Detection of Mycobacterium avium subsp. paratuberculosis in Australian Cattle and Sheep by Analysing Volatile Organic Compounds in Faeces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Panel of Samples, Culture and PCR Preparation
2.2. Sample Preparation
2.2.1. Cultures
2.2.2. Faeces
2.3. VOC Analysis
2.4. Data Analysis
= 1 − (sensitivity + specificity)/2.
2.5. Identification of Putative Biomarkers
2.6. Validation of GC-MS-Based Models for Faeces
2.6.1. eNose Data Collection and Model Training
2.6.2. Validation of eNose
3. Results
3.1. Culture and qPCR
3.2. Assessment of Volatile Organic Compounds from Cultures
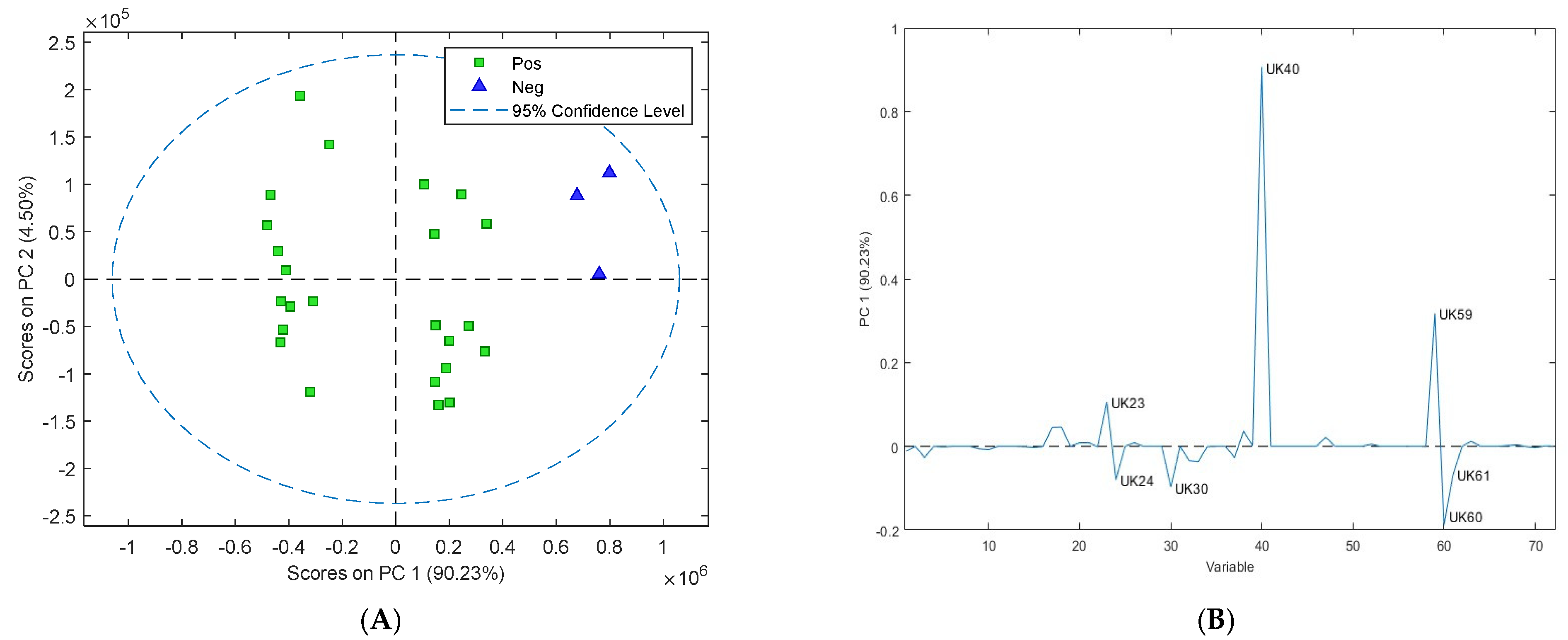
3.3. Assessment of Volatile Organic Compounds from Cattle Faeces
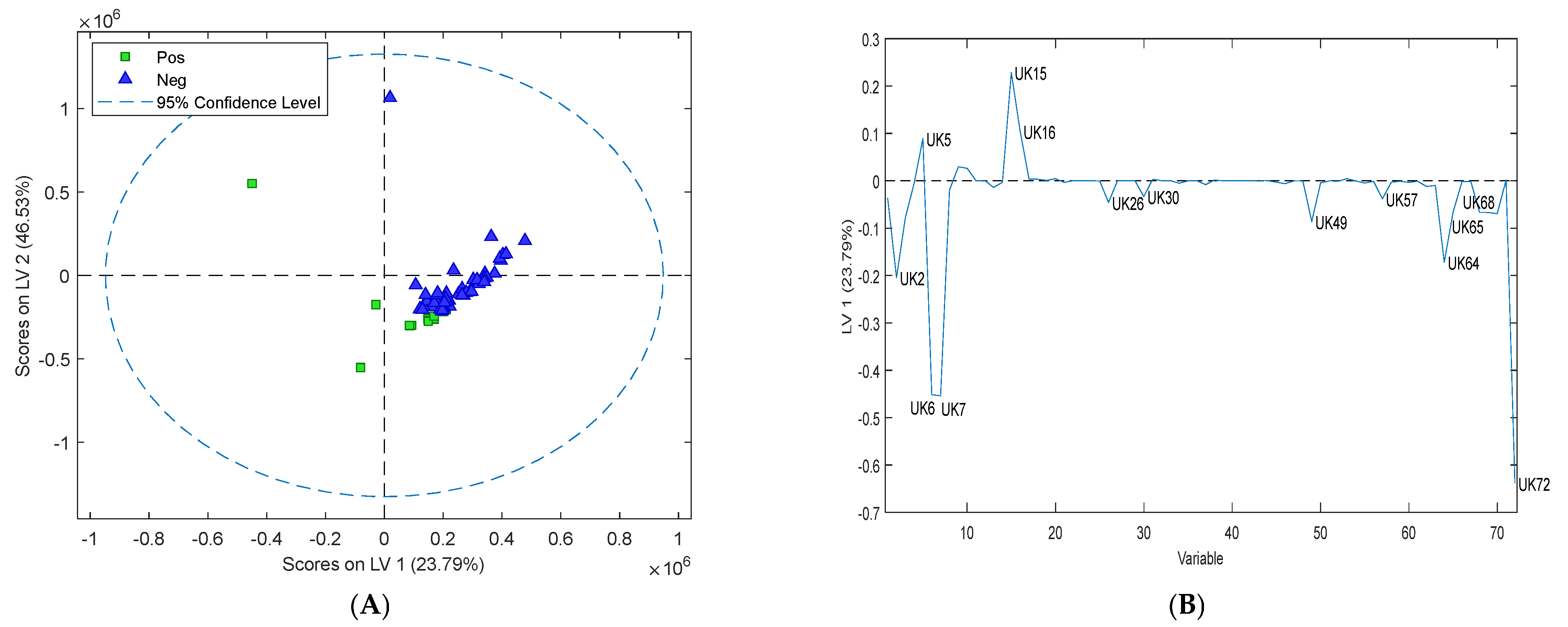
3.4. Assessment of Volatile Organic Compounds in Sheep Faeces
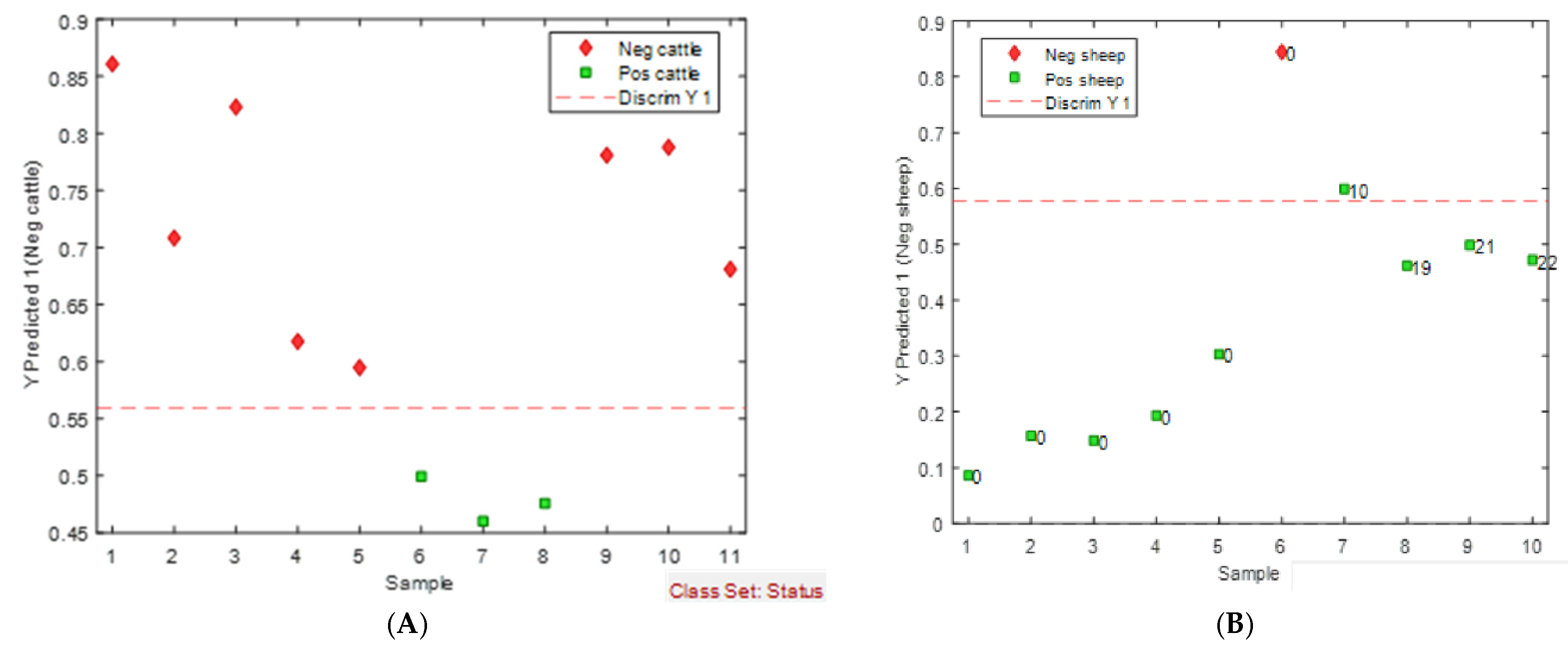
3.5. Assessment and Validation of the eNose
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Buck, J.; Shaykhutdinov, R.; Barkema, H.W.; Vogel, H.J. Metabolomic profiling in cattle experimentally infected with Mycobacterium avium subsparatuberculosis. PLoS ONE 2014, 9, e111872. [Google Scholar] [CrossRef] [PubMed]
- Djonne, B. Paratuberculosis in Goats. In Paratuberculosis: Organism, Disease, Control; Behr, M.A., Collins, D.M., Eds.; CAB International: Wallingford, UK, 2010; pp. 169–178. [Google Scholar]
- Mortier, R.A.R.; Barkema, H.W.; Orsel, K.; Muench, G.P.; Bystrom, J.M.; Illanes, O.; De Buck, J. Longitudinal evaluation of diagnostics in experimentally infected young calves during subclinical and clinical paratuberculosis. Can. Vet. J. 2015, 56, 1266–1270. [Google Scholar]
