A Case Study on EEG Signal Correlation Towards Potential Epileptic Foci Triangulation
Abstract
:1. Introduction
2. Temporal and Spatial Design Considerations
3. Experimental Results
4. Discussion
5. Conclusions
- (a)
- Linearly simplified neural signaling (weighted signal speed and transition delays) for small-area, underlying source localizations;
- (b)
- A set of “brain state models” according to a) that merge into one another;
- (c)
- An adaptive, machine-learning mechanism that develops those brain states (within recurring 200–300 msec sequences) and recognizes those states.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mégevand, P.; Hamid, L.; Dümpelmann, M.; Heers, M. New horizons in clinical electric source imaging. Z. Epileptol. 2019, 32, 187–193. [Google Scholar] [CrossRef]
- Kaiboriboon, K.; Lüders, H.O.; Hamaneh, M.; Turnbull, J.; Lhatoo, S.D. EEG source imaging in epilepsy—Practicalities and pitfalls. Nat. Rev. Neurol. 2012, 8, 498–507. [Google Scholar] [CrossRef]
- Vorwerk, J.; Wolters, C.H.; Baumgarten, D. Global sensitivity of EEG source analysis to tissue conductivity uncertainties. Front. Hum. Neurosci. 2024, 18, 1335212. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.C.; Stefan, H.; Holtkamp, M. (Eds.) Epileptische Anfälle und Epilepsien im Erwachsenenalter: Diagnostik und Therapie; Springer: Berlin/Heidelberg, Germany, 2021; pp. 21–28. [Google Scholar]
- Tamilia, E.; Madsen, J.R.; Grant, P.E.; Pearl, P.L.; Papadelis, C. Current and Emerging Potential of Magnetoencephalography in the Detection and Localization of High-Frequency Oscillations in Epilepsy. Front. Neurology 2017, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, H.; Li, C. Effect of Inverse Solutions, Connectivity Measures, and Node Sizes on EEG Source Network: A Simultaneous EEG Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 2644–2653. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef]
- Dümpelmann, M. Early seizure detection for closed loop direct neurostimulation devices in epilepsy. J. Neural Eng. 2019, 16, 41001. [Google Scholar] [CrossRef]
