Electrochemical Sensors Based on Transition Metal Materials for Phenolic Compound Detection
Abstract
:1. Introduction
2. Transition Metal-Based Electrocatalysts
2.1. Transition Metal Oxides
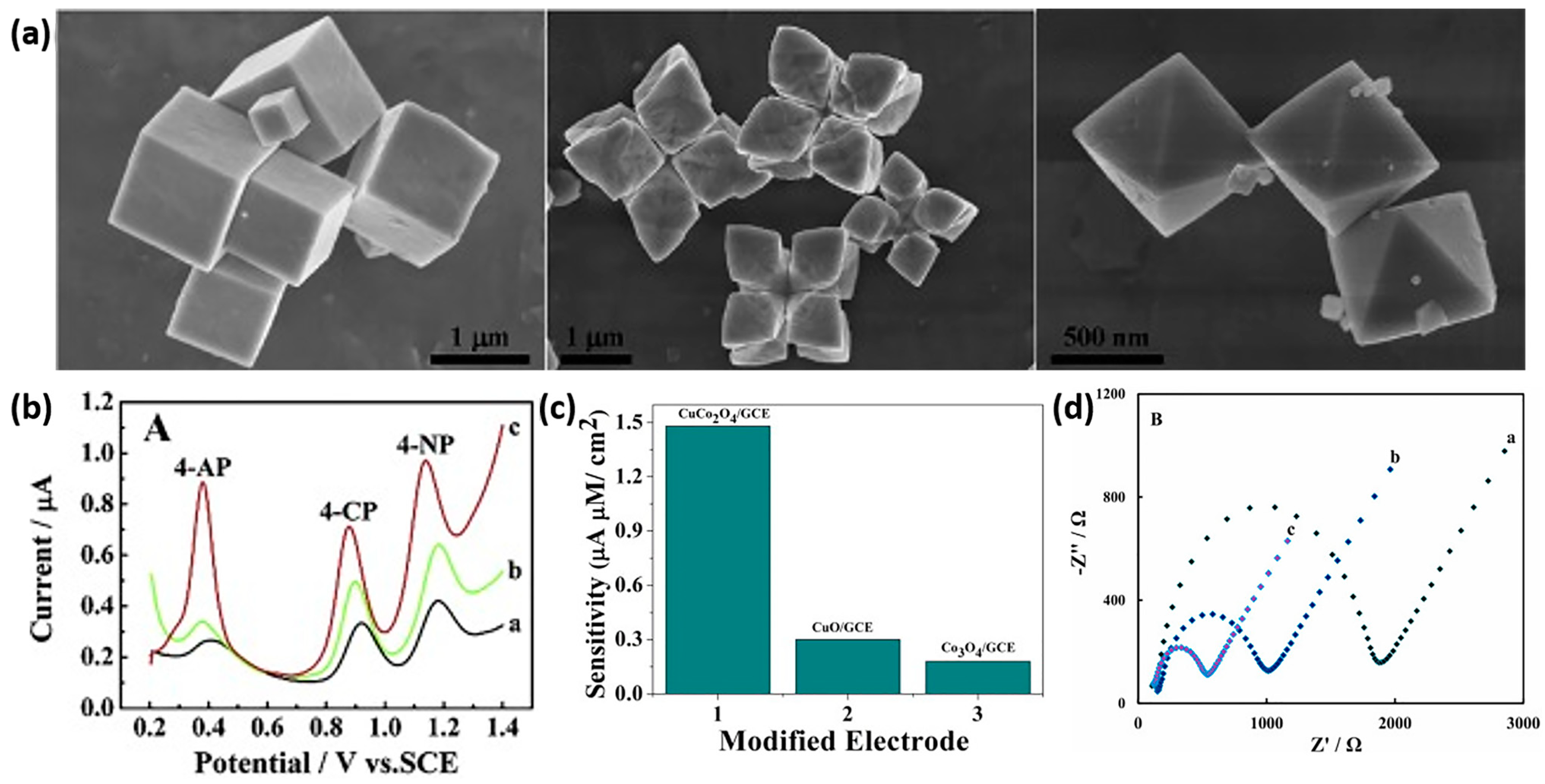
2.2. Transition Metal Chalcogenides
2.2.1. Transition Metal Sulfides


2.2.2. Transition Metal Selenides

2.2.3. Transition Metal Tellurides
2.3. Transition Metal Phosphides

| Electrode Material | Phenolic Compound | Method | Linear Range (µM) | Sensitivity (A M−1 cm−2) | LOD (µM) | Ref. |
|---|---|---|---|---|---|---|
| O–Cu2O/GCE | 4-aminophenol | DPV | 0.008–9 | - | 0.0018 | [39] |
| 4-chlorophenol | 0.01–4 | - | 0.0027 | |||
| 4-nitrophenol | 0.08–30 | - | 0.0085 | |||
| Co3O4/GCE | Uric acid | DPV | 0–1500 | 2.16 | 1.6 | [40] |
| Co3O4/GCE | Hydroquinone | DPV | 1–500 | 0.721 | 0.1 | [41] |
| Catechol | 1–500 | 0.354 | 0.1 | |||
| CuCo2O4/GCE | Metol | DPV | 0.02–1000 | 1.45 | 0.006 | [43] |
| CoFe2O4/GCE | Bisphenol A | DPV | 0.05–10 | 0.815 | 0.0036 | [44] |
| ZnFe2O4/g-C3N4/GCE | 4-nitrophenol | Amp. | 0.015–724 | 1.68 | 0.0042 | [45] |
| ZnFe2O4/PANI@rGO/GCE | 4-nitrophenol | DPV | 1–100 | 36.9 | 0.083 | [46] |
| MWCNTs/CuFe2O4/GCE | Bisphenol A | DPV | 0.01–120 | 5.07 | 0.0032 | [47] |
| MoS2/GCE | Ascorbic acid | DPV | 5–1200 | 0.16 | 0.82 | [49] |
| Dopamine | 1–900 | 0.72 | 0.15 | |||
| Uric acid | 1–60 | 10.13 | 0.06 | |||
| MoS2/GCE | 4-aminophenol | DPV | 0.04–7 | 0.0043 | 0.03 | [50] |
| GNS-CNTs/MoS2/GCE | Dopamine | DPV | 0.1–100 | 10.81 | 0.05 | [51] |
| MoS2-Gr/GCE | Acetaminophen | DPV | 0.1–100 | 3.51 | 0.02 | [52] |
| NiS2/MoS2/rGO/GCE | Bisphenol A | DPV | 0.02–200 | 0.2646 A M−1 | 0.0021 | [59] |
| Ni(OH)2/MoS2/GCE | Dopamine | DPV | 0.75–95 | 0.0284 A M−1 | 0.056 | [60] |
| MPL-NiS/rGO | Bisphenol A | ASV | 0.043–0.26 | - | 1.75 | [54] |
| MnS/GCE | Bisphenol A | DPV | 0.02–109 | - | 0.0065 | [55] |
| WS2-Gr/GCE | Catechol | DPV | 1–100 | 0.447 | 0.2 | [57] |
| Resorcinol | 0.206 | 0.1 | ||||
| Hydroquinone | 0.380 | 0.1 | ||||
| FeSe2/GCE | 4-nitrophenol | DPV | 1–10 | 0.397 A M−1 | 0.030 | [61] |
| 2-nitrophenol | 1–10 | 0.377 A M−1 | 0.034 | |||
| Cu2Se/CC | Dopamine | Amp. | 0.002–30 | 12.4 | 0.084 | [62] |
| MoSe2-graphene/Ni foam | Dopamine | DPV | 0.01–10 | 0.104 A M−1 | 0.001 | [65] |
| Pt/Co0.85Se/GCE | Dopamine | DPV | 0.5–22 | 2.31 | 0.39 | [66] |
| S-MoSe2/NSG/Au/MIPs/GCE | Dopamine | DPV | 0.05–100 | 0.101 A M−1 | 0.02 | [67] |
| Ni3Se2/rGO/ITO | 4-nitrophenol | DPV | 0.05–5 | 3.06 A M−1 | 0.017 | [68] |
| V2Se9/rGO/GCE | 2,4,6-trichlorophenol | DPV | 0.001– 1150 | 0.0184 A M−1 | 0.035 | [69] |
| GO@CoMoSe2/GCE | Metol | DPV | 0.04–40 | 2.39 | 0.009 | [70] |
