A Systematic Review on the Advancements in Remote Sensing and Proximity Tools for Grapevine Disease Detection
Abstract
:1. Introduction
2. Systematic Review Methodology
2.1. Review Methodology
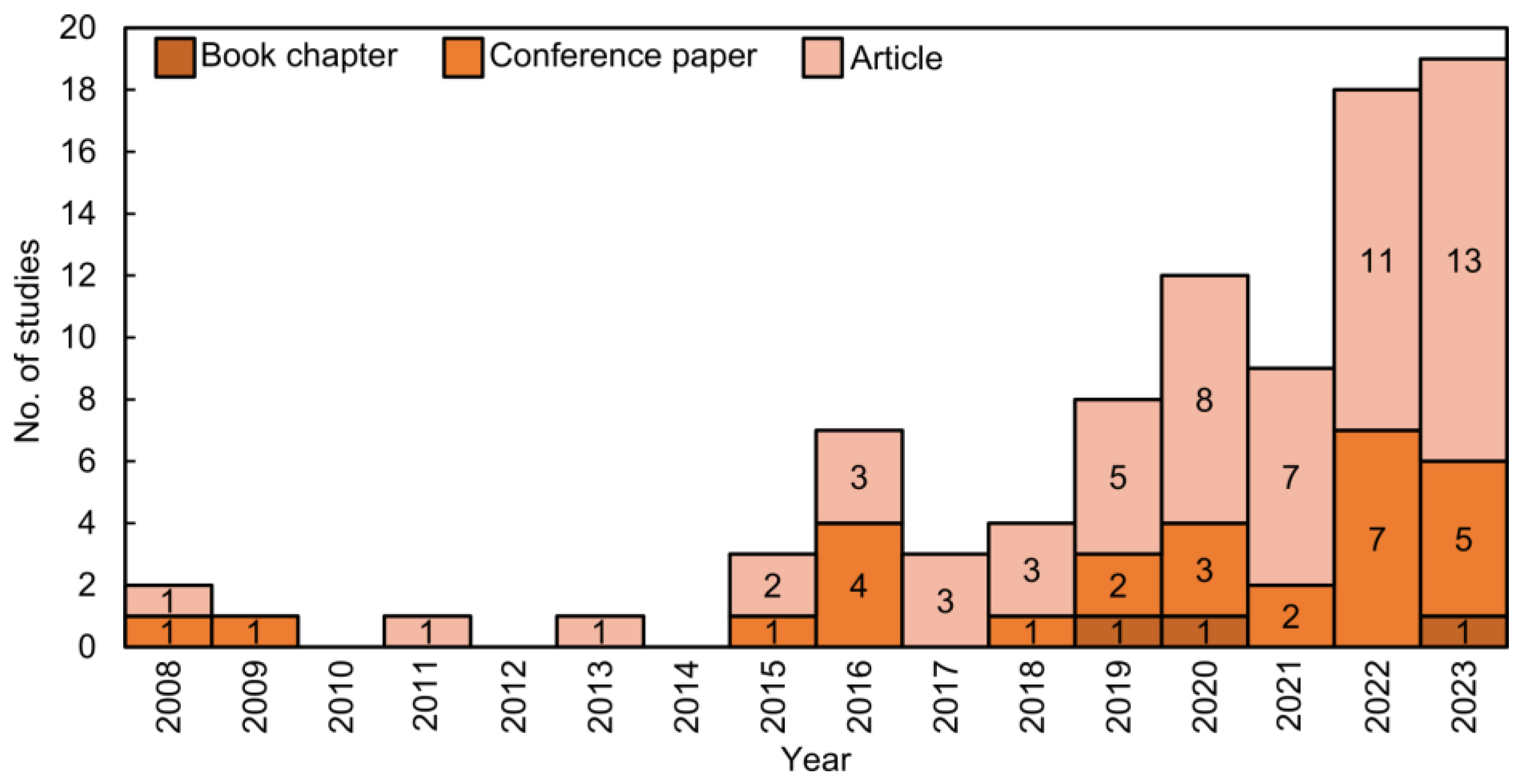
2.2. Bibliometric Analysis
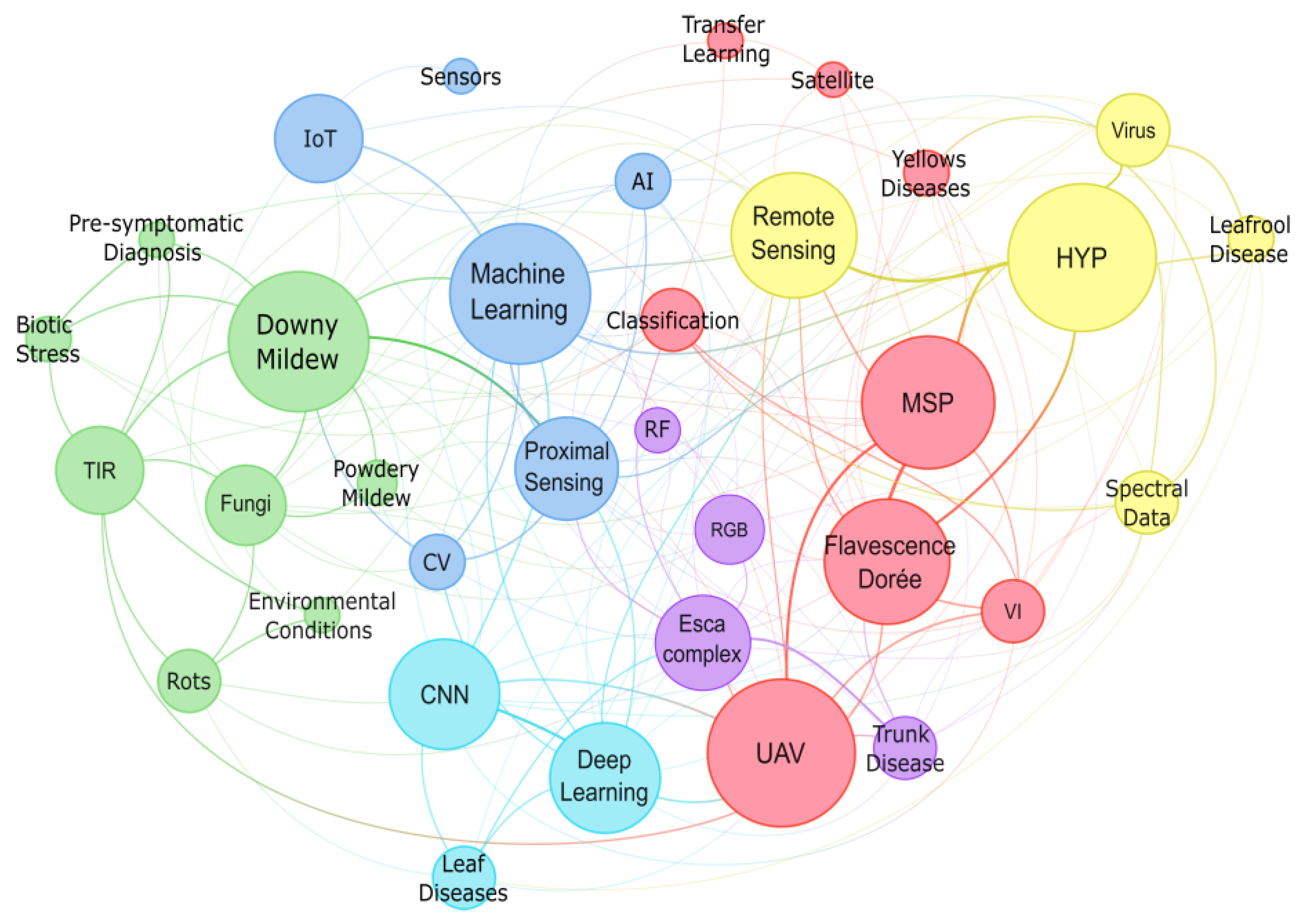
3. Overview of Platforms, Sensors, Diseases and Data Processing Techniques
3.1. Platforms Capabilities and Applications
3.2. Sensors for Grapevine Disease Detection
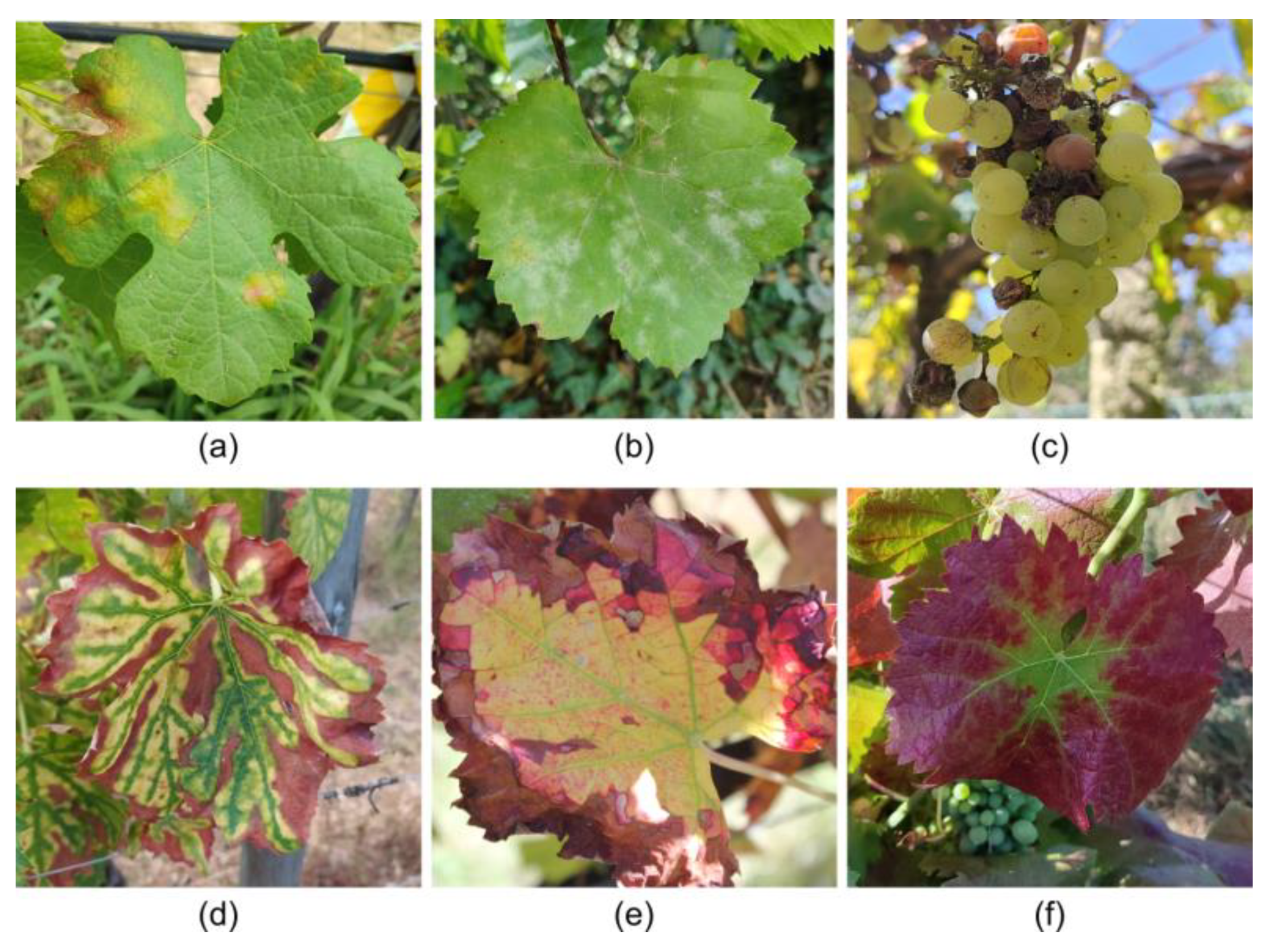
3.3. Characteristics and Impacts of Grapevine Diseases
3.4. Data Processing and Analysis Techniques
4. Results
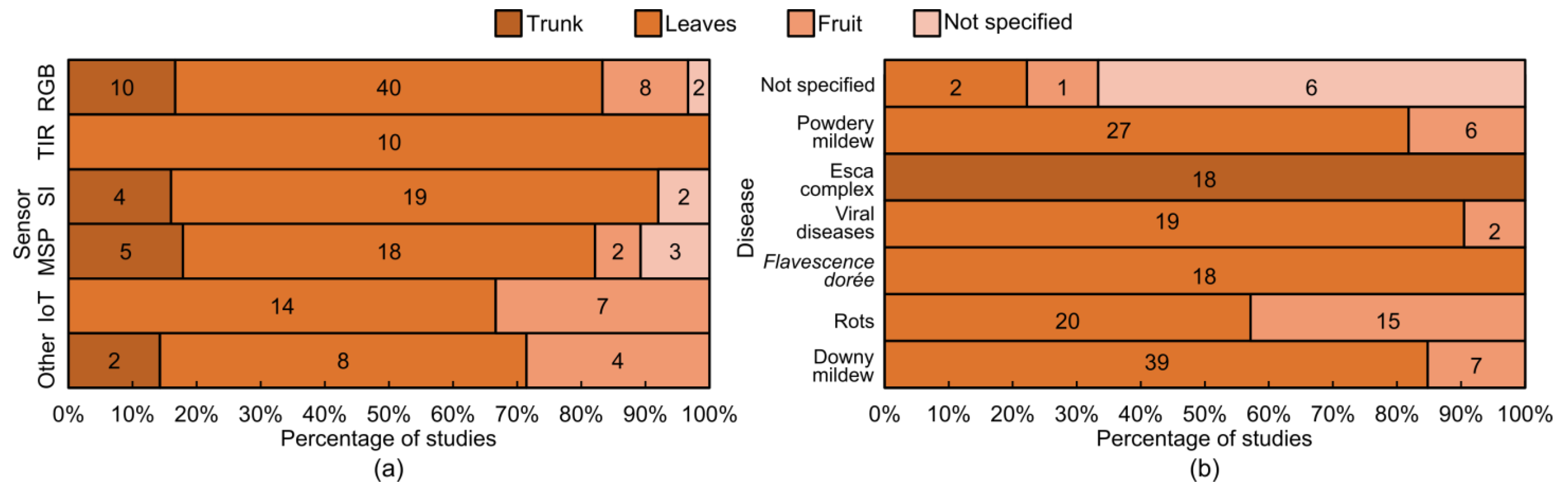
4.1. Overview of the Reviewed Studies
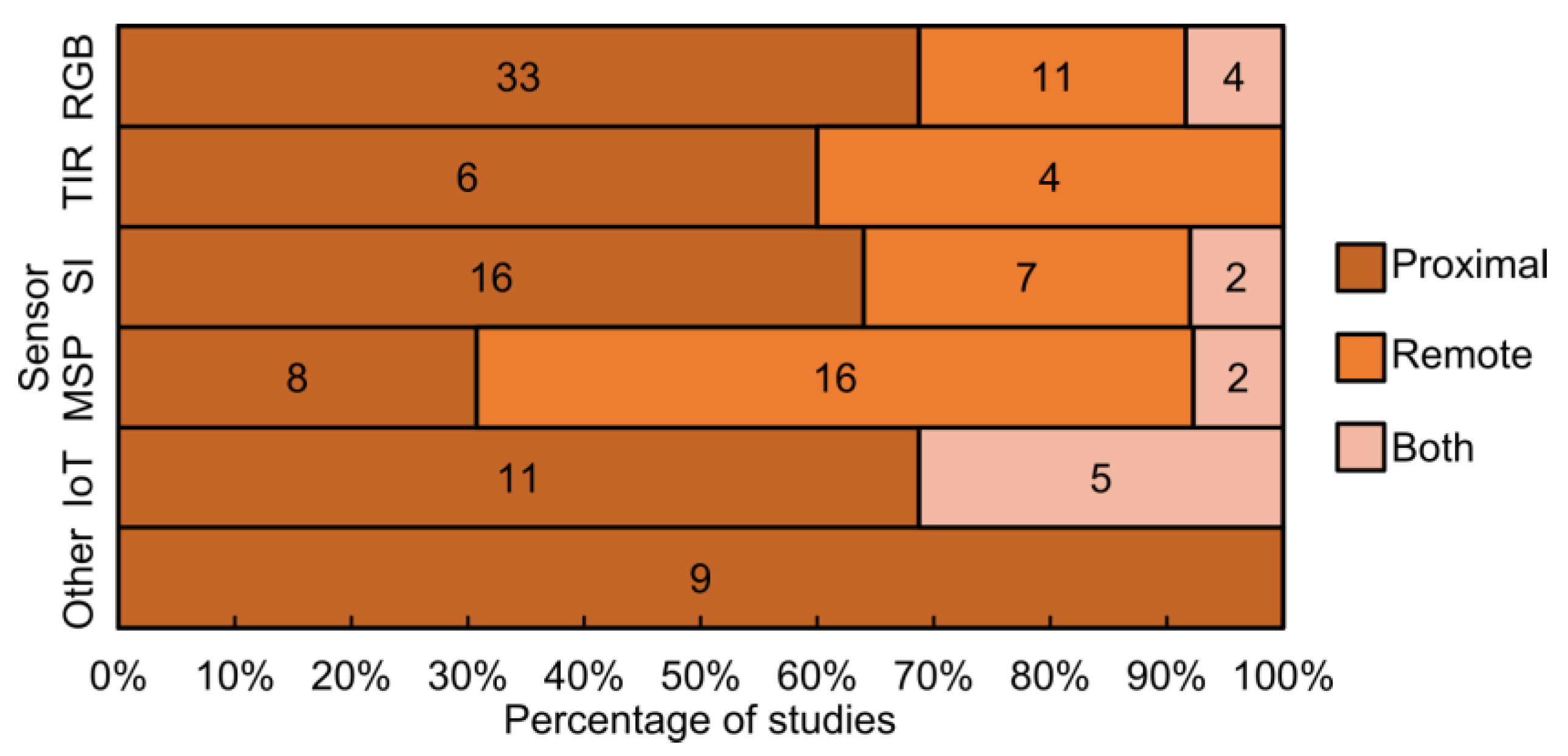
4.2. Overview of Sensors Used in Proximal and Remote Sensing
4.3. Techniques and Advancements in Field-Based Grapevine Disease Detection
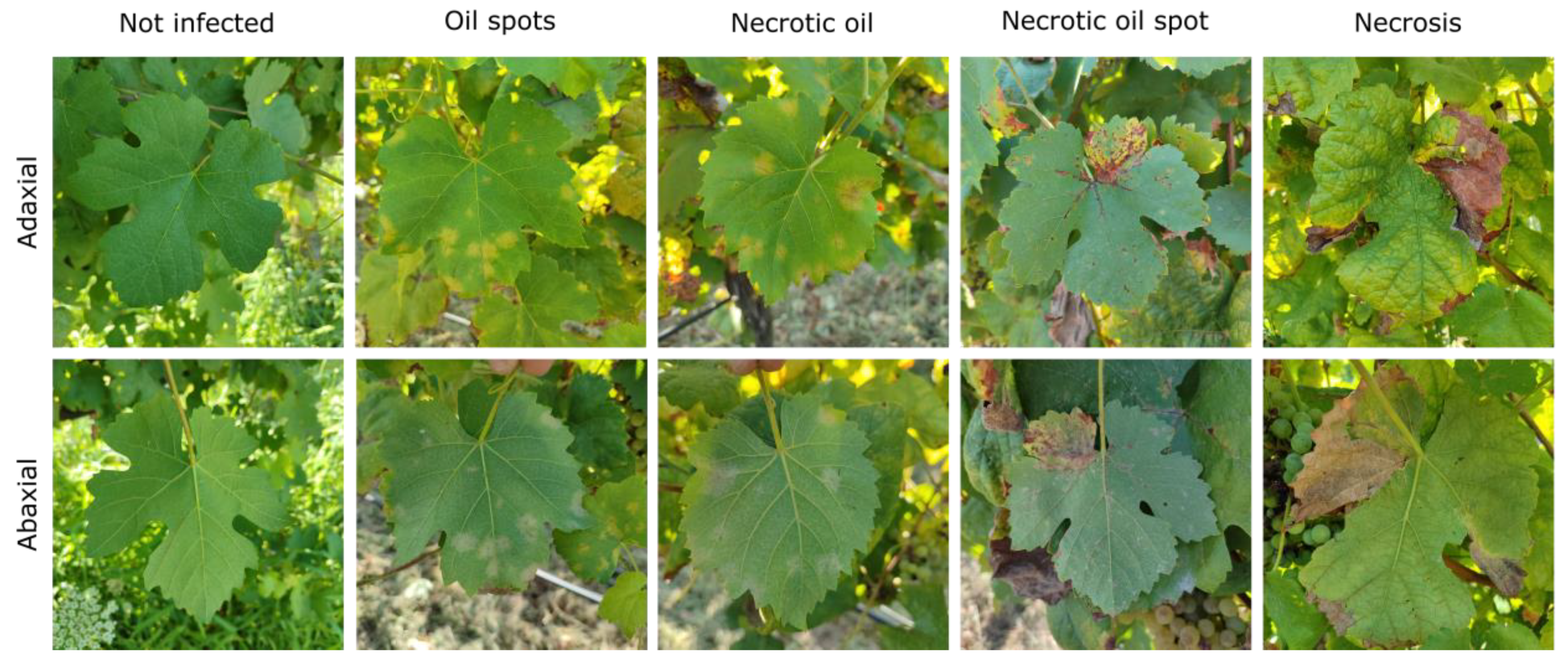
4.3.1. Downy Mildew
4.3.2. Fruit and Leaf Rots
4.3.3. Flavescence Dorée
4.3.4. Powdery Mildew
4.3.5. Esca Complex
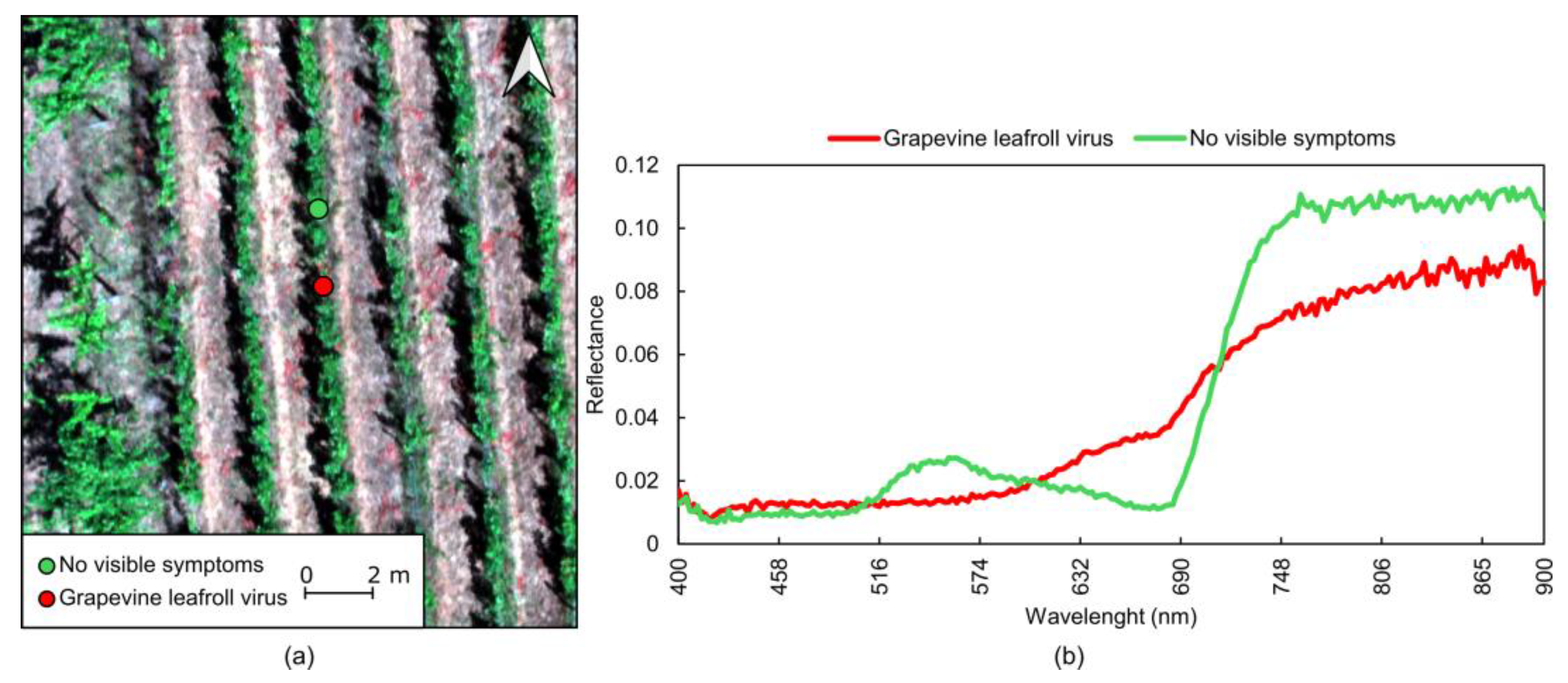
4.3.6. Viral Diseases
5. Advances, Limitations and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV. Database|OIV. Available online: https://www.oiv.int/what-we-do/data-discovery-report?oiv (accessed on 12 March 2024).
- Sun, Q.; Granco, G.; Groves, L.; Voong, J.; Van Zyl, S. Viticultural Manipulation and New Technologies to Address Environmental Challenges Caused by Climate Change. Climate 2023, 11, 83. [Google Scholar] [CrossRef]
- Cohen, B.; Edan, Y.; Levi, A.; Alchanatis, V. Early Detection of Grapevine (Vitis vinifera) Downy Mildew (Peronospora) and Diurnal Variations Using Thermal Imaging. Sensors 2022, 22, 3585. [Google Scholar] [CrossRef]
- Juroszek, P.; Bartsch, L.; Fontaine, J.F.; Racca, P.; Kleinhenz, B. Summary of the Worldwide Available Crop Disease Risk Simulation Studies That Were Driven by Climate Change Scenarios and Published during the Past 20 Years. Plant Pathol. 2022, 71, 1815–1838. [Google Scholar] [CrossRef]
- Frem, M.; Petrontino, A.; Fucilli, V.; Sansiviero, C.; Bozzo, F. Sustainable Viticulture of Italian Grapevines: Environmental Evaluation and Societal Cost Estimation Using EU Farm Accountancy Data Network Data. Horticulturae 2023, 9, 1239. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Darriet, P. The Impact of Climate Change on Viticulture and Wine Quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Jones, G.V.; Webb, L.B. Climate Change, Viticulture, and Wine: Challenges and Opportunities. J. Wine Res. 2010, 21, 103–106. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Abernethy, K.E.; Smart, R.E.; Wilson, R.J.; Maclean, I.M.D. Climate Change Impacts and Adaptive Strategies: Lessons from the Grapevine. Glob. Change Biol. 2016, 22, 3814–3828. [Google Scholar] [CrossRef] [PubMed]
- Mira de Orduña, R. Climate Change Associated Effects on Grape and Wine Quality and Production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate Change, Wine, and Conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Mozell, M.R.; Thach, L. The Impact of Climate Change on the Global Wine Industry: Challenges & Solutions. Wine Econ. Policy 2014, 3, 81–89. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Araujo, E.S. Optimization of Vineyard Water Management: Challenges, Strategies, and Perspectives. Water 2021, 13, 746. [Google Scholar] [CrossRef]
- Bavaresco, L. Impact of Grapevine Breeding for Disease Resistance on the Global Wine Industry. Acta Hortic. 2019, 1248, 7–14. [Google Scholar] [CrossRef]
- Mannini, F.; Digiaro, M. The Effects of Viruses and Viral Diseases on Grapes and Wine. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 453–482. ISBN 978-3-319-57706-7. [Google Scholar]
- Chakraborty, S.; Tiedemann, A.V.; Teng, P.S. Climate Change: Potential Impact on Plant Diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Scott, E.S.; Dambergs, R.G.; Stummer, B.E.; Petrovic, T. 13—Fungal Contaminants in the Vineyard and Wine Quality and Safety. In Managing Wine Quality, 2nd ed.; Reynolds, A.G., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2022; pp. 587–623. ISBN 978-0-08-102067-8. [Google Scholar]
- Griggs, R.G.; Steenwerth, K.L.; Mills, D.A.; Cantu, D.; Bokulich, N.A. Sources and Assembly of Microbial Communities in Vineyards as a Functional Component of Winegrowing. Front. Microbiol. 2021, 12, 673810. [Google Scholar] [CrossRef] [PubMed]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A Review of Fifteen Years of Trials for Their Control with Chemicals and Biocontrol Agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced Methods of Plant Disease Detection. A Review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Fang, Y.; Ramasamy, R.P. Current and Prospective Methods for Plant Disease Detection. Biosensors 2015, 5, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.H.; Chiang, K.-S.; Del Ponte, E.M. Plant Disease Severity Estimated Visually: A Century of Research, Best Practices, and Opportunities for Improving Methods and Practices to Maximize Accuracy. Trop. Plant Pathol. 2022, 47, 25–42. [Google Scholar] [CrossRef]
- Borges, D.F.; Preising, S.; de Queiroz Ambrósio, M.M.; da Silva, W.L. Detection of Multiple Grapevine Viruses in New England Vineyards. Crop Prot. 2020, 132, 105143. [Google Scholar] [CrossRef]
- Chiumenti, M.; Mohorianu, I.; Roseti, V.; Saldarelli, P.; Dalmay, T.; Minafra, A. High-Throughput-Sequencing-Based Identification of a Grapevine Fanleaf Virus Satellite RNA in Vitis vinifera. Arch. Virol. 2016, 161, 1401–1403. [Google Scholar] [CrossRef]
- Trippa, D.; Scalenghe, R.; Basso, M.F.; Panno, S.; Davino, S.; Morone, C.; Giovino, A.; Oufensou, S.; Luchi, N.; Yousefi, S.; et al. Next-Generation Methods for Early Disease Detection in Crops. Pest Manag. Sci. 2024, 80, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Molitor, D.; Baus, O.; Hoffmann, L.; Beyer, M. Meteorological Conditions Determine the Thermaltemporal Position of the Annual Botrytis Bunch Rot Epidemic on Vitis vinifera L. Cv. Riesling Grapes. OENO One 2016, 50. [Google Scholar] [CrossRef]
- Zherdev, A.V.; Vinogradova, S.V.; Byzova, N.A.; Porotikova, E.V.; Kamionskaya, A.M.; Dzantiev, B.B. Methods for the Diagnosis of Grapevine Viral Infections: A Review. Agriculture 2018, 8, 195. [Google Scholar] [CrossRef]
- Pithan, P.A.; Ducati, J.R.; Garrido, L.R.; Arruda, D.C.; Thum, A.B.; Hoff, R. Spectral Characterization of Fungal Diseases Downy Mildew, Powdery Mildew, Black-Foot and Petri Disease on Vitis vinifera Leaves. Int. J. Remote Sens. 2021, 42, 5680–5697. [Google Scholar] [CrossRef]