- Whitlock, R.H.; Wells, S.J.; Sweeney, R.W.; Van Tiem, J. ELISA and fecal culture for paratuberculosis (Johne’s disease): Sensitivity and specificity of each method. Vet. Microbiol. 2000, 77, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Dane, H.; Stewart, L.D.; Grant, I.R. Culture of Mycobacterium avium subsparatuberculosis: Challenges, limitations and future prospects. J. Appl. Microbiol. 2022, 134, lxac017. [Google Scholar]
- Stabel, J.R. An improved method for cultivation of Mycobacterium paratuberculosis from bovine fecal samples and comparison to three other methods. J. Vet. Diagn. Investig. 1997, 9, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Vitense, P.; Kasbohm, E.; Klassen, A.; Gierschner, P.; Trefz, P.; Weber, M.; Miekisch, W.; Schubert, J.K.; Möbius, P.; Reinhold, P.; et al. Detection of Mycobacterium avium ssparatuberculosis in Cultures from Fecal and Tissue Samples Using VOC Analysis and Machine Learning Tools. Front. Vet. Sci. 2021, 8, 620327. [Google Scholar] [CrossRef]
- Saktiawati, A.M.; Putera, D.D.; Setyawan, A.; Mahendradhata, Y.; van der Werf, T.S. Diagnosis of tuberculosis through breath test: A systematic review. EBioMedicine 2019, 46, 202–214. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Condos, R.; Erickson, G.A.R.; Greenberg, J.; La Bombardi, V.; Munawar, M.I.; Tietje, O. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis 2007, 87, 44–52. [Google Scholar] [CrossRef]
- Nol, P.; Ionescu, R.; Welearegay, T.G.; Barasona, J.A.; Vicente, J.; Beleño-Sáenz, K.d.J.; Barrenetxea, I.; Torres, M.J.; Ionescu, F.; Rhyan, J. Evaluation of Volatile Organic Compounds Obtained from Breath and Feces to Detect Mycobacterium tuberculosis Complex in Wild Boar (Sus scrofa) in Doñana National Park, Spain. Pathogens 2020, 9, 346. [Google Scholar] [CrossRef]