- Bauer, S.; Rosenow, F.; Anfälle, I.; Diener, H.-C.; Steinmetz, H.; Kastrup, O. (Eds.) Referenz Neurologie; Georg Thieme Verlag: Stuttgart, NY, USA, 2019; pp. 158–172. [Google Scholar]
- Todaro, C.; Marzetti, L.; Sosa, P.A.V.; Valdés-Hernandez, P.A.; Pizzella, V. Mapping Brain Activity with Electrocorticography: Resolution Properties and Robustness of Inverse Solutions. Brain Topogr. 2019, 32, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pizarro, M.; Spencer, D.D.; Damisah, E.C. Recent advances in epilepsy surgery. Curr. Opin. Neurol. 2023, 36, 95. [Google Scholar] [CrossRef]
- Sun, J.; Barth, K.; Qiao, S.; Chiang, C.H.; Wang, C.; Rahimpour, S.; Trumpis, M.; Duraivel, S.; Dubey, A.; Wingel, K.E.; et al. Intraoperative microseizure detection using a high-density micro-electrocorticography electrode array. Brain Commun. 2022, 4, fcac122. [Google Scholar] [CrossRef]
- Jobst, B.C.; Bartolomei, F.; Diehl, B.; Frauscher, B.; Kahane, P.; Minotti, L.; Sharan, A.; Tardy, N.; Worrell, G.; Gotman, J. Intracranial EEG in the 21st Century. Epilepsy Curr. 2020, 20, 180–188. [Google Scholar] [CrossRef]
- Parvizi, J.; Kastner, S. Promises and limitations of human intracranial electroencephalography. Nat. Neurosci. 2018, 21, 474–483. [Google Scholar] [CrossRef]
- Mercier, M.R.; Dubarry, A.S.; Tadel, F.; Avanzini, P.; Axmacher, N.; Cellier, D.; Del Vecchio, M.; Hamilton, L.S.; Hermes, D.; Kahana, M.J.; et al. Advances in human intracranial electroencephalography research, guidelines and good practices. NeuroImage 2022, 260, 119438. [Google Scholar] [CrossRef] [PubMed]
- Lhatoo, S.D.; Kahane, P.; Lüders, H.O. (Eds.) Invasive Studies of the Human Epileptic Brain: Principles and Practice; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Lee, K.; Paulk, A.C.; Ro, Y.G.; Cleary, D.R.; Tonsfeldt, K.J.; Kfir, Y.; Pezaris, J.S.; Tchoe, Y.; Lee, J.; Bourhis, A.M.; et al. Flexible, scalable, high channel count stereo-electrode for recording in the human brain. Nat. Commun. 2024, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, S.W.; Yeom, H.G.; Bang, M.S.; Kim, J.S.; Chung, C.K.; Kim, S. A study on a robot arm driven by three-dimensional trajectories predicted from non-invasive neural signals. Biomed. Eng. Online 2015, 14, 81. [Google Scholar] [CrossRef]