| H-FeMoSe2/SPCE | Mesalazine | DPV | 0.004–57 | 0.24 | 0.008 | [71] |
| CoFe2Se4/PCF/GCE | Hydroquinone | DPV | 0.5–200 | 0.814 | 0.13 | [13] |
| Catechol | 0.5–190 | 0.829 | 0.15 | |||
| Resorcinol | 5–350 | 0.357 | 1.36 | |||
| CoFeSe2/f-CNF/GCE | Caffeic acid | DPV | 0.01–264 | 2.04 | 0.002 | [72] |
| MoCuSe-rGO/GCE | Bisphenol A | DPV | 0.003–0.9 | 12.86 | 0.0009 | [73] |
| W-Co0.5Ni0.5Se2/Ni foam | Gallic acid | DPV | 1–36.2 | 1.33 A M−1 | 0.120 | [74] |
| FeTe2/GP | Dopamine | DPV | 5–120 | 7.29 | 0.028 | [75] |
| Uric acid | 3–120 | 6.36 | 0.042 | |||
| NiTe/GP | Uric acid | DPV | 3–200 | 0.108 A M−1 | 0.095 | [76] |
| 5%Pt-doped CoTe/GCE | Dopamine | DPV | 0.049–0.84 | 342.2 | 0.025 | [77] |
| Cu MOF/ZnTe/Au/GCE | Catechol | DPV | 0.25–300 | 0.142 A M−1 | 0.016 | [78] |
| NiTe2/CPE | Morin | DPV | 0.014–32 | 5.42 A M−1 | 0.0133 | [79] |
| CPE/Ni3-xTe2 | Dopamine | SWV | 4–31 | 1.12 A M−1 | 0.15 | [80] |
| Adrenaline | 4–31 | 0.64 A M−1 | 0.35 | |||
| CoTe2/GCE | Ferulic Acid | SWV | 0.04–28 | 1.096 A M−1 | 0.013 | [81] |
| CoxP/NC/GCE | 4-nitrophenol | DPV | 0.05–1 | 20.9 A M−1 | 0.002 | [82] |
| CoPx@NCNTs/GCE | 4-nitrophenol | LSV | 0.0025–1 | 802 | 0.00079 | [84] |
| G-CoP/N,P–C/GCE | Hydroquinone | DPV | 0.8–900 | 0.541 | 0.18 | [85] |
| Catechol | DPV | 0.6–800 | 0.986 | 0.12 | ||
| Ni2P/GCE | Acetaminophen | Amp. | 0.5–4500 | 0.131 | 0.107 | [86] |
| MnCo-P/S-RGO/RRDE | Acetaminophen | Amp. | 0.05–1.94 | 0.658 | 0.00139 | [87] |
| CoP-NiCoP/GFs/GCE | Hydroquinone | DPV | 1–101 | 0.18 A M−1 | 0.256 | [88] |
| Catechol | 2–102 | 0.21 A M−1 | 0.379 | |||
| CC/Ti3C2Tx/NiCoP | Dopamine | Amp. | 0.17–785 | 31.4 | 0.00018 | [89] |
| NiFeP/Whatman filter paper | Dopamine | SWV | 0.01–10 | 756 | 0.0001 | [90] |
| CoP/Ti mesh | Dopamine | Amp. | 1–3000 | 3.36 | 0.356 | [91] |
| NiCoP/Ni foam | Dopamine | Amp. | 0.5–2350 | 5.26 | 1 | [92] |
| NiCoP/GF microelectrode | Dopamine | DPV | 0.5–200 | 5.56 | 0.014 | [93] |
| ZnNiP/GCE | isoprenaline | Amp. | 0.2–5000 | 0.0668 A M−1 | 0.06 | [94] |
| NiCo-P@NiCo-LDH/GCE | isoprenaline | Amp. | 0.5–2110 | 21.4 | 0.17 | [95] |
| CoPx-N-C/GCE | chloramphenicol | DPV | 0.2–40 | 0.181 A M−1 | 0.044 | [96] |
| CoNiP@rGO/GCE | Hydroquinone | DPV | 0.5–25 | 36.4 | 0.5 | [98] |
| Bisphenol A | 0.001–8 | 96.4 | 0.38 |
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varguez, P.E.M.; Brunel, F.; Raimundo, J.M. Recent Electrochemical/Electrical Microfabricated Sensor Devices for Ionic and Polyionic Analytes. ACS Omega 2020, 5, 4733–4742. [Google Scholar] [CrossRef] [PubMed]
- Jeerapan, I.; Poorahong, S. Review-Flexible and Stretchable Electrochemical Sensing Systems: Materials, Energy Sources, and Integrations. J. Electrochem. Soc. 2020, 167, 037573. [Google Scholar] [CrossRef]