- Bergmann, A.; Trefz, P.; Fischer, S.; Klepik, K.; Walter, G.; Steffens, M.; Ziller, M.; Schubert, J.C.; Reinhold, P.; Köhler, H.; et al. In Vivo Volatile Organic Compound Signatures of Mycobacterium avium subsparatuberculosis. PLoS ONE 2015, 10, e0123980. [Google Scholar] [CrossRef] [PubMed]
- Maddula, S.; Blank, L.M.; Schmid, A.; Baumbach, J.I. Detection of volatile metabolites of Escherichia coli by multi capillary column coupled ion mobility spectrometry. Anal. Bioanal. Chem. 2009, 394, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Syhre, M.; Scotter, J.M.; Chambers, S.T. Investigation into the production of 2-Pentylfuran by Aspergillus fumigatus and other respiratory pathogens in vitro and human breath samples. Med. Mycol. 2008, 46, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Rock, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef]
- Lai, S.Y.; Deffenderfer, O.F.; Hanson, W.; Phillips, M.P.; Thaler, E.R. Deffenderfer BS, William Hanson MD, Marguerite P. Phillips RN, BSN, Erica R. Thaler MD, Identification of Upper Respiratory Bacterial Pathogens with the Electronic Nose. Laryngoscope 2009, 112, 975–979. [Google Scholar] [CrossRef]
- Saktiawati AM, I.; Triyana, K.; Wahyuningtias, S.D.; Dwihardiani, B.; Julian, T.; Hidayat, S.N.; Mahendradhata, Y. eNose-TB: A trial study protocol of electronic nose for tuberculosis screening in Indonesia. PLoS ONE 2021, 16, e0249689. [Google Scholar] [CrossRef]