- Rampp, S.; Scherg, M. Grundlagen der Quellenlokalisation. Z. Für Epileptol. 2018, 31, 170–178. [Google Scholar] [CrossRef]
- Moller, C. Behandlung von Potentialsingularitaten bei der EEG/MEG-Basierten Rekonstruktion Dipolarer Stromquellen in Finite-Elemente-Volumenleitermodellen des Menschlichen Kopfes. Ph.D. Thesis, Erlangen University, Erlangen, Germany, 2005. [Google Scholar]
- Leski, S.; Wójcik, D.K.; Tereszczuk, J.; Swiejkowski, D.A.; Kublik, E.; Wróbel, A. Inverse Current-Source Density in three dimensions. Neuroinformatics 2007, 5, 207. [Google Scholar]
- Chintaluri, C.; Bejtka, M.; Średniawa, W.; Czerwiński, M.; Dzik, J.M.; Jędrzejewska-Szmek, J.; Kondrakiewicz, K.; Kublik, E.; Wójcik, D.K. What we can and what we cannot see with extracellular multielectrodes. PLoS Comput. Biol. 2021, 17, e1008615. [Google Scholar] [CrossRef]
- Robinson, J.T.; Xie, C.; Pohlmeyer, E.; Gather, M.C.; Kemere, C.; Kitching, J.E.; Malliaras, G.G.; Marblestone, A.; Shepard, K.L.; Stieglitz, T. Developing Next-Generation Brain Sensing Technologies—A Review. IEEE Sens. J. 2019, 18, 10163–10175. [Google Scholar] [CrossRef] [PubMed]
- Zijlmans, M.; Worrell, G.; Duempelmann, M.; Stieglitz, T.; Barborica, A.; Heers, M.; Ikeda, A.; Usui, N.; Le Van Quyen, M. How to record high frequency oscillations in epilepsy: A practical guideline. Epilepsia 2017, 58, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Cimbalnik, J.; Klimes, P.; Sladky, V.; Nejedly, P.; Jurak, P.; Pail, M.; Roman, R.; Daniel, P.; Guragain, H.; Brinkmann, B.; et al. Multi-Feature Localization of Epileptic Foci from Interictal, Intracranial EEG. Clin. Neurophysiol. 2019, 130, 1945–1953. [Google Scholar] [CrossRef]