- Vélez, S.; Barajas, E.; Rubio, J.A.; Pereira-Obaya, D.; Rodríguez-Pérez, J.R. Field-Deployed Spectroscopy from 350 to 2500 Nm: A Promising Technique for Early Identification of Powdery Mildew Disease (Erysiphe Necator) in Vineyards. Agronomy 2024, 14, 634. [Google Scholar] [CrossRef]
- Ganeva, D.; Filchev, L.; Roumenina, E.; Dragov, R.; Nedyalkova, S.; Bozhanova, V. Winter Durum Wheat Disease Severity Detection with Field Spectroscopy in Phenotyping Experiment at Leaf and Canopy Level. Remote Sens. 2024, 16, 1762. [Google Scholar] [CrossRef]
- Nguyen, C.; Sagan, V.; Maimaitiyiming, M.; Maimaitijiang, M.; Bhadra, S.; Kwasniewski, M.T. Early Detection of Plant Viral Disease Using Hyperspectral Imaging and Deep Learning. Sensors 2021, 21, 742. [Google Scholar] [CrossRef] [PubMed]
- Rubambiza, G.; Romero Galvan, F.; Pavlick, R.; Weatherspoon, H.; Gold, K.M. Toward Cloud-Native, Machine Learning Base Detection of Crop Disease with Imaging Spectroscopy. J. Geophys. Res. Biogeosci. 2023, 128, e2022JG007342. [Google Scholar] [CrossRef]
- Hruska, J.; Adao, T.; Padua, L.; Marques, P.; Peres, E.; Sousa, A.; Morais, R.; Sousa, J.J. Deep Learning-Based Methodological Approach for Vineyard Early Disease Detection Using Hyperspectral Data. In Proceedings of the IGARSS 2018—2018 IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain, 22–27 July 2018; pp. 9063–9066. [Google Scholar]
- Lloret, J.; Bosch, I.; Sendra, S.; Serrano, A. A Wireless Sensor Network for Vineyard Monitoring That Uses Image Processing. Sensors 2011, 11, 6165–6196. [Google Scholar] [CrossRef]
- Kerkech, M.; Hafiane, A.; Canals, R. Deep Leaning Approach with Colorimetric Spaces and Vegetation Indices for Vine Diseases Detection in UAV Images. Comput. Electron. Agric. 2018, 155, 237–243. [Google Scholar] [CrossRef]
- Aruna, M.G.; Silvia, E.; Al-Fatlawy, R.R.; Rao, H.K.; Sowmya, M. Vine Disease Detection UAV Multi Spectral Image Using Segnet and Mobilenet Method. In Proceedings of the 2024 International Conference on Distributed Computing and Optimization Techniques (ICDCOT), Bengaluru, India, 15–16 March 2024; pp. 1–4. [Google Scholar]
- Hnatiuc, M.; Ghita, S.; Alpetri, D.; Ranca, A.; Artem, V.; Dina, I.; Cosma, M.; Abed Mohammed, M. Intelligent Grapevine Disease Detection Using IoT Sensor Network. Bioengineering 2023, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Y.; Cui, P.; Fang, Y.; Su, B. Diagnosis of Grapevine Leafroll Disease Severity Infection via UAV Remote Sensing and Deep Learning. Smart Agric. 2023, 5, 49–61. [Google Scholar] [CrossRef]
- Barjaktarovic, M.; Santoni, M.; Faralli, M.; Bertamini, M.; Bruzzone, L. A Multispectral Acquisition System for Potential Detection of Flavescence Dorée. In Proceedings of the 2022 30th Telecommunications Forum (TELFOR), Belgrade, Serbia, 15–16 November 2022; pp. 1–4. [Google Scholar]
- Liu, E.; Gold, K.M.; Combs, D.; Cadle-Davidson, L.; Jiang, Y. Deep Learning-Based Autonomous Downy Mildew Detection and Severity Estimation in Vineyards. In Proceedings of the 2021 ASABE Annual International Virtual Meeting, Online, 12–16 July 2021. [Google Scholar] [CrossRef]
- Stoll, M.; Schultz, H.R.; Berkelmann-Loehnertz, B. Exploring the Sensitivity of Thermal Imaging for Plasmopara Viticola Pathogen Detection in Grapevines under Different Water Status. Funct. Plant Biol. 2008, 35, 281–288. [Google Scholar] [CrossRef]
- Sun, H.; Li, M.; Zhang, Q.; Rayapati, N.A. Segmentation of Grapevine Leafroll Disease Characteristic Based on Multi-Spectral Image. Sens. Lett. 2013, 11, 1245–1250. [Google Scholar] [CrossRef]
- Žibrat, U.; Knapič, M. Chapter 18—Detection of Grapevine Yellows Using Multispectral Imaging. In Remote Sensing in Precision Agriculture; Lamine, S., Srivastava, P.K., Kayad, A., Muñoz-Arriola, F., Pandey, P.C., Eds.; Earth Observation; Academic Press: Cambridge, MA, USA, 2024; pp. 367–386. ISBN 978-0-323-91068-2. [Google Scholar]
- Mehrubeoglu, M.; Orlebeck, K.; Zemlan, M.J.; Autran, W. Detecting Red Blotch Disease in Grape Leaves Using Hyperspectral Imaging. In Proceedings of the Algorithms and Technologies for Multispectral, Hyperspectral, and Ultraspectral Imagery XXII, Baltimore, MD, USA, 17–21 April 2016; SPIE: Bellingham, WA, USA, 23 May 2016; Volume 9840, pp. 73–80. [Google Scholar]
- Gambhir, N.; Paul, A.; Qiu, T.; Combs, D.B.; Hosseinzadeh, S.; Underhill, A.; Jiang, Y.; Cadle-Davidson, L.E.; Gold, K.M. Non-Destructive Monitoring of Foliar Fungicide Efficacy with Hyperspectral Sensing in Grapevine. Phytopathology 2024, 114, 464–473. [Google Scholar] [CrossRef]
- Sandika, B.; Avil, S.; Sanat, S.; Srinivasu, P. Random Forest Based Classification of Diseases in Grapes from Images Captured in Uncontrolled Environments. In Proceedings of the 2016 IEEE 13th International Conference on Signal Processing (ICSP), Chengdu, China, 6–10 November 2016; pp. 1775–1780. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; He, T.; Huang, J.; Jia, A.; Zhang, Y.; Cao, Y.; Chen, X.; Chen, X.; Cheng, J.; Jiang, B.; et al. Advancements in High-Resolution Land Surface Satellite Products: A Comprehensive Review of Inversion Algorithms, Products and Challenges. Sci. Remote Sens. 2024, 10, 100152. [Google Scholar] [CrossRef]
- Cheng, J.; Meng, X.; Dong, S.; Liang, S. Generating the 30-m Land Surface Temperature Product over Continental China and USA from Landsat 5/7/8 Data. Sci. Remote Sens. 2021, 4, 100032. [Google Scholar] [CrossRef]
- Manivasagam, V.S. Remote Sensing of Irrigation: Research Trends and the Direction to next-Generation Agriculture through Data-Driven Scientometric Analysis. Water Secur. 2024, 21, 100161. [Google Scholar] [CrossRef]
- Allen, R.; Irmak, A.; Trezza, R.; Hendrickx, J.M.H.; Bastiaanssen, W.; Kjaersgaard, J. Satellite-Based ET Estimation in Agriculture Using SEBAL and METRIC. Hydrol. Process. 2011, 25, 4011–4027. [Google Scholar] [CrossRef]
- Islam, M.M. Unravelling the Complexities of Wetland Agriculture, Climate Change, and Coping Mechanisms: An Integrative Review Using Economics and Satellite Approaches. Environ. Dev. Sustain. 2024. [Google Scholar] [CrossRef]
- Matese, A.; Toscano, P.; Di Gennaro, S.F.; Genesio, L.; Vaccari, F.P.; Primicerio, J.; Belli, C.; Zaldei, A.; Bianconi, R.; Gioli, B. Intercomparison of UAV, Aircraft and Satellite Remote Sensing Platforms for Precision Viticulture. Remote Sens. 2015, 7, 2971–2990. [Google Scholar] [CrossRef]
- Marino, S.; Alvino, A. Detection of Spatial and Temporal Variability of Wheat Cultivars by High-Resolution Vegetation Indices. Agronomy 2019, 9, 226. [Google Scholar] [CrossRef]