- Fend, R.; Kolk, A.H.; Bessant, C.; Buijtels, P.; Klatser, P.R.; Woodman, A.C. Prospects for clinical application of electronic-nose technology to early detection of Mycobacterium tuberculosis in culture and sputum. J. Clin. Microbiol. 2006, 44, 2039–2045. [Google Scholar] [CrossRef]
- Fend, R.; Geddes, R.; Lesellier, S.; Vordermeier, H.-M.; Corner, L.A.L.; Gormley, E.; Costello, E.; Hewinson, R.G.; Marlin, D.J.; Woodman, A.C.; et al. Use of an electronic nose to diagnose Mycobacterium bovis infection in badgers and cattle. J. Clin. Microbiol. 2005, 43, 1745–1751. [Google Scholar] [CrossRef]
- Hodgeman, R.; Mann, R.; Savin, K.; Djitro, N.; Rochfort, S.; Rodoni, B. Molecular characterisation of Mycobacterium avium subsparatuberculosis in Australia. BMC Microbiol. 2021, 21, 101. [Google Scholar] [CrossRef]
- Hodgeman, R.; Liu, Y.; Rochfort, S.; Rodoni, B. Development and evaluation of genomics informed real-time PCR assays for the detection and strain typing of Mycobacterium avium subsparatuberculosis. J. Appl. Microbiol. 2024, 135, lxae107. [Google Scholar] [CrossRef]
- MathWorks Inc. MATLAB Version: 9.13. 0 (R2022b); MathWorks Inc.: Natick, MA, USA, 2022. [Google Scholar]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8 (Suppl. S1), 3–16. [Google Scholar] [CrossRef] [PubMed]
- Cyrano Sciences. The Practical Guide to the Cyranose 320; Cyrano Sciences: Pasadena, CA, USA, 2013. [Google Scholar]
- Fecteau, M.; Whitlock, R.H. Paratuberculosis in Cattle. In Paratuberculosis: Organism, Disease, Control; Behr, M.A., Collins, D.M., Eds.; CAB International: Wallingford, UK, 2010; pp. 144–156. [Google Scholar]