- Daoud, H.; Bayoumi, M. Deep Learning Approach for Epileptic Focus Localization. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.M.; Liu, A.K.; Fischl, B.R.; Buckner, R.L.; Belliveau, J.W.; Lewine, J.D.; Halgren, E. Dynamic statistical parametric mapping: Combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron 2000, 26, 55–67. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp. Clin. Pharmacol. 2002, 24, 5–12. [Google Scholar] [PubMed]
- Clarke, C.; Janday, B. The solution of the biomagnetic inverse problem by maximum statistical entropy. Inverse Prob. 1989, 5, 483. [Google Scholar] [CrossRef]
- Chintaluri, C.; Bejtka, M.; Średniawa, W.; Czerwiński, M.; Dzik, J.M.; Jędrzejewska-Szmek, J.; Wójcik, D.K. kCSD-python, reliable current source density estimation with quality control. PLoS Comput. Biol. 2024, 20, e1011941. [Google Scholar] [CrossRef] [PubMed]
- Wendling, F.; Ansari-Asl, K.; Bartolomei, F.; Senhadji, L. From EEG signals to brain connectivity: A model-based evaluation of interdependence measures. J. Neurosci. Methods 2009, 183, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Dickten, H.; Porz, S.; Elger, C.E.; Lehnertz, K. Weighted and Directed Interactions in Evolving Large-Scale Epileptic Brain Networks. Sci. Rep. 2016, 6, 34824. [Google Scholar] [CrossRef]
- Russo, A.; Jayakar, P.; Lallas, M.; Miller, I.; Hyslop, A.; Korman, B.; Dunoyer, C.; Resnick, T.; Duchowny, M. The Diagnostic Utility of 3D Electroencephalography Source Imaging in Pediatric Epilepsy Surgery. Epilepsia 2016, 57, 24–31. [Google Scholar] [CrossRef]
- Hassan, M.; Merlet, I.; Mheich, A.; Kabbara, A.; Biraben, A.; Nica, A.; Wendling, F. Identification of Interictal Epileptic Networks from Dense-EEG. Brain Topogr. 2017, 30, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Nemtsas, P.; Birot, G.; Pittau, F.; Michel, C.M.; Schaller, K.; Vulliemoz, S.; Kimiskidis, V.K.; Seeck, M. Source Localization of Ictal Epileptic Activity Based on High-Density Scalp EEG Data. Epilepsia 2017, 58, 1027–1036. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, X.; Cai, D.; Du, J.; Liu, W. An epileptic focus location method based on ECoG. Biomed. Mater. Eng. 2015, 26 (Suppl. S1), 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Ju, C.; Joo, J.; Lee, J.; Shon, Y.-M.; Park, S.-M. Closed-Loop Direct Control of Seizure Focus in a Rodent Model of Temporal Lobe Epilepsy via Localized Electric Fields Applied Sequentially. Nat. Commun. 2022, 13, 7805. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Harangozó, M.; Pedraza, L.; Földi, T.; Kozák, G.; Li, Q.; Berényi, A. Closed-loop stimulation of the medial septum terminates epileptic seizures. Brain 2021, 144, 885–908. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E. The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 2007, 7, 348–354. [Google Scholar] [CrossRef]
- Matsumoto, R.; Ajmone-Marsan, C. Cortical cellular phenomena in experimental epilepsy: Ictal manifestations. Exp. Neurol. 1964, 80, 305–326. [Google Scholar] [CrossRef]
- Cobb, S.R.; Buhl, E.H.; Halasy, K.; Paulsen, O.; Somogyi, P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 1996, 378, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.D.; Michelson-Law, H.; Bibbig, A.E.; Buhl, E.H.; Whittington, M.A. Gap junctions, fast oscillations and the initiation of seizures. Adv. Exp. Med. Biol. 2004, 548, 110–122. [Google Scholar] [PubMed]
- Sutula, T.P.; Dudek, F.E. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: An emergent property of a complex system. In The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications; Scharfman, H.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Johnston, D.; Brown, T.H. The synaptic nature of the paroxysmal depolarization shift in hippocampal neurons. Ann. Neurol. 1984, 16, S65–S71. [Google Scholar] [CrossRef]
- Sabatini, B.L.; Regher, W.G. Timing of synaptic transmission. Annu. Rev. Physiol. 1999, 61, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Bröhl, T.; Rings, T.; Lehnertz, K. Von Interaktionen zu Interaktionsnetzwerken: Zeitabhängige funktionelle Netzwerke am Beispiel der Epilepsie. Klin. Neurophysiol. 2020, 51, 132–143. [Google Scholar] [CrossRef]