- Hassler, S.C.; Baysal-Gurel, F. Unmanned Aircraft System (UAS) Technology and Applications in Agriculture. Agronomy 2019, 9, 618. [Google Scholar] [CrossRef]
- Valasek, J.; Lu, H.-H.; Shi, Y. Development and Testing of a Customized Low-Cost Unmanned Aircraft System Based on Multispectral and Thermal Sensing for Precision Agriculture Applications. In Proceedings of the 2017 International Conference on Unmanned Aircraft Systems (ICUAS), Miami, FL, USA, 13–16 June 2017; pp. 1208–1216. [Google Scholar]
- Galvan, F.E.R.; Pavlick, R.; Trolley, G.; Aggarwal, S.; Sousa, D.; Starr, C.; Forrestel, E.; Bolton, S.; del Mar Alsina, M.; Dokoozlian, N.; et al. Scalable Early Detection of Grapevine Viral Infection with Airborne Imaging Spectroscopy. Phytopathology 2023, 113, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.K.-K.; Hoekstra, M.; Foroutan, M.; Chegoonian, A.M.; Zolfaghari, K.; Duguay, C.R. Operating Procedures and Calibration of a Hyperspectral Sensor Onboard a Remotely Piloted Aircraft System for Water and Agriculture Monitoring. In Proceedings of the IGARSS 2019 IEEE International Geoscience and Remote Sensing Symposium, Yokohama, Japan, 28 July–2 August 2019; pp. 9200–9203. [Google Scholar]
- Zhang, C.; Kovacs, J.M. The Application of Small Unmanned Aerial Systems for Precision Agriculture: A Review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Tsouros, D.C.; Bibi, S.; Sarigiannidis, P.G. A Review on UAV-Based Applications for Precision Agriculture. Information 2019, 10, 349. [Google Scholar] [CrossRef]
- Velusamy, P.; Rajendran, S.; Mahendran, R.K.; Naseer, S.; Shafiq, M.; Choi, J.-G. Unmanned Aerial Vehicles (UAV) in Precision Agriculture: Applications and Challenges. Energies 2022, 15, 217. [Google Scholar] [CrossRef]
- Gennaro, S.D.; Battiston, E.; Marco, S.D.; Facini, O.; Matese, A.; Nocentini, M.; Palliotti, A.; Mugnai, L. Unmanned Aerial Vehicle (UAV)-Based Remote Sensing to Monitor Grapevine Leaf Stripe Disease within a Vineyard Affected by Esca Complex. Phytopathol. Mediterr. 2016, 55, 262–275. [Google Scholar] [CrossRef]
- Araújo-Paredes, C.; Portela, F.; Mendes, S.; Valín, M.I. Using Aerial Thermal Imagery to Evaluate Water Status in Vitis vinifera Cv. Loureiro. Sensors 2022, 22, 8056. [Google Scholar] [CrossRef]
- García-Fernández, M.; Sanz-Ablanedo, E.; Rodríguez-Pérez, J.R. High-Resolution Drone-Acquired RGB Imagery to Estimate Spatial Grape Quality Variability. Agronomy 2021, 11, 655. [Google Scholar] [CrossRef]
- Ahmed, F.; Mohanta, J.C.; Keshari, A.; Yadav, P.S. Recent Advances in Unmanned Aerial Vehicles: A Review. Arab. J. Sci. Eng. 2022, 47, 7963–7984. [Google Scholar] [CrossRef]
- Morales-Rodríguez, P.A.; Cano Cano, E.; Villena, J.; López-Perales, J.A. A Comparison between Conventional Sprayers and New UAV Sprayers: A Study Case of Vineyards and Olives in Extremadura (Spain). Agronomy 2022, 12, 1307. [Google Scholar] [CrossRef]
- Sassu, A.; Psiroukis, V.; Bettucci, F.; Ghiani, L.; Fountas, S.; Gambella, F. Unmanned Aerial System Plant Protection Products Spraying Performance Evaluation on a Vineyard. Precis. Agric. 2024, 25, 2082–2112. [Google Scholar] [CrossRef]
- Librán-Embid, F.; Klaus, F.; Tscharntke, T.; Grass, I. Unmanned Aerial Vehicles for Biodiversity-Friendly Agricultural Landscapes—A Systematic Review. Sci. Total Environ. 2020, 732, 139204. [Google Scholar] [CrossRef]
- Pascoal, D.; Silva, N.; Adão, T.; Lopes, R.D.; Peres, E.; Morais, R. A Technical Survey on Practical Applications and Guidelines for IoT Sensors in Precision Agriculture and Viticulture. Sci. Rep. 2024, 14, 29793. [Google Scholar] [CrossRef]
- Abdelghafour, F.; Keresztes, B.; Germain, C.; Da Costa, J.-P. In Field Detection of Downy Mildew Symptoms with Proximal Colour Imaging. Sensors 2020, 20, 4380. [Google Scholar] [CrossRef] [PubMed]
- Ammoniaci, M.; Kartsiotis, S.-P.; Perria, R.; Storchi, P. State of the Art of Monitoring Technologies and Data Processing for Precision Viticulture. Agriculture 2021, 11, 201. [Google Scholar] [CrossRef]
- Moses, J.C.; Adibi, S.; Wickramasinghe, N.; Nguyen, L.; Angelova, M.; Islam, S.M.S. Smartphone as a Disease Screening Tool: A Systematic Review. Sensors 2022, 22, 3787. [Google Scholar] [CrossRef]
- Sassu, A.; Gambella, F.; Ghiani, L.; Mercenaro, L.; Caria, M.; Pazzona, A.L. Advances in Unmanned Aerial System Remote Sensing for Precision Viticulture. Sensors 2021, 21, 956. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Bachik, N.A.; Muhadi, N. ‘A.; Tuan Yusof, T.N.; Gomes, C. Non-Destructive Techniques of Detecting Plant Diseases: A Review. Physiol. Mol. Plant Pathol. 2019, 108, 101426. [Google Scholar] [CrossRef]
- Kior, A.; Yudina, L.; Zolin, Y.; Sukhov, V.; Sukhova, E. RGB Imaging as a Tool for Remote Sensing of Characteristics of Terrestrial Plants: A Review. Plants 2024, 13, 1262. [Google Scholar] [CrossRef]
- Ndlovu, H.S.; Odindi, J.; Sibanda, M.; Mutanga, O. A Systematic Review on the Application of UAV-Based Thermal Remote Sensing for Assessing and Monitoring Crop Water Status in Crop Farming Systems. Int. J. Remote Sens. 2024, 45, 4923–4960. [Google Scholar] [CrossRef]
- De la Fuente, C.P.; Valdés-Gómez, H.; Roudet, J.; Verdugo-Vásquez, N.; Mirabal, Y.; Laurie, V.F.; Goutouly, J.P.; Opazo, C.A.; Fermaud, M. Vigor Thresholded NDVI Is a Key Early Risk Indicator of Botrytis Bunch Rot in Vineyards. OENO One 2020, 54, 279–297. [Google Scholar] [CrossRef]
- Giovos, R.; Tassopoulos, D.; Kalivas, D.; Lougkos, N.; Priovolou, A. Remote Sensing Vegetation Indices in Viticulture: A Critical Review. Agriculture 2021, 11, 457. [Google Scholar] [CrossRef]
- Tardaguila, J.; Stoll, M.; Gutiérrez, S.; Proffitt, T.; Diago, M.P. Smart Applications and Digital Technologies in Viticulture: A Review. Smart Agric. Technol. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Farooq, M.S.; Riaz, S.; Abid, A.; Umer, T.; Zikria, Y.B. Role of IoT Technology in Agriculture: A Systematic Literature Review. Electronics 2020, 9, 319. [Google Scholar] [CrossRef]
- Molitor, D.; Baus, O.; Didry, Y.; Junk, J.; Hoffmann, L.; Beyer, M. BotRisk: Simulating the Annual Bunch Rot Risk on Grapevines (Vitis vinifera L. Cv. Riesling) Based on Meteorological Data. Int. J. Biometeorol. 2020, 64, 1571–1582. [Google Scholar] [CrossRef]
- Clippinger, J.I.; Dobry, E.P.; Laffan, I.; Zorbas, N.; Hed, B.; Campbell, M.A. Traditional and Emerging Approaches for Disease Management of Plasmopara Viticola, Causal Agent of Downy Mildew of Grape. Agriculture 2024, 14, 406. [Google Scholar] [CrossRef]