- Crossley, B.M.; Zagmutt-Vergara, F.J.; Fyock, T.L.; Whitlock, R.H.; Gardner, I.A. Fecal shedding of Mycobacterium avium subsparatuberculosis by dairy cows. Vet. Microbiol. 2005, 107, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.B.; Robbe-Austerman, S.; Payeur, J.B. Effect of egg yolk on the detection of Mycobacterium avium subsparatuberculosis using the ESP II liquid culture system. J. Vet. Diagn. Investig. 2005, 17, 554–560. [Google Scholar] [CrossRef]
- Chaubey, K.K.; Gupta, R.D.; Gupta, S.; Singh, S.V.; Bhatia, A.K.; Jayaraman, S.; Kumar, N.; Goel, A.; Rathore, A.S.; Sahzad; et al. Trends and advances in the diagnosis and control of paratuberculosis in domestic livestock. Vet. Q. 2016, 36, 203–227. [Google Scholar] [CrossRef]
- Trefz, P.; Koehler, H.; Klepik, K.; Moebius, P.; Reinhold, P.; Schubert, J.K.; Miekisch, W. Volatile emissions from Mycobacterium avium subsparatuberculosis mirror bacterial growth and enable distinction of different strains. PLoS ONE 2013, 8, e76868. [Google Scholar] [CrossRef]
- Küntzel, A.; Fischer, S.; Bergmann, A.; Oertel, P.; Steffens, M.; Trefz, P.; Köhler, H. Effects of biological and methodological factors on volatile organic compound patterns during cultural growth of Mycobacterium avium ssparatuberculosis. J. Breath. Res. 2016, 10, 037103. [Google Scholar] [CrossRef]
- The Department of Agriculture, Fisheries and Forestry. Aquatic and Terrestrial Australian and New Zealand Standard Diagnostic Procedures, in Paratuberculosis (Johne’s Disease); The Department of Agriculture, Fisheries and Forestry: Canberra, Australia, 2015. [Google Scholar]
- Metabolon. Partial Least-Squares Discriminant Analysis (PLS-DA). 2024. Available online: https://www.metabolon.com/bioinformatics/pls-da/#:~:text=PLS%2DDA%20is%20a%20supervised,vs%20diseased)%20in%20the%20data (accessed on 12 July 2024).