- Boudkkazi, S.; Carlier, E.; Ankri, N.; Caillard, O.; Giraud, P.; Fronzaroli-Molinieres, L.; Debanne, D. Release-Dependent Variations in Synaptic Latency: A Putative Code for Short- and Long-Term Synaptic Dynamics. Neuron 2007, 56, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Seek, M.; Gschwind, M. High-Density-Elektroenzephalografie. In Epileptische Anfälle und Epilepsien im Erwachsenenalter: Diagnostik und Therapie; Schmitt, F.C., Stefan, H., Holtkamp, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 21–28. [Google Scholar]
- Marcoleta, J.P.; Nogueira, W.; Doll, T. Distributed mixed signal demultiplexer for electrocorticography electrodes. Biomed. Phys. Eng. Express 2020, 6, 55006. [Google Scholar] [CrossRef]
- Ha, S.; Akinin, A.; Park, J.; Kim, C.; Wang, H.; Maier, C.; Mercier, P.P.; Cauwenberghs, G. Silicon-Integrated High-Density Electrocortical Interfaces. Proc. IEEE 2017, 105, 11–33. [Google Scholar] [CrossRef]
- Kiral-Kornek, I.; Roy, S.; Nurse, E.; Mashford, B.; Karoly, P.; Carroll, T.; Payne, D.; Saha, S.; Baldassano, S.; O’Brien, T.; et al. Epileptic seizure prediction using big data and deep learning: Toward a mobile system. EBioMedicine 2017, 27, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adeli, H. Deep convolutional neural network for the automated detection and diagnosis of seizure using EEG signals. Comput. Biol. Med. 2017, 415–416, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Deppisch, J. Die Zeitstruktur Neuronaler Repräsentation: Modellierung und Identifikation Neuronaler Assemblies als Kollektive Erregungszustände des Visuellen Systems. Ph.D. Thesis, Frankfurt am Main University (Physics), Frankfurt, Germany, 1994. [Google Scholar]
- Bruns, A. Amplitudenkopplung Zwischen Kortexsignalen: Ein Bislang Ungenutzter Indikator für Kooperative Hirnprozesse im Menschen. Ph.D. Thesis, Marburg University, Marburg, Germany, 2003. [Google Scholar]