- Velasquez-Camacho, L.; Otero, M.; Basile, B.; Pijuan, J.; Corrado, G. Current Trends and Perspectives on Predictive Models for Mildew Diseases in Vineyards. Microorganisms 2023, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Legler, S.E.; Caffi, T.; Rossi, V. A Nonlinear Model for Temperature-Dependent Development of Erysiphe Necator Chasmothecia on Grapevine Leaves. Plant Pathol. 2012, 61, 96–105. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Cadle-Davidson, L.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine Powdery Mildew (Erysiphe Necator): A Fascinating System for the Study of the Biology, Ecology and Epidemiology of an Obligate Biotroph. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef]
- Perria, R.; Ciofini, A.; Petrucci, W.A.; D’Arcangelo, M.E.M.; Valentini, P.; Storchi, P.; Carella, G.; Pacetti, A.; Mugnai, L. A Study on the Efficiency of Sustainable Wine Grape Vineyard Management Strategies. Agronomy 2022, 12, 392. [Google Scholar] [CrossRef]
- Rahman, M.U.; Liu, X.; Wang, X.; Fan, B. Grapevine Gray Mold Disease: Infection, Defense and Management. Hortic. Res. 2024, 11, uhae182. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.; Csikász-Krizsics, A.; Dula, T.; Farkas, E.; Roznik, D.; Kozma, P.; Deák, T. Black Rot of Grapes (Guignardia Bidwellii)—A Comprehensive Overview. Horticulturae 2023, 9, 130. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Li, P.; Sun, L.; Jiang, J.; Fan, X.; Liu, C.; Zhang, Y. Genome Assembly and Transcriptome Analysis of the Fungus Coniella Diplodiella During Infection on Grapevine (Vitis vinifera L.). Front. Microbiol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Del Frari, G.; Oliveira, H.; Boavida Ferreira, R. White Rot Fungi (Hymenochaetales) and Esca of Grapevine: Insights from Recent Microbiome Studies. J. Fungi 2021, 7, 770. [Google Scholar] [CrossRef] [PubMed]
- Tessitori, M.; La Rosa, R.; Marzachì, C. Flavescence Dorée and Bois Noir Diseases of Grapevine Are Evolving Pathosystems. Plant Health Prog. 2018, 19, 136–138. [Google Scholar] [CrossRef]
- Chuche, J.; Thiéry, D. Biology and Ecology of the Flavescence Dorée Vector Scaphoideus Titanus: A Review. Agron. Sustain. Dev. 2014, 34, 381–403. [Google Scholar] [CrossRef]
- Maree, H.J.; Almeida, R.P.; Bester, R.; Chooi, K.M.; Cohen, D.; Dolja, V.V.; Fuchs, M.F.; Golino, D.A.; Jooste, A.E.; Martelli, G.P.; et al. Grapevine Leafroll-Associated Virus 3. Front. Microbiol. 2013, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, J.; Stephan, D.; Mawassi, M.; Burger, J.T. The Grapevine-Infecting Vitiviruses, with Particular Reference to Grapevine Virus A. Arch. Virol. 2011, 156, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Cieniewicz, E.J.; Qiu, W.; Saldarelli, P.; Fuchs, M. Believing Is Seeing: Lessons from Emerging Viruses in Grapevine. J. Plant Pathol. 2020, 102, 619–632. [Google Scholar] [CrossRef]
- Nex, F.; Remondino, F. UAV for 3D Mapping Applications: A Review. Appl. Geomat. 2014, 6, 1–15. [Google Scholar] [CrossRef]
- Sousa, J.J.; Toscano, P.; Matese, A.; Di Gennaro, S.F.; Berton, A.; Gatti, M.; Poni, S.; Pádua, L.; Hruška, J.; Morais, R.; et al. UAV-Based Hyperspectral Monitoring Using Push-Broom and Snapshot Sensors: A Multisite Assessment for Precision Viticulture Applications. Sensors 2022, 22, 6574. [Google Scholar] [CrossRef]
- Daniels, L.; Eeckhout, E.; Wieme, J.; Dejaegher, Y.; Audenaert, K.; Maes, W.H. Identifying the Optimal Radiometric Calibration Method for UAV-Based Multispectral Imaging. Remote Sens. 2023, 15, 2909. [Google Scholar] [CrossRef]
- González-García, J.; Swenson, R.L.; Gómez-Espinosa, A. Real-Time Kinematics Applied at Unmanned Aerial Vehicles Positioning for Orthophotography in Precision Agriculture. Comput. Electron. Agric. 2020, 177, 105695. [Google Scholar] [CrossRef]
- Stolarski, O.; Lourenço, J.M.; Peres, E.; Morais, R.; Sousa, J.; Pádua, L. The Impact of Ground Control Points for the 3D Study of Grapevines in Steep Slope Vineyards. Procedia Comput. Sci. 2023, 239, 2301–2307. [Google Scholar] [CrossRef]
- Villanueva, J.K.S.; Blanco, A.C. Optimization of Ground Control Point (GCP) Configuration for Unmanned Aerial Vehicle (UAV) Survey Using Structure from Motion (SFM). Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2019, XLII-4-W12, 167–174. [Google Scholar] [CrossRef]
- Nasiri, V.; Darvishsefat, A.A.; Arefi, H.; Pierrot-Deseilligny, M.; Namiranian, M.; Le Bris, A. Unmanned Aerial Vehicles (UAV)-Based Canopy Height Modeling under Leaf-on and Leaf-off Conditions for Determining Tree Height and Crown Diameter (Case Study: Hyrcanian Mixed Forest). Can. J. For. Res. 2021, 51, 962–971. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T. A Comprehensive Review of Assessing Storm Surge Disasters: From Traditional Statistical Methods to Artificial Intelligence-Based Techniques. Atmosphere 2024, 15, 359. [Google Scholar] [CrossRef]
- Carlin, J.B.; Gurrin, L.C.; Sterne, J.A.; Morley, R.; Dwyer, T. Regression Models for Twin Studies: A Critical Review. Int. J. Epidemiol. 2005, 34, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Saed-Moucheshi, A.; Fasihfar, E.; Hasheminasab, H.; Rahmani, A.; Ahmadi, A. A Review on Applied Multivariate Statistical Techniques in Agriculture and Plant Science. Int. J. Agron. Plant Prod. 2013, 4, 127–141. [Google Scholar]
- Demšar, U.; Harris, P.; Brunsdon, C.; Fotheringham, A.S.; McLoone, S. Principal Component Analysis on Spatial Data: An Overview. Ann. Assoc. Am. Geogr. 2013, 103, 106–128. [Google Scholar] [CrossRef]
- Sharma, A.; Paliwal, K.K. Linear Discriminant Analysis for the Small Sample Size Problem: An Overview. Int. J. Mach. Learn. Cyber. 2015, 6, 443–454. [Google Scholar] [CrossRef]
- Guezenoc, J.; Gallet-Budynek, A.; Bousquet, B. Critical Review and Advices on Spectral-Based Normalization Methods for LIBS Quantitative Analysis. Spectrochim. Acta Part B At. Spectrosc. 2019, 160, 105688. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, B. The Diversity in the Applications of Partial Least Squares: An Overview. J. Chemom. 2016, 30, 4–17. [Google Scholar] [CrossRef]
- Sharma, R. Artificial Intelligence in Agriculture: A Review. In Proceedings of the 2021 5th International Conference on Intelligent Computing and Control Systems (ICICCS), Madurai, India, 6–8 May 2021; pp. 937–942. [Google Scholar]
- Ray, S. A Quick Review of Machine Learning Algorithms. In Proceedings of the 2019 International Conference on Machine Learning, Big Data, Cloud and Parallel Computing (COMITCon), Faridabad, India, 14–16 February 2019; pp. 35–39. [Google Scholar]