- Kasbohm, E.; Fischer, S.; Küntzel, A.; Oertel, P.; Bergmann, A.; Trefz, P.; Miekisch, W.; Schubert, J.K.; Reinhold, P.; Ziller, M.; et al. Strategies for the identification of disease-related patterns of volatile organic compounds: Prediction of paratuberculosis in an animal model using random forests. J. Breath. Res. 2017, 11, 047105. [Google Scholar] [CrossRef]
- Weber, M.; Gierschner, P.; Klassen, A.; Kasbohm, E.; Schubert, J.K.; Miekisch, W.; Reinhold, P.; Köhler, H. Detection of Paratuberculosis in Dairy Herds by Analyzing the Scent of Feces, Alveolar Gas, and Stable Air. Molecules 2021, 26, 2854. [Google Scholar] [CrossRef]
- Forney, F.; Markovetz, A. The biology of methyl ketones. J. Lipid Res. 1971, 12, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Lukins, H.B.; Foster, J.W. Methyl Ketone Metabolism in Hydrocarbon-Utilizing Mycobacteria. J. Bacteriol. 1963, 85, 1074–1087. [Google Scholar] [CrossRef]
- Gross, J.J.; Bruckmaier, R.M. Review: Metabolic challenges in lactating dairy cows and their assessment via established and novel indicators in milk. Animal 2019, 13, s75–s81. [Google Scholar] [CrossRef]
- Anderson, J.C. Measuring breath acetone for monitoring fat loss: Review. Obesity 2015, 12, 2327–2334. [Google Scholar] [CrossRef]
- Begg, D.J.; Whittington, R. Paratuberculosis in Sheep. In Paratuberculosis: Organism, Disease, Control; Behr, M.A., Collins, D.M., Eds.; CAB International: Wallingford, UK, 2010; pp. 157–168. [Google Scholar]
- Links, I.J.; Denholm, L.J.; Evers, M.; Kingham, L.J.; Greenstein, R.J. Is vaccination a viable method to control Johne′s disease caused by Mycobacterium avium subsparatuberculosis? Data from 12 million ovine vaccinations and 7.6 million carcass examinations in New South Wales, Australia from 1999–2009. PLoS ONE 2021, 16, e0246411. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, R.; Kawakami, K.; Chiba, K. Gut bacteria producing phenols disturb keratinocyte differentiation in human skin. Microb. Ecol. Health Dis. 2009, 21, 221–227. [Google Scholar]
- Filipiak, W.; Sponring, A.; Filipiak, A.; Baur, M.; Ager, C.; Wiesenhofer, H.; Amann, A. Volatile Organic Compounds (VOCs) Reelased by Pathogenic Microorganisms in vitro: Potential Breath Biomarkers for Early-Stage Diagnosis of Disease. In Volatile Biomarkers; Elsevier: Amsterdam, The Netherlands, 2013; pp. 463–512. [Google Scholar]




| Flow Settings | ||
|---|---|---|
| Time (s) | Pump Speed | |
| Baseline purge | 40 | Medium |
| Sample draw | 30 | Medium |
| Sample draw 2 | 0 | Medium |
| Snout removal | 0 | |
| 1st sample gas purge | 45 | High |
| 1st air intake purge | 10 | High |
| 2nd sample gas purge | 45 | High |
| 2nd air intake purge | 0 | High |
| Digital filtering | On | |
| Substrate heater | On 35C | |
| Training repeat count | 1 | |
| Identifying repeat count | 1 | |
| Data Processing | ||
| Active sensors | All | |
| Algorithm | Canonical | |
| Pre-processing | Auto-scaling | |
| Normalisation | Normalisation 1 | |