- Buledi, J.A.; Amin, S.; Haider, S.I.; Bhanger, M.I.; Solangi, A.R. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environ. Sci. Pollut. Res. 2021, 28, 58994–59002. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Q.; Liu, Q.; Cheng, N.Y.; Asiri, A.M.; Sun, X.P. Self-Supported Cu3P Nanowire Arrays as an Integrated High-Performance Three-Dimensional Cathode for Generating Hydrogen from Water. Angew. Chem. Int. Ed. 2014, 53, 9577–9581. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.J.; Li, X.X.; Li, Y.; Zheng, M.B.; Pang, H. Applications of MxSey (M = Fe, Co, Ni) and Their Composites in Electrochemical Energy Storage and Conversion. Nano-Micro Lett. 2019, 11, 40. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds-Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.d.R., Eds.; InTech: Rijeka, Yugoslavia, 2017; p. 17. [Google Scholar]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Fauzi, N.I.M.; Daniyal, W.M.E.M.M. Recent advances of priority phenolic compounds detection using phenol oxidases-based electrochemical and optical sensors. Measurement 2021, 184, 109855. [Google Scholar] [CrossRef]
- Salehi, S.; Abdollahi, K.; Panahi, R.; Rahmanian, N.; Shakeri, M.; Mokhtarani, B. Applications of Biocatalysts for Sustainable Oxidation of Phenolic Pollutants: A Review. Sustainability 2021, 13, 8620. [Google Scholar] [CrossRef]
- Yin, D.D.; Liu, J.; Bo, X.J.; Guo, L.P. Cobalt-iron selenides embedded in porous carbon nanofibers for simultaneous electrochemical detection of trace of hydroquinone, catechol and resorcinol. Anal. Chim. Acta 2020, 1093, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Shen, Y.L.; Ouyang, H.Y.; Long, Y.M.; Li, W.F. A voltammetric sensor for simultaneous determination of hydroquinone and catechol by using a heterojunction prepared from gold nanoparticle and graphitic carbon nitride. Microchim. Acta 2019, 186, 819. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.R.; Song, D.N.; Zhao, X. Potential applications and preliminary mechanism of action of dietary polyphenols against hyperuricemia: A review. Food Biosci. 2021, 43, 101297. [Google Scholar] [CrossRef]
- Schindler, S.; Bechtold, T. Mechanistic insights into the electrochemical oxidation of dopamine by cyclic voltammetry. J. Electroanal. Chem. 2019, 836, 94–101. [Google Scholar] [CrossRef]
- Mikkelsen, O.; Schroder, K.H. Amalgam electrodes for electroanalysis. Electroanalysis 2003, 15, 679–687. [Google Scholar] [CrossRef]