- Parmar, A.; Katariya, R.; Patel, V. A Review on Random Forest: An Ensemble Classifier. In Proceedings of the International Conference on Intelligent Data Communication Technologies and Internet of Things (ICICI), Coimbatore, India, 7–8 August 2018; Hemanth, J., Fernando, X., Lafata, P., Baig, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 758–763. [Google Scholar]
- Segal, M.; Xiao, Y. Multivariate Random Forests. WIREs Data Min. Knowl. Discov. 2011, 1, 80–87. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2015, arXiv:1409.1556. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the Inception Architecture for Computer Vision. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 2818–2826. [Google Scholar]
- Aguiar, A.S.; Santos, F.N.D.; De Sousa, A.J.M.; Oliveira, P.M.; Santos, L.C. Visual Trunk Detection Using Transfer Learning and a Deep Learning-Based Coprocessor. IEEE Access 2020, 8, 77308–77320. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, A. Era of Deep Neural Networks: A Review. In Proceedings of the 2017 8th International Conference on Computing, Communication and Networking Technologies (ICCCNT), Delhi, India, 3–5 July 2017; pp. 1–5. [Google Scholar]
- Jiang, P.; Ergu, D.; Liu, F.; Cai, Y.; Ma, B. A Review of Yolo Algorithm Developments. Procedia Comput. Sci. 2022, 199, 1066–1073. [Google Scholar] [CrossRef]
- Mezei, I.; Lukić, M.; Berbakov, L.; Pavković, B.; Radovanović, B. Grapevine Downy Mildew Warning System Based on NB-IoT and Energy Harvesting Technology. Electronics 2022, 11, 356. [Google Scholar] [CrossRef]
- Marcu, I.; Drăgulinescu, A.-M.; Oprea, C.; Suciu, G.; Bălăceanu, C. Predictive Analysis and Wine-Grapes Disease Risk Assessment Based on Atmospheric Parameters and Precision Agriculture Platform. Sustainability 2022, 14, 11487. [Google Scholar] [CrossRef]
- Kleb, M.; Merkt, N.; Zörb, C. New Aspects of In Situ Measurements for Downy Mildew Forecasting. Plants 2022, 11, 1807. [Google Scholar] [CrossRef] [PubMed]
- Sanna, F.; Deboli, R.; Calvo, A.; Merlone, A. Influence of Sensor Calibration on Forecasting Models for Vineyard Disease Detection. IOP Conf. Ser. Earth Environ. Sci. 2019, 275, 012020. [Google Scholar] [CrossRef]
- Cohen, B.; Edan, Y.; Levi, A.; Alchanatis, V. 33. Early Detection of Grapevine Downy Mildew Using Thermal Imaging. In Precision Agriculture’21; Wageningen Academic Publishers: Wageningen, The Netherlands, 2021; pp. 283–290. ISBN 978-90-8686-363-1. [Google Scholar]
- Zia-Khan, S.; Kleb, M.; Merkt, N.; Schock, S.; Müller, J. Application of Infrared Imaging for Early Detection of Downy Mildew (Plasmopara Viticola) in Grapevine. Agriculture 2022, 12, 617. [Google Scholar] [CrossRef]
- Stoll, M.; Schultz, H.R.; Baecker, G.; Berkelmann-Loehnertz, B. Early Pathogen Detection under Different Water Status and the Assessment of Spray Application in Vineyards through the Use of Thermal Imagery. Precis. Agric. 2008, 9, 407–417. [Google Scholar] [CrossRef]
- Liu, E.; Gold, K.M.; Combs, D.; Cadle-Davidson, L.; Jiang, Y. Deep Semantic Segmentation for the Quantification of Grape Foliar Diseases in the Vineyard. Front. Plant Sci. 2022, 13, 978761. [Google Scholar] [CrossRef]
- Poblete-Echeverría, C.; Hernández, I.; Gutiérrez, S.; Iñiguez, R.; Barrio, I.; Tardaguila, J. Using Artificial Intelligence (AI) for Grapevine Disease Detection Based on Images. BIO Web Conf. 2023, 68, 01021. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Hernández, I.; Ceballos, S.; Barrio, I.; Díez-Navajas, A.M.; Tardaguila, J. Deep Learning for the Differentiation of Downy Mildew and Spider Mite in Grapevine under Field Conditions. Comput. Electron. Agric. 2021, 182, 105991. [Google Scholar] [CrossRef]
- Padol, P.B.; Yadav, A.A. SVM Classifier Based Grape Leaf Disease Detection. In Proceedings of the 2016 Conference on Advances in Signal Processing (CASP), Pune, India, 9–11 June 2016; pp. 175–179. [Google Scholar]
- Latouche, G.; Debord, C.; Raynal, M.; Milhade, C.; Cerovic, Z.G. First Detection of the Presence of Naturally Occurring Grapevine Downy Mildew in the Field by a Fluorescence-Based Method. Photochem. Photobiol. Sci. 2015, 14, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, S.; Nuzillard, D.; Goupil, A. Construction of a Grape Quality Index from RGB Images of Crates. In Proceedings of the Sixteenth International Conference on Quality Control by Artificial Vision, Albi, France, 6–8 June 2023; SPIE: Bellingham, WA, USA, 28 July 2023; Volume 12749, pp. 17–24. [Google Scholar]
- Yang, R.; Lu, X.; Huang, J.; Zhou, J.; Jiao, J.; Liu, Y.; Liu, F.; Su, B.; Gu, P. A Multi-Source Data Fusion Decision-Making Method for Disease and Pest Detection of Grape Foliage Based on ShuffleNet V2. Remote Sens. 2021, 13, 5102. [Google Scholar] [CrossRef]
- Kanaley, K.; Combs, D.B.; Paul, A.; Jiang, Y.; Bates, T.; Gold, K.M. Assessing the Capacity of High-Resolution Commercial Satellite Imagery for Grapevine Downy Mildew Detection and Surveillance in New York State. In Phytopathology; The American Phytopathological Society: St. Paul, MN, USA, 2023. [Google Scholar] [CrossRef]
- Kerkech, M.; Hafiane, A.; Canals, R. Vine Disease Detection in UAV Multispectral Images Using Optimized Image Registration and Deep Learning Segmentation Approach. Comput. Electron. Agric. 2020, 174, 105446. [Google Scholar] [CrossRef]
- Kerkech, M.; Hafiane, A.; Canals, R. VddNet: Vine Disease Detection Network Based on Multispectral Images and Depth Map. Remote Sens. 2020, 12, 3305. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Kotelnikov, D.Y.; Shchekin, V.Y.; Koltsov, A.D.; Kabankova, E.N. Intelligent Complex of Monitoring and Diagnostics of Grape Plantations. IOP Conf. Ser. Earth Environ. Sci. 2022, 981, 032020. [Google Scholar] [CrossRef]
- Balaceanu, C.; Streche, R.; Roscaneanu, R.; Osiac, F.; Orza, O.; Bosoc, S.; Suciu, G. Diseases Detection System Based on Machine Learning Algorithms and Internet of Things Technology Used in Viticulture. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–19 November 2022; pp. 1–4. [Google Scholar]
- Roşcăneanu, R.; Streche, R.; Osiac, F.; Bălăceanu, C.; Suciu, G.; Drăgulinescu, A.M.; Marcu, I. Detection of Vineyard Diseases Using the Internet of Things Technology and Machine Learning Algorithms. In Proceedings of the “2022 Air and Water—Components of the Environment” Conference Proceedings, Cluj-Napoca, Romania, 18–20 March 2022; pp. 128–139. [Google Scholar]
- Ouhami, M.; Es-saady, Y.; Hajj, M.E.; Canals, R.; Hafiane, A. Meteorological Data and UAV Images for the Detection and Identification of Grapevine Disease Using Deep Learning. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–19 November 2022; pp. 1–4. [Google Scholar]