| Identification quality | Medium | |
| Acceptance threshold | 99.900% | |
| ID No UK# | Name | m/z | RT (min) | Match | RMatch | Probability | Identified from | Identification Status |
|---|---|---|---|---|---|---|---|---|
| 2 | methanethiol | 48.0014 | 3.949 | 510 | 765 | 68.3 | SF, CF | LM |
| 3 | dimethylamine | 45.0333 | 5.387 | 987 | 996 | 45.8 | SC | LM |
| 5 | acetone | 58.0414 | 6.223 | 728 | 948 | 51.9 | CF | Confirmed |
| 6 | dimethyl sulfide | 62.0176 | 6.315 | 896 | 901 | 71.6 | CF, SF | LM |
| 7 | ethanthiol | 62.0167 | 6.322 | 810 | 810 | 22.2 | CF, SF | Confirmed |
| 15 | 2-butanone | 72.0570 | 10.771 | 699 | 879 | 56.7 | CF | LM |
| 16 | 2-butanol | 45.0334 | 11.389 | 851 | 936 | 69.6 | CF | Confirmed |
| 23 | pentanal | 58.0411 | 16.489 | 874 | 881 | 40.7 | CC, SC | Confirmed |
| 24 | 3-pentanone | 57.0322 | 16.673 | 855 | 912 | 67.8 | SC, CC | LM |
| 26 | disulfide dimethyl | 93.9902 | 19.015 | 868 | 868 | 93.3 | SF | LM |
| 30 | 1-butanol 3-methyl | 55.0539 | 19.953 | 896 | 900 | 39.9 | SC, CC, SF | Confirmed |
| 38 | 1-butanol 2-methyl | 55.0540 | 21.988 | 870 | 927 | 48.4 | SC | Confirmed |
| 40 | Hexanal | 56.0617 | 23.190 | 932 | 945 | 94.4 | CC, SC | Confirmed |
| 49 | 3-carene | 93.0679 | 28.772 | 882 | 886 | 9.17 | CF | Confirmed |
| 57 | dimethyl trisulfide | 125.9597 | 30.746 | 762 | 867 | 94.4 | SF | LM |
| 59 | 1-octen-3-ol | 57.0322 | 30.967 | 754 | 836 | 64.4 | CC | Confirmed |
| 60 | 6-octen-2-one (Z) | 67.0528 | 31.053 | 780 | 805 | 54.8 | SC, CC | LM |
| 61 | 2-octanone | 58.0400 | 31.094 | 858 | 858 | 71.8 | SC, CC | Confirmed |
| 64 | p-cymene | 119.0852 | 31.325 | 889 | 933 | 39.1 | CF | Confirmed |
| 65 | D-limonene | 93.0695 | 31.352 | 719 | 865 | 10.1 | CF | Confirmed |
| 70 | phenol | 94.0393 | 31.989 | 920 | 932 | 69.6 | SF | Confirmed |
| 72 | phenol 3-methyl | 107.0468 | 32.9 | 793 | 885 | 34.5 | CF, SF | LM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodgeman, R.; Krill, C.; Rochfort, S.; Rodoni, B. Detection of Mycobacterium avium subsp. paratuberculosis in Australian Cattle and Sheep by Analysing Volatile Organic Compounds in Faeces. Sensors 2024, 24, 7443. https://doi.org/10.3390/s24237443
Hodgeman R, Krill C, Rochfort S, Rodoni B. Detection of Mycobacterium avium subsp. paratuberculosis in Australian Cattle and Sheep by Analysing Volatile Organic Compounds in Faeces. Sensors. 2024; 24(23):7443. https://doi.org/10.3390/s24237443
Chicago/Turabian StyleHodgeman, Rachel, Christian Krill, Simone Rochfort, and Brendan Rodoni. 2024. "Detection of Mycobacterium avium subsp. paratuberculosis in Australian Cattle and Sheep by Analysing Volatile Organic Compounds in Faeces" Sensors 24, no. 23: 7443. https://doi.org/10.3390/s24237443
APA StyleHodgeman, R., Krill, C., Rochfort, S., & Rodoni, B. (2024). Detection of Mycobacterium avium subsp. paratuberculosis in Australian Cattle and Sheep by Analysing Volatile Organic Compounds in Faeces. Sensors, 24(23), 7443. https://doi.org/10.3390/s24237443






