| Repetitions | ||||||
| Men | 13.4 ± 2.7 | 7.8 ± 2.2 | 5.2 ± 1.9 | 4.6 ± 1.5 | 4.3 ± 1.6 | 7.1 ± 3.8 |
| Women | 13.4 ± 3.1 | 9.2 ± 1.6 | 6.8 ± 1.2 | 5.9 ± 1.7 | 4.9 ± 1.4 | 8.0 ± 3.4 * |
| All | 13.4 ± 2.8 | 8.2 ± 2.1 # | 5.7 ± 1.9 # | 5.0 ± 1.6 # | 4.4 ± 1.5 # | 7.3 ± 3.7 |
| Volume Load (kg) | ||||||
| Men | 1061 ± 230 | 617 ± 179 # | 419 ± 175 # | 363 ± 124 # | 339 ± 129 | 560 ± 301 |
| Women | 497 ± 133 * | 346 ± 96 *# | 259 ± 83 * # | 224 ± 81 * | 187 ± 73 * | 303 ± 124 * |
| All | 904 ± 328 | 541 ± 201 | 375 ± 171 | 324 ± 129 | 297 ± 135 | 488 ± 251 |
| ∆%SmO2 (%) | ||||||
| Men | 74.7 ± 16.1 | 74.3 ± 18.5 | 69.7 ± 19.7 | 70.5 ± 19.6 | 74.2 ± 18.2 | 72.7 ± 2.4 |
| Women | 65.8 ± 18.1 | 68.1 ± 14.4 | 70.5 ± 17.6 | 67.7 ± 13.0 | 66.3 ± 13.3 | 67.7 ± 1.8 |
| All | 72.3 ± 17.0 | 72.6 ± 17.5 | 69.9 ± 19.0 | 69.8 ± 17.9 | 72.0 ± 17.3 | 71.3 ± 1.4 |
| SmO2RecT (sec) | ||||||
| Men | 52.4 ± 14.2 | 51.7 ± 12.4 | 50.6 ± 12.4 | 54.3 ± 14.1 | 61.6 ± 19.0 | 54.1 ± 4.4 |
| Women | 55.4 ± 18.8 | 58.0 ± 24.6 | 60.1 ± 16.0 | 54.5 ± 15.7 | 55.1 ± 17.6 | 56.6 ± 2.4 |
| All | 53.4 ± 15.7 | 53.8 ± 17.3 | 53.7 ± 14.2 | 54.4 ± 14.5 | 59.4 ± 18.7 | 54.9 ± 2.5 |
| SmO2RecSlope | ||||||
| Men | 1.12 ± 0.54 | 1.12 ± 0.57 | 0.98 ± 0.50 | 0.92 ± 0.53 | 0.80 ± 0.57 | 0.99 ± 0.14 |
| Women | 1.00 ± 0.69 | 0.82 ± 0.52 | 0.75 ± 0.53 | 0.74 ± 0.50 | 0.77 ± 0.55 | 0.82 ± 0.11 |
| All | 1.08 ± 0.59 | 1.02 ± 0.57 | 0.91 ± 0.51 | 0.86 ± 0.52 # | 0.79 ± 0.56 # | 0.93 ± 0.12 |
| SmO2Peak (%) | ||||||
| Men | 80.7 ± 12.1 | 79.9 ± 11.9 | 79.3 ± 12.1 | 80.8 ± 9.4 | 77.7 ± 12.3 | 79.7 ± 1.3 |
| Women | 81.3 ± 11.8 | 80.8 ± 10.9 | 80.8 ± 11.8 | 79.4 ± 11.8 | 76.3 ± 14.1 | 79.7 ± 2.0 |
| All | 80.9 ± 11.9 | 80.2 ± 11.5 | 79.8 ± 11.9 | 80.3 ± 10.2 | 77.2 ± 12.8 # | 79.7 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, A.M.; Mangine, G.T.; Pinzone, A.G.; Beyer, K.S.; Townsend, J.R. Muscle Oxygen Saturation Dynamics During Upper-Body Resistance Exercise. Sensors 2024, 24, 6668. https://doi.org/10.3390/s24206668
Gonzalez AM, Mangine GT, Pinzone AG, Beyer KS, Townsend JR. Muscle Oxygen Saturation Dynamics During Upper-Body Resistance Exercise. Sensors. 2024; 24(20):6668. https://doi.org/10.3390/s24206668
Chicago/Turabian StyleGonzalez, Adam M., Gerald T. Mangine, Anthony G. Pinzone, Kyle S. Beyer, and Jeremy R. Townsend. 2024. "Muscle Oxygen Saturation Dynamics During Upper-Body Resistance Exercise" Sensors 24, no. 20: 6668. https://doi.org/10.3390/s24206668
APA StyleGonzalez, A. M., Mangine, G. T., Pinzone, A. G., Beyer, K. S., & Townsend, J. R. (2024). Muscle Oxygen Saturation Dynamics During Upper-Body Resistance Exercise. Sensors, 24(20), 6668. https://doi.org/10.3390/s24206668