- Pernet, C.; Garrido, M.I.; Gramfort, A.; Maurits, N.; Michel, C.M.; Pang, E.; Salmelin, R.; Schoffelen, J.M.; Valdes-Sosa, P.A.; Puce, A. Issues and recommendations from the OHBM COBIDAS MEEG committee for reproducible EEG and MEG research. Nat. Neurosci. 2020, 23, 1473–1483. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Deco, G. Brain States and Transitions: Insights from Computational Neuroscience. Cell Rep. 2020, 32, 108128. [Google Scholar] [CrossRef]
- Korzeniewska, A.; Mańczak, M.; Kamiński, M.; Blinowska, K.J.; Kasicki, S. Determination of information flow direction among brain structures by a modified directed transfer function (dDTF) method. J. Neurosci. Methods 2003, 125, 195–207. [Google Scholar] [CrossRef]
- Kamiński, M.; Ding, M.; Truccolo, W.A.; Bressler, S.L. Evaluating causal relations in neural systems: Granger causality, directed transfer function and statistical assessment of significance. Biol. Cybern. 2001, 85, 145–157. [Google Scholar] [CrossRef]
- Wilke, C.; Van Drongelen, W.; Kohrman, M.; He, B. Neocortical seizure foci localization by means of a directed transfer function method. Epilepsia 2010, 51, 564–572. [Google Scholar] [CrossRef]
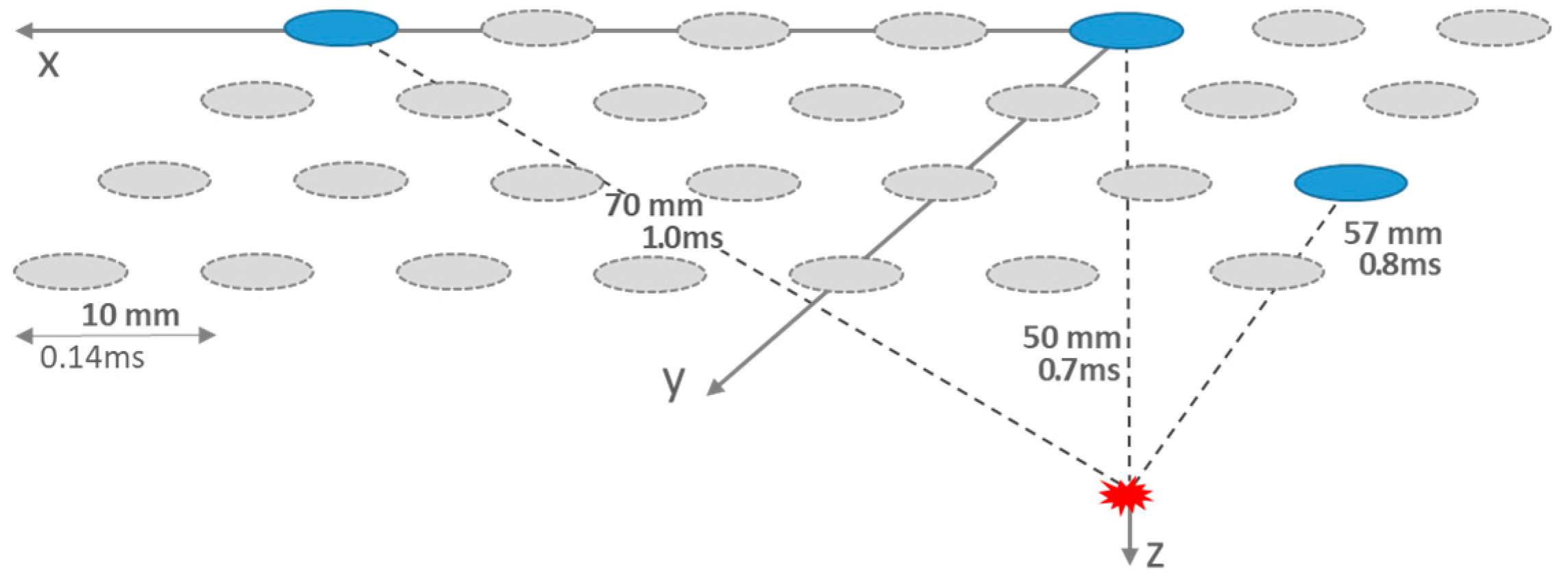
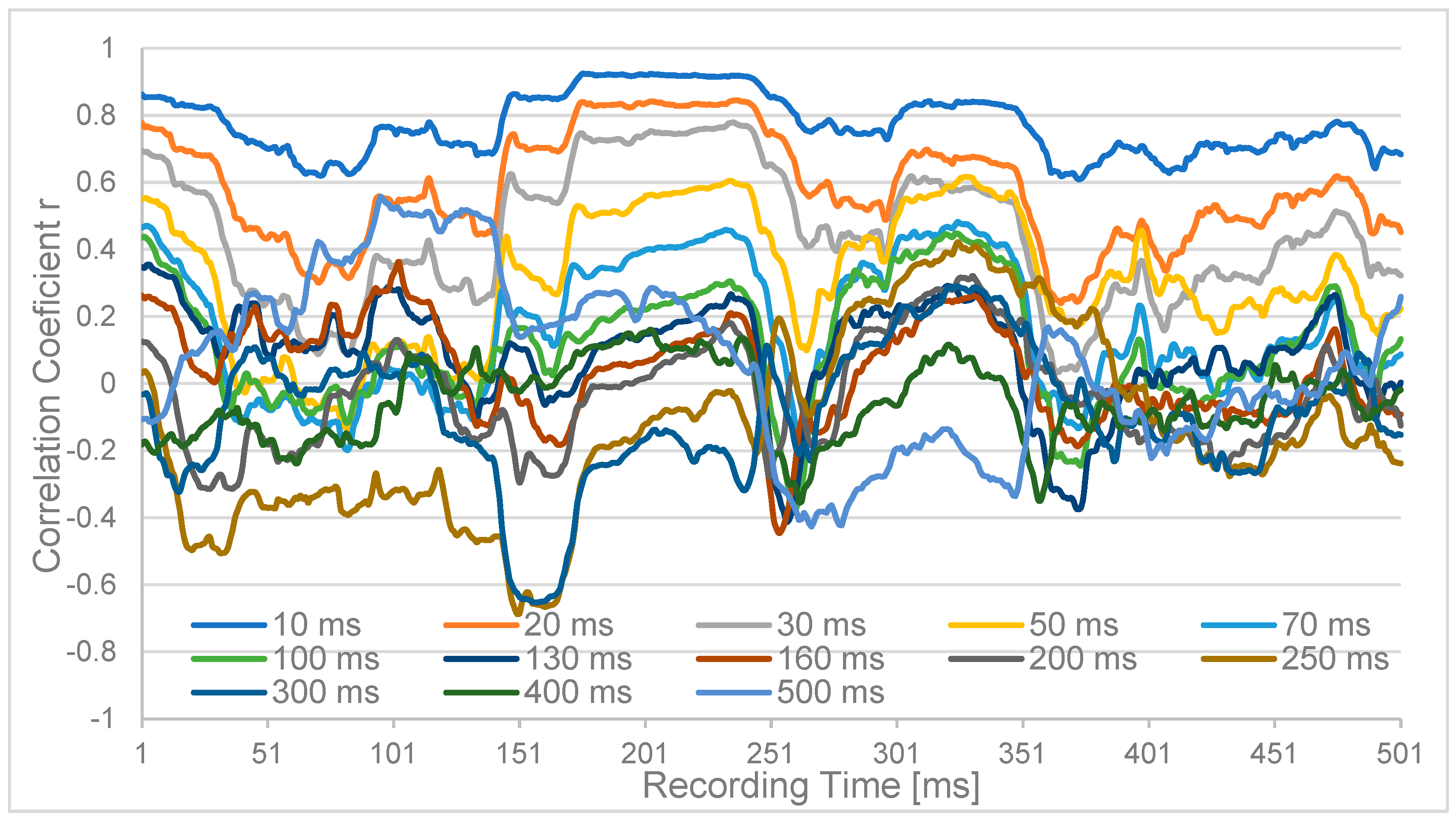

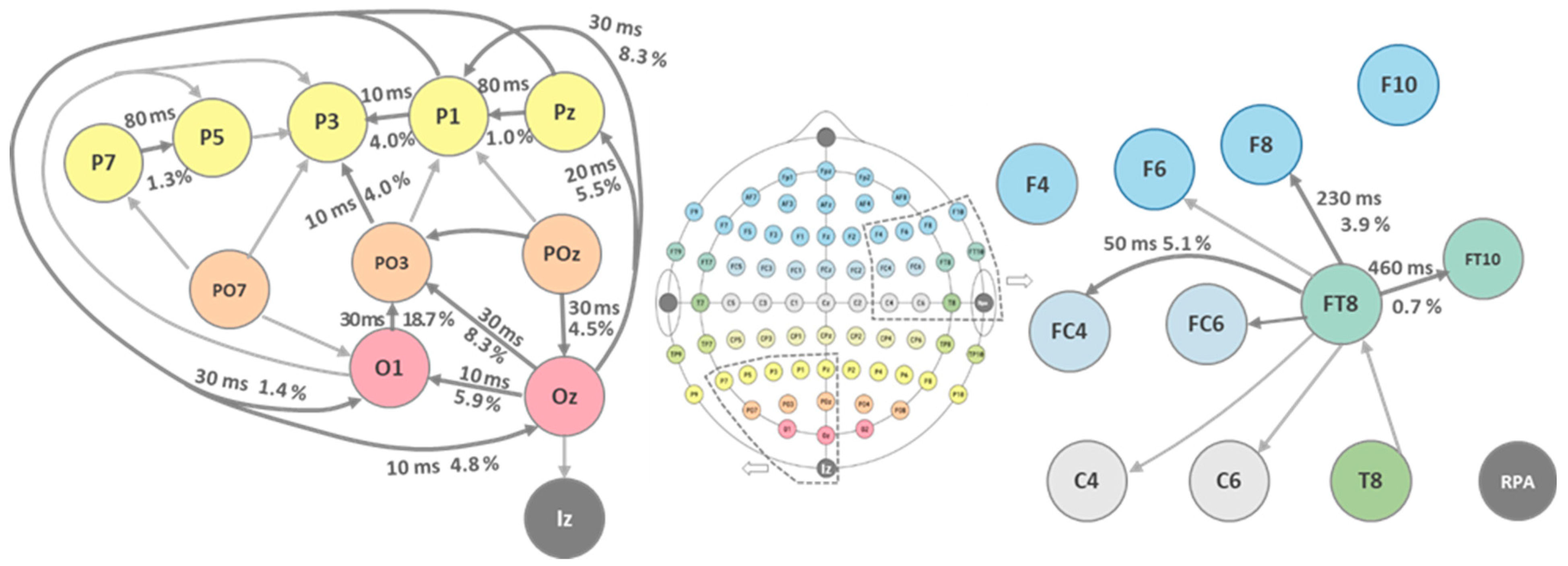
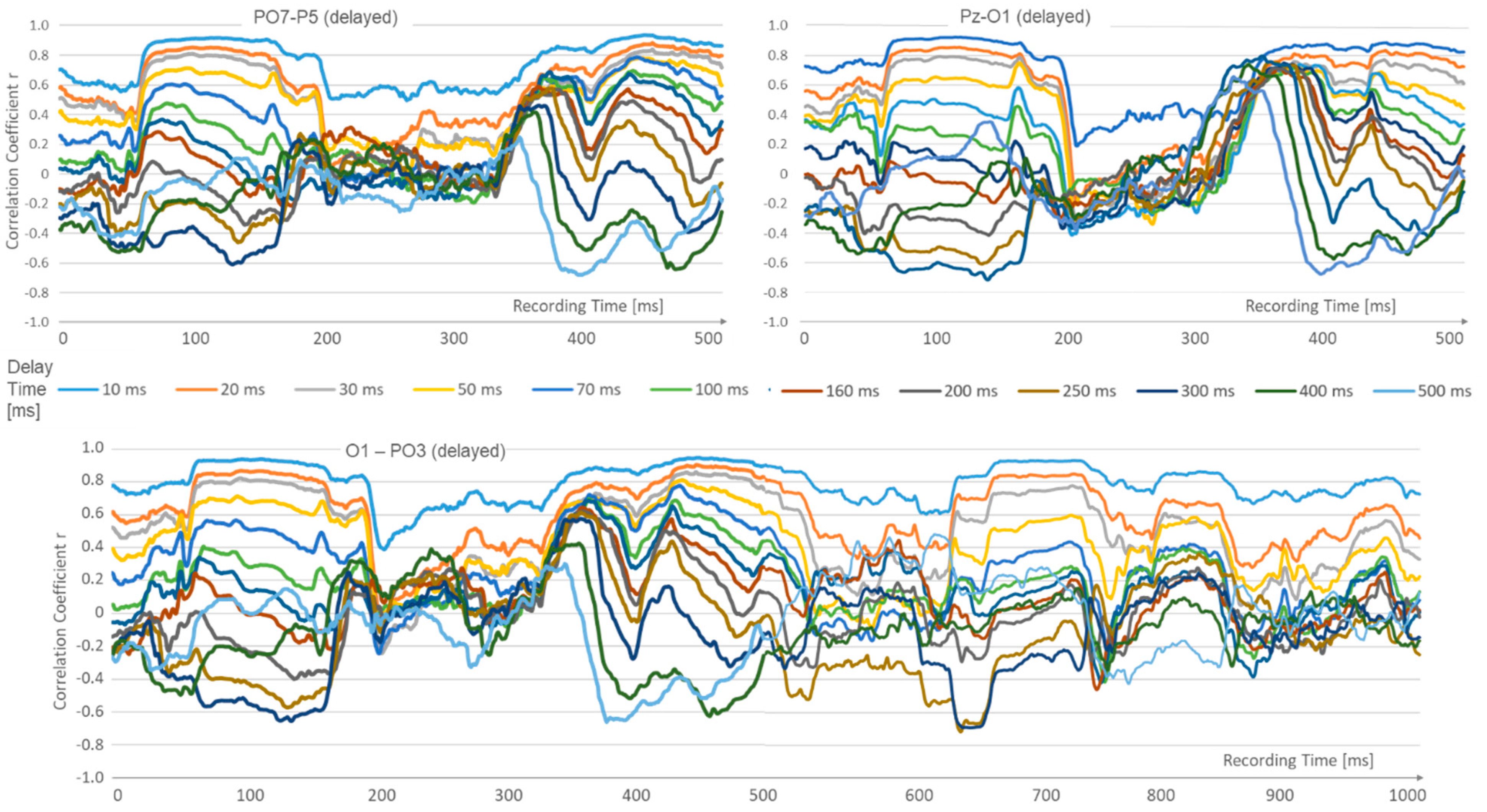
| Locked Channel | Iz | Oz | Oz | Oz | O1 | PO3 | POz | POz | PO3 | PO3 | O1 | PO7 | PO7 | PO3 | PO7 | P7 | P5 |
| Delayed Channel | Oz | O1 | POz | PO3 | PO3 | POz | Pz | P1 | P1 | P3 | PO7 | P7 | P5 | PO7 | P3 | P5 | P3 |
| Corr. Coeff. @ 10 ms | 0.58 | 0.7 | 0.75 | 0.76 | 0.75 | 0.76 | 0.77 | 0.66 | 0.63 | 0.71 | 0.49 | 0.7 | 0.83 | 0.59 | 0.59 | 0.81 | 0.67 |
| Latency @r = 0.7/ms | 0 | 10 | 30 | 30 | 30 | 30 | 30 | 0 | 0 | 10 | 0 | 0 | 80 | 0 | 0 | 80 | 0 |
| Locked Channel | P3 | P1 | P7 | P5 | P3 | P1 | Pz | Pz | P1 | FT10 | FT8 | FT8 | FT8 | C6 | FT8 | FC4 | FT8 |
| Delayed Channel | P1 | Pz | O1 | O1 | O1 | O1 | O1 | Oz | Oz | FT8 | F8 | FC6 | F6 | FT8 | T8 | FT8 | C4 |