- Wang, J. Stripping analysis at bismuth electrodes: A review. Electroanalysis 2005, 17, 1341–1346. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, T.Y.; Bootharaju, M.S.; Kim, J.H.; Chung, D.Y.; Hyeon, T. Noble Metal-Based Multimetallic Nanoparticles for Electrocatalytic Applications. Adv. Sci. 2022, 9, 2104054. [Google Scholar] [CrossRef]
- Lu, L. Nanoporous noble metal-based alloys: A review on synthesis and applications to electrocatalysis and electrochemical sensing. Microchim. Acta 2019, 186, 664. [Google Scholar] [CrossRef]
- Niu, X.H.; Li, X.; Pan, J.M.; He, Y.F.; Qiu, F.X.; Yan, Y.S. Recent advances in non-enzymatic electrochemical glucose sensors based on non-precious transition metal materials: Opportunities and challenges. RSC Adv. 2016, 6, 84893–84905. [Google Scholar] [CrossRef]
- Wu, Z.P.; Lu, X.F.; Zang, S.Q.; Lou, X.W. Non-Noble-Metal-Based Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Wang, J.J.; Yue, X.Y.; Yang, Y.Y.; Sirisomboonchai, S.; Wang, P.F.; Ma, X.L.; Abudula, A.; Guan, G.Q. Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: A review. J. Alloys Compd. 2020, 819, 153346. [Google Scholar] [CrossRef]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Liu, X.H.; Wang, H.D.; Ma, R.Z.; Sasaki, T. Recent advances in developing high-performance nanostructured electrocatalysts based on 3d transition metal elements. Nanoscale Horiz. 2019, 4, 789–808. [Google Scholar] [CrossRef]
- Hu, Y.; Hojamberdiev, M.; Geng, D.S. Recent advances in enzyme-free electrochemical hydrogen peroxide sensors based on carbon hybrid nanocomposites. J. Mater. Chem. C 2021, 9, 6970–6990. [Google Scholar] [CrossRef]
- Shamkhalichenar, H.; Choi, J.W. Review-Non-Enzymatic Hydrogen Peroxide Electrochemical Sensors Based on Reduced Graphene Oxide. J. Electrochem. Soc. 2020, 167, 037531. [Google Scholar] [CrossRef]
- Dhara, K.; Mahapatra, D.R. Electrochemical nonenzymatic sensing of glucose using advanced nanomaterials. Microchim. Acta 2018, 185, 49. [Google Scholar] [CrossRef]
- Zhang, J.X.; Bian, Y.X.; Liu, D.; Zhu, Z.W.; Shao, Y.H.; Li, M.X. Detection of Phosphate in Human Blood Based on a Catalytic Hydrogen Wave at a Molybdenum Phosphide Modified Electrode. Anal. Chem. 2019, 91, 14666–14671. [Google Scholar] [CrossRef]
- Sari, S.R.; Tsushida, M.; Sato, T.; Tominaga, M. Highly sensitive detection of phosphate using well-ordered crystalline cobalt oxide nanoparticles supported by multi-walled carbon nanotubes. Mater. Adv. 2022, 3, 2018–2025. [Google Scholar] [CrossRef]