- Kontogiannis, S.; Konstantinidou, M.; Tsioukas, V.; Pikridas, C. A Cloud-Based Deep Learning Framework for Downy Mildew Detection in Viticulture Using Real-Time Image Acquisition from Embedded Devices and Drones. Information 2024, 15, 178. [Google Scholar] [CrossRef]
- Patil, S.S.; Thorat, S.A. Early Detection of Grapes Diseases Using Machine Learning and IoT. In Proceedings of the 2016 Second International Conference on Cognitive Computing and Information Processing (CCIP), Mysuru, India, 12–13 August 2016; pp. 1–5. [Google Scholar]
- Spachos, P. Towards a Low-Cost Precision Viticulture System Using Internet of Things Devices. IoT 2020, 1, 5–20. [Google Scholar] [CrossRef]
- Sai Reddy, B.; Neeraja, S. Plant Leaf Disease Classification and Damage Detection System Using Deep Learning Models. Multimed. Tools Appl. 2022, 81, 24021–24040. [Google Scholar] [CrossRef]
- Zahra, U.; Khan, M.A.; Alhaisoni, M.; Alasiry, A.; Marzougui, M.; Masood, A. An Integrated Framework of Two-Stream Deep Learning Models Optimal Information Fusion for Fruits Disease Recognition. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2024, 17, 3038–3052. [Google Scholar] [CrossRef]
- Huang, Z.; Qin, A.; Lu, J.; Menon, A.; Gao, J. Grape Leaf Disease Detection and Classification Using Machine Learning. In Proceedings of the 2020 International Conferences on Internet of Things (iThings) and IEEE Green Computing and Communications (GreenCom) and IEEE Cyber, Physical and Social Computing (CPSCom) and IEEE Smart Data (SmartData) and IEEE Congress on Cybermatics (Cybermatics), Rhodes, Greece, 2–6 November 2020; pp. 870–877. [Google Scholar]
- Ferentinos, K.P. Deep Learning Models for Plant Disease Detection and Diagnosis. Comput. Electron. Agric. 2018, 145, 311–318. [Google Scholar] [CrossRef]
- Miranda, M.; Zabawa, L.; Kicherer, A.; Strothmann, L.; Rascher, U.; Roscher, R. Detection of Anomalous Grapevine Berries Using Variational Autoencoders. Front. Plant Sci. 2022, 13, 729097. [Google Scholar] [CrossRef]
- Strothmann, L.; Rascher, U.; Roscher, R. Detection of Anomalous Grapevine Berries Using All-Convolutional Autoencoders. In Proceedings of the IGARSS 2019 IEEE International Geoscience and Remote Sensing Symposium, Yokohama, Japan, 28 July–2 August 2019; pp. 3701–3704. [Google Scholar]
- Rist, F.; Gabriel, D.; Mack, J.; Steinhage, V.; Töpfer, R.; Herzog, K. Combination of an Automated 3D Field Phenotyping Workflow and Predictive Modelling for High-Throughput and Non-Invasive Phenotyping of Grape Bunches. Remote Sens. 2019, 11, 2953. [Google Scholar] [CrossRef]
- Ariza-Sentís, M.; Vélez, S.; Valente, J. BBR: An Open-Source Standard Workflow Based on Biophysical Crop Parameters for Automatic Botrytis Cinerea Assessment in Vineyards. SoftwareX 2023, 24, 101542. [Google Scholar] [CrossRef]
- Vélez, S.; Ariza-Sentís, M.; Valente, J. Mapping the Spatial Variability of Botrytis Bunch Rot Risk in Vineyards Using UAV Multispectral Imagery. Eur. J. Agron. 2023, 142, 126691. [Google Scholar] [CrossRef]
- Tardif, M.; Amri, A.; Keresztes, B.; Deshayes, A.; Martin, D.; Greven, M.; Da Costa, J.-P. Two-Stage Automatic Diagnosis of Flavescence Dorée Based on Proximal Imaging and Artificial Intelligence: A Multi-Year and Multi-Variety Experimental Study. OENO One 2022, 56, 371–384. [Google Scholar] [CrossRef]
- Musci, M.A.; Persello, C.; Lingua, A.M. Uav Images and Deep-Learning Algorithms for Detecting Flavescence Doree Disease in Grapevine Orchards. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2020, XLIII-B3-2020, 1483–1489. [Google Scholar] [CrossRef]
- Barjaktarovic, M.; Santoni, M.; Faralli, M.; Bertamini, M.; Bruzzone, L. Potential Detection of Flavescence Dorée in the Vineyard Using Close-Range Hyperspectral Imaging. In Proceedings of the 2023 3rd International Conference on Electrical, Computer, Communications and Mechatronics Engineering (ICECCME), Tenerife, Spain, 19–21 July 2023; pp. 1–6. [Google Scholar]
- Barjaktarović, M.; Santoni, M.; Faralli, M.; Bertamini, M.; Bruzzone, L. Data Acquisition for Testing Potential Detection of Flavescence Dorée with a Designed, Affordable Multispectral Camera. Telfor J. 2023, 15, 2–7. [Google Scholar] [CrossRef]
- Zottele, F.; Crocetta, P.; Baiocchi, V. How Important Is UAVs RTK Accuracy for the Identification of Certain Vine Diseases? In Proceedings of the 2022 IEEE Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Perugia, Italy, 3–5 November 2022; pp. 239–243. [Google Scholar]
- Daglio, G.; Cesaro, P.; Todeschini, V.; Lingua, G.; Lazzari, M.; Berta, G.; Massa, N. Potential Field Detection of Flavescence Dorée and Esca Diseases Using a Ground Sensing Optical System. Biosyst. Eng. 2022, 215, 203–214. [Google Scholar] [CrossRef]
- AL-Saddik, H.; Simon, J.-C.; Cointault, F. Development of Spectral Disease Indices for ‘Flavescence Dorée’ Grapevine Disease Identification. Sensors 2017, 17, 2772. [Google Scholar] [CrossRef] [PubMed]
- Al-Saddik, H.; Simon, J.C.; Cointault, F. Assessment of the Optimal Spectral Bands for Designing a Sensor for Vineyard Disease Detection: The Case of ‘Flavescence Dorée’. Precis. Agric. 2019, 20, 398–422. [Google Scholar] [CrossRef]
- Al-Saddik, H.; Laybros, A.; Simon, J.C.; Cointault, F. Protocol for the Definition of a Multi-Spectral Sensor for Specific Foliar Disease Detection: Case of “Flavescence Dorée”. In Phytoplasmas: Methods and Protocols; Musetti, R., Pagliari, L., Eds.; Springer: New York, NY, USA, 2019; pp. 213–238. ISBN 978-1-4939-8837-2. [Google Scholar]
- Albetis, J.; Duthoit, S.; Guttler, F.; Jacquin, A.; Goulard, M.; Poilvé, H.; Féret, J.-B.; Dedieu, G. Detection of Flavescence Dorée Grapevine Disease Using Unmanned Aerial Vehicle (UAV) Multispectral Imagery. Remote Sens. 2017, 9, 308. [Google Scholar] [CrossRef]
- Albetis, J.; Jacquin, A.; Goulard, M.; Poilvé, H.; Rousseau, J.; Clenet, H.; Dedieu, G.; Duthoit, S. On the Potentiality of UAV Multispectral Imagery to Detect Flavescence Dorée and Grapevine Trunk Diseases. Remote Sens. 2018, 11, 23. [Google Scholar] [CrossRef]
- Imran, H.A.; Zeggada, A.; Ianniello, I.; Melgani, F.; Polverari, A.; Baroni, A.; Danzi, D.; Goller, R. Low-Cost Handheld Spectrometry for Detecting Flavescence Dorée in Vineyards. Appl. Sci. 2023, 13, 2388. [Google Scholar] [CrossRef]
- Gallo, R.; Ristorto, G.; Daglio, G.; Massa, M.; Berta, G.; Lazzari, M.; Mazzetto, F. New Solutions for the Automatic Early Detection of Diseases in Vineyards through Ground Sensing Approaches Integrating Lidar and Optical Sensors. Chem. Eng. Trans. 2017, 58, 673–678. [Google Scholar] [CrossRef]