| Corr. Coeff. @ 10 ms | 0.71 | 0.83 | 0.78 | 0.64 | 0.57 | 0.75 | 0.71 | 0.73 | 0.76 | 0.89 | 0.8 | 0.69 | 0.29 | 0.69 | 0.5 | 0.74 | 0.64 |
| Latency @r = 0.7/ms | 10 | 80 | 60 | 0 | 0 | 30 | 10 | 20 | 30 | 460 | 230 | 0 | 0 | 0 | 0 | 50 | 0 |
| Locked Channel | Iz | Oz | Oz | Oz | O1 | PO3 | POz | POz | PO3 | PO3 | O1 | PO7 | PO7 | PO3 | PO7 | P7 | P5 |
| Delayed Channel | Oz | O1 | POz | PO3 | PO3 | POz | Pz | P1 | P1 | P3 | PO7 | P7 | P5 | PO7 | P3 | P5 | P3 |
| Directionality [%] | 6.8 | 5.9 | 4.5 | 8.3 | 19.7 | 4.3 | 5.9 | 8.9 | 4.4 | 4.0 | 7.6 | 6.9 | 2.6 | 2.7 | 8.0 | 1.3 | 10.6 |
| Source | Oz | Oz | POz | Oz | O1 | POz | POz | POz | PO3 | PO3 | PO7 | PO7 | PO7 | PO3 | PO7 | P7 | P5 |
| Locked Channel | P3 | P1 | P7 | P5 | P3 | P1 | Pz | Pz | P1 | FT10 | FT8 | FT8 | FT8 | C6 | FT8 | FC4 | FT8 |
| Delayed Channel | P1 | Pz | O1 | O1 | O1 | O1 | O1 | Oz | Oz | FT8 | F8 | FC6 | F6 | FT8 | T8 | FT8 | C4 |
| Directionality [%] | 4.0 | 1.0 | 2.3 | 1.5 | 10.4 | 1.4 | 2.7 | 5.5 | 8.3 | 0.7 | 3.9 | 3.4 | 23.5 | 3.8 | 1.9 | 5.1 | 12.6 |
| Source | P1 | Pz | O1 | O1 | O1 | P1 | Pz | Oz | Oz | FT9 | FT8 | FT8 | FT8 | FT8 | T8 | FT8 | FT8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doll, T.; Stieglitz, T.; Heumann, A.S.; Wójcik, D.K. A Case Study on EEG Signal Correlation Towards Potential Epileptic Foci Triangulation. Sensors 2024, 24, 8116. https://doi.org/10.3390/s24248116
Doll T, Stieglitz T, Heumann AS, Wójcik DK. A Case Study on EEG Signal Correlation Towards Potential Epileptic Foci Triangulation. Sensors. 2024; 24(24):8116. https://doi.org/10.3390/s24248116
Chicago/Turabian StyleDoll, Theodor, Thomas Stieglitz, Anna Sophie Heumann, and Daniel K. Wójcik. 2024. "A Case Study on EEG Signal Correlation Towards Potential Epileptic Foci Triangulation" Sensors 24, no. 24: 8116. https://doi.org/10.3390/s24248116
APA StyleDoll, T., Stieglitz, T., Heumann, A. S., & Wójcik, D. K. (2024). A Case Study on EEG Signal Correlation Towards Potential Epileptic Foci Triangulation. Sensors, 24(24), 8116. https://doi.org/10.3390/s24248116