- Elsherbiny, O.; Elaraby, A.; Alahmadi, M.; Hamdan, M.; Gao, J. Rapid Grapevine Health Diagnosis Based on Digital Imaging and Deep Learning. Plants 2024, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Morellos, A.; Pantazi, X.E.; Paraskevas, C.; Moshou, D. Comparison of Deep Neural Networks in Detecting Field Grapevine Diseases Using Transfer Learning. Remote Sens. 2022, 14, 4648. [Google Scholar] [CrossRef]
- Suciu, G.; Vulpe, A.; Fratu, O.; Suciu, V. M2M Remote Telemetry and Cloud IoT Big Data Processing in Viticulture. In Proceedings of the 2015 International Wireless Communications and Mobile Computing Conference (IWCMC), Dubrovnik, Croatia, 24–28 August 2015; pp. 1117–1121. [Google Scholar]
- Oriolani, E.J.A.; Moschini, R.C.; Salas, S.; Martinez, M.; Banchero, S. Weather-Based Models for Predicting Grape Powdery Mildew (Uncinula Necator (Schwein) Burrill) Epidemics. Rev. Fac. Cienc. Agrar. Univ. Nac. Cuyo 2015, 47, 197–211. [Google Scholar]
- Pero, C.; Bakshi, S.; Nappi, M.; Tortora, G. IoT-Driven Machine Learning for Precision Viticulture Optimization. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2024, 17, 2437–2447. [Google Scholar] [CrossRef]
- Li, W.; Yu, X.; Chen, C.; Gong, Q. Identification and Localization of Grape Diseased Leaf Images Captured by UAV Based on CNN. Comput. Electron. Agric. 2023, 214, 108277. [Google Scholar] [CrossRef]
- Rançon, F.; Bombrun, L.; Keresztes, B.; Germain, C. Comparison of SIFT Encoded and Deep Learning Features for the Classification and Detection of Esca Disease in Bordeaux Vineyards. Remote Sens. 2018, 11, 1. [Google Scholar] [CrossRef]
- Daglio, G.; Gallo, R.; Rinaldi, M.F.; Massa, N.; Todeschini, V.; Mazzetto, F. Use of a Multirotor-UAV Equipped with a Multispectral Camera to Detect Vineyard Diseases: A Case Study on Barbera and Dolcetto Cultivars. In Innovative Biosystems Engineering for Sustainable Agriculture, Forestry and Food Production; Coppola, A., Di Renzo, G.C., Altieri, G., D’Antonio, P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 803–809. [Google Scholar]
- Bendel, N.; Kicherer, A.; Backhaus, A.; Klück, H.-C.; Seiffert, U.; Fischer, M.; Voegele, R.T.; Töpfer, R. Evaluating the Suitability of Hyper- and Multispectral Imaging to Detect Foliar Symptoms of the Grapevine Trunk Disease Esca in Vineyards. Plant Methods 2020, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Al-Saddik, H.; Laybros, A.; Billiot, B.; Cointault, F. Using Image Texture and Spectral Reflectance Analysis to Detect Yellowness and Esca in Grapevines at Leaf-Level. Remote Sens. 2018, 10, 618. [Google Scholar] [CrossRef]
- Wang, Y.M.; Ostendorf, B.; Pagay, V. Detecting Grapevine Virus Infections in Red and White Winegrape Canopies Using Proximal Hyperspectral Sensing. Sensors 2023, 23, 2851. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Khot, L.R.; Rathnayake, A.P.; Gao, Z.; Naidu, R.A. Visible-near Infrared Spectroradiometry-Based Detection of Grapevine Leafroll-Associated Virus 3 in a Red-Fruited Wine Grape Cultivar. Comput. Electron. Agric. 2019, 162, 165–173. [Google Scholar] [CrossRef]
- Ampatzidis, Y.; Cruz, A.; Pierro, R.; Materazzi, A.; Panattoni, A.; De Bellis, L.; Luvisi, A. Vision-Based System for Detecting Grapevine Yellow Diseases Using Artificial Intelligence. In Proceedings of the XXX International Horticultural Congress IHC2018: VII Conference on Landscape and Urban Horticulture, IV Conference on Turfgrass Management and Science for Sports Fields and II Symposium on Mechanization, Precision Horticulture, and Robotics, Istanbul, Turkey, 12–16 August 2018; pp. 225–230. [Google Scholar] [CrossRef]
- Mickey Wang, Y.; Ostendorf, B.; Pagay, V. Evaluating the Potential of High-Resolution Hyperspectral UAV Imagery for Grapevine Viral Disease Detection in Australian Vineyards. Int. J. Appl. Earth Obs. Geoinf. 2024, 130, 103876. [Google Scholar] [CrossRef]
- Wang, Y.M.; Ostendorf, B.; Pagay, V. Evaluating the Potential of High-Resolution Visible Remote Sensing to Detect Shiraz Disease in Grapevines. Aust. J. Grape Wine Res. 2023, 2023, 7376153. [Google Scholar] [CrossRef]
- MacDonald, S.L.; Staid, M.; Staid, M.; Cooper, M.L. Remote Hyperspectral Imaging of Grapevine Leafroll-Associated Virus 3 in Cabernet Sauvignon Vineyards. Comput. Electron. Agric. 2016, 130, 109–117. [Google Scholar] [CrossRef]
- Nižetić, S.; Šolić, P.; López-de-Ipiña González-de-Artaza, D.; Patrono, L. Internet of Things (IoT): Opportunities, Issues and Challenges towards a Smart and Sustainable Future. J. Clean. Prod. 2020, 274, 122877. [Google Scholar] [CrossRef]
- Calderón, R.; Montes-Borrego, M.; Landa, B.B.; Navas-Cortés, J.A.; Zarco-Tejada, P.J. Detection of Downy Mildew of Opium Poppy Using High-Resolution Multi-Spectral and Thermal Imagery Acquired with an Unmanned Aerial Vehicle. Precis. Agric. 2014, 15, 639–661. [Google Scholar] [CrossRef]
- Fahey, T.; Pham, H.; Gardi, A.; Sabatini, R.; Stefanelli, D.; Goodwin, I.; Lamb, D.W. Active and Passive Electro-Optical Sensors for Health Assessment in Food Crops. Sensors 2021, 21, 171. [Google Scholar] [CrossRef]
- Eh Teet, S.; Hashim, N. Recent Advances of Application of Optical Imaging Techniques for Disease Detection in Fruits and Vegetables: A Review. Food Control 2023, 152, 109849. [Google Scholar] [CrossRef]
- Kouadio, L.; El Jarroudi, M.; Belabess, Z.; Laasli, S.-E.; Roni, M.Z.K.; Amine, I.D.I.; Mokhtari, N.; Mokrini, F.; Junk, J.; Lahlali, R. A Review on UAV-Based Applications for Plant Disease Detection and Monitoring. Remote Sens. 2023, 15, 4273. [Google Scholar] [CrossRef]
- Morais, R.; Mendes, J.; Silva, R.; Silva, N.; Sousa, J.J.; Peres, E. A Versatile, Low-Power and Low-Cost IoT Device for Field Data Gathering in Precision Agriculture Practices. Agriculture 2021, 11, 619. [Google Scholar] [CrossRef]
- Lee, L.; Reynolds, A.; Lan, Y.; Meng, B. Identification of Unique Electromagnetic Signatures from GLRaV-3 Infected Grapevine Leaves in Different Stages of Virus Development. Smart Agric. Technol. 2024, 8, 100464. [Google Scholar] [CrossRef]
- Madeira, M.; Porfírio, R.P.; Santos, P.A.; Madeira, R.N. AI-Powered Solution for Plant Disease Detection in Viticulture. Procedia Comput. Sci. 2024, 238, 468–475. [Google Scholar] [CrossRef]
- Abdelghafour, F.; Keresztes, B.; Deshayes, A.; Germain, C.; Da Costa, J.-P. An Annotated Image Dataset of Downy Mildew Symptoms on Merlot Grape Variety. Data Brief 2021, 37, 107250. [Google Scholar] [CrossRef] [PubMed]
- Vélez, S.; Ariza-Sentís, M.; Valente, J. Dataset on Unmanned Aerial Vehicle Multispectral Images Acquired over a Vineyard Affected by Botrytis Cinerea in Northern Spain. Data Brief 2023, 46, 108876. [Google Scholar] [CrossRef] [PubMed]
- Vélez, S.; Ariza-Sentís, M.; Valente, J. EscaYard: Precision Viticulture Multimodal Dataset of Vineyards Affected by Esca Disease Consisting of Geotagged Smartphone Images, Phytosanitary Status, UAV 3D Point Clouds and Orthomosaics. Data Brief 2024, 54, 110497. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.P.; Salathe, M. An Open Access Repository of Images on Plant Health to Enable the Development of Mobile Disease Diagnostics. arXiv 2015, arXiv:1511.08060. [Google Scholar]
- Mendes, J.; Peres, E.; Neves dos Santos, F.; Silva, N.; Silva, R.; Sousa, J.J.; Cortez, I.; Morais, R. VineInspector: The Vineyard Assistant. Agriculture 2022, 12, 730. [Google Scholar] [CrossRef]
- Cubero, S.; Marco-Noales, E.; Aleixos, N.; Barbé, S.; Blasco, J. RobHortic: A Field Robot to Detect Pests and Diseases in Horticultural Crops by Proximal Sensing. Agriculture 2020, 10, 276. [Google Scholar] [CrossRef]
- Azevedo, F.; Shinde, P.; Santos, L.; Mendes, J.; Santos, F.N.; Mendonça, H. Parallelization of a Vine Trunk Detection Algorithm for a Real Time Robot Localization System. In Proceedings of the 2019 IEEE International Conference on Autonomous Robot Systems and Competitions (ICARSC), Porto, Portugal, 24–26 April 2019; pp. 1–6. [Google Scholar]
- Mendes, J.M.; dos Santos, F.N.; Ferraz, N.A.; do Couto, P.M.; dos Santos, R.M. Localization Based on Natural Features Detector for Steep Slope Vineyards. J. Intell. Robot. Syst. 2019, 93, 433–446. [Google Scholar] [CrossRef]
- Székely, D.E.; Dobra, D.; Dobre, A.E.; Domşa, V.; Drăghici, B.G.; Ileni, T.-A.; Konievic, R.; Molnár, S.; Sucala, P.; Zah, E.; et al. Bacterial-Fungicidal Vine Disease Detection with Proximal Aerial Images. Heliyon 2024, 10, e34017. [Google Scholar] [CrossRef] [PubMed]





| Platform | Spatial Coverage | Availability | Costs | Capacity |
|---|---|---|---|---|
| Satellite Platforms | >10 m | Regular revisit periods | Low | Broad overview of vineyard conditions |
| Manned Aircrafts | Sub-meter | Subject to logistical limitations | High | Detailed capture of smaller areas |
| Unmanned Aerial Vehicles | Very high (cm level) | On-demand, flexible | Moderate | High-resolution but limited spatial coverage (vineyard plot level) |
| Weather Stations and IoT Devices | Varies by number of sensors/devices | Continuous, real-time data | Varies | Focused on specific environmental parameters |
| Proximity Sensing Platforms (Agricultural Machinery, Smartphones) | Plant and leaf level | As needed during operations | Low to Moderate | Continuous, real-time data collection during operations |
| Sensor Type | Spectrum/Measurement | Applications in Viticulture |
|---|---|---|
| RGB | Visible Spectrum | Monitoring leaf color, density and general growth assessment |
| Thermal Infrared | Thermal Infrared Radiation (8 to 14 µm) | Water stress detection; irrigation scheduling; canopy temperature monitoring |
| Multispectral | Visible to Near-Infrared (0.4 to 1 µm) | Plant vigor and health assessment; nutrient management; yield estimation; water management |
| Spectroscopic Equipment | Wide range of wavelengths | Grape chemical composition and ripeness; variety classification; water status; detecting nutrient deficiencies |
| Weather and Environmental Sensors | Climate, plant, and soil parameters | Real-time vineyard microclimate and environmental monitoring; irrigation support |
| Pathogen Type | Disease | Main Symptoms | Spreading/Occurrence Condition(s) | Management Practices | Main Affected Plant Parts |
|---|---|---|---|---|---|
| Fungal | Downy Mildew | Oily spots, necrotic lesions, white sporulating layer, shriveling clusters | Humid, mild temperatures, leaf wetness | Fungicides, improve air circulation, monitoring climatic conditions | Leaves, stems, grapes |
| Powdery Mildew | White-grayish powdery coating on leaves and berries | Warm, dry climates with high humidity | |||
| Grey Rot | Brown spots on flowers and leaves, grey sporulation on berries | High humidity, precipitation | Fungicides, removal of infected material | Grapes, flowers, leaves | |
| Black Rot | Brown spots with black borders on leaves, black spots on berries | Warm, humid climates | Removal of infected plant debris, fungicides, pruning | Leaves, grapes | |
| Sour Rot | Vinegary odor, berry splits | High humidity during ripening | Removal of infected material, insect vector control | Grapes | |
| White Rot | Watery rot in grapes | High humidity | Removal of infected material, fungicides | ||
| Esca Complex | Chlorotic streaks, vascular discoloration, necrosis, apoplexy | Pruning activities or plant damage | Prevent pruning wounds, remove infected plants | Trunk, leaves | |
| Bacterial | Flavescence Dorée | Yellowing and curling leaves, stunted shoots, necrosis of inflorescences | Leafhopper | Insect vector control, remove infected plants | Leaves, shoots, inflorescences |
| Viral | GLRaV | Leaf discoloration, rolling, stunted growth, reduced yield | Climatic conditions, insect vectors, grafting | Use of virus-free planting material and grafting practices, control insect vectors, management of rugose wood complex | Leaves, grapes, trunk, vascular tissues |
| GVA | Bark cracking, vascular tissue damage, reduced yield | ||||
| GVCV | Vein clearing in leaves, deformities, decreased vigor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portela, F.; Sousa, J.J.; Araújo-Paredes, C.; Peres, E.; Morais, R.; Pádua, L. A Systematic Review on the Advancements in Remote Sensing and Proximity Tools for Grapevine Disease Detection. Sensors 2024, 24, 8172. https://doi.org/10.3390/s24248172
Portela F, Sousa JJ, Araújo-Paredes C, Peres E, Morais R, Pádua L. A Systematic Review on the Advancements in Remote Sensing and Proximity Tools for Grapevine Disease Detection. Sensors. 2024; 24(24):8172. https://doi.org/10.3390/s24248172
Chicago/Turabian StylePortela, Fernando, Joaquim J. Sousa, Cláudio Araújo-Paredes, Emanuel Peres, Raul Morais, and Luís Pádua. 2024. "A Systematic Review on the Advancements in Remote Sensing and Proximity Tools for Grapevine Disease Detection" Sensors 24, no. 24: 8172. https://doi.org/10.3390/s24248172
APA StylePortela, F., Sousa, J. J., Araújo-Paredes, C., Peres, E., Morais, R., & Pádua, L. (2024). A Systematic Review on the Advancements in Remote Sensing and Proximity Tools for Grapevine Disease Detection. Sensors, 24(24), 8172. https://doi.org/10.3390/s24248172