- Zhu, D.; Zhen, Q.F.; Xin, J.J.; Ma, H.Y.; Tan, L.C.; Pang, H.J.; Wang, X.M. A free-standing and flexible phosphorus/nitrogen dual-doped three-dimensional reticular porous carbon frameworks encapsulated cobalt phosphide with superior performance for nitrite detection in drinking water and sausage samples. Sens. Actuators B Chem. 2020, 321, 128541. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Wang, X.M.; He, W.; Pathak, R.; Dong, X.X.; Jia, H.X.; Gu, Z.R. Communication—In Situ Electrodeposition of Nickel Phosphide on Ni Foam for Non-Enzymatic Detection of Nitrite. J. Electrochem. Soc. 2020, 167, 146517. [Google Scholar] [CrossRef]
- Nithyayini, K.N.; Harish, M.N.K.; Nagashree, K.L. Electrochemical detection of nitrite at NiFe2O4 nanoparticles synthesised by solvent deficient method. Electrochim. Acta 2019, 317, 701–710. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Hwang, S.K.; Lee, M.J.; Park, B.; Huh, Y.S.; Han, Y.K. Gold nanoparticle decorated patronite on rGO for the quantification of sulfadiazine at nanomolar levels in contaminated water. Chem. Eng. J. 2022, 439, 135782. [Google Scholar] [CrossRef]
- Vinoth, S.; Govindasamy, M.; Wang, S.F.; Alothman, A.A.; Alshgari, R.A. Hydrothermally synthesized cubical zinc manganite nanostructure for electrocatalytic detection of sulfadiazine. Microchim. Acta 2021, 188, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Ye, C.; Bao, S.J.; Zhang, Y.; Xu, M.W.; Lia, Z. Bimetal-organic-frameworks-derived yolk-shell-structured porous Co2P/ZnO@PC/CNTs hybrids for highly sensitive non-enzymatic detection of superoxide anion released from living cells. Chem. Commun. 2016, 52, 12442–12445. [Google Scholar] [CrossRef]
- Leong, K.L.; Ho, M.Y.; Lee, X.Y.; Yee, M.S.L. A Review on the Development of Non-Enzymatic Glucose Sensor Based on Graphene-Based Nanocomposites. Nano 2020, 15, 2030004. [Google Scholar] [CrossRef]
- Yan, Y.T.; Wang, P.C.; Lin, J.H.; Cao, J.; Qi, J.L. Modification strategies on transition metal-based electrocatalysts for efficient water splitting. J. Energy Chem. 2021, 58, 446–462. [Google Scholar] [CrossRef]
- Gan, T.; Wang, Z.K.; Gap, J.Y.; Sun, J.Y.; Wu, K.B.; Wang, H.B.; Liu, Y.M. Morphology-dependent electrochemical activity of Cu2O polyhedrons and construction of sensor for simultaneous determination of phenolic compounds with graphene oxide as reinforcement. Sens. Actuators B Chem. 2019, 282, 549–558. [Google Scholar] [CrossRef]
- Nagal, V.; Masrat, S.; Khan, M.; Alam, S.; Ahmad, A.; Alshammari, M.B.; Bhat, K.S.; Novikov, S.M.; Mishra, P.; Khosla, A.; et al. Highly Sensitive Electrochemical Non-Enzymatic Uric Acid Sensor Based on Cobalt Oxide Puffy Balls-like Nanostructure. Biosensors 2023, 13, 375. [Google Scholar] [CrossRef]
- Cui, S.Q.; Li, L.; Ding, Y.P.; Zhang, J.J. Mesoporous cobalto-cobaltic oxide modified glassy carbon electrode for simultaneous detection of hydroquinone and catechol. J. Electroanal. Chem. 2016, 782, 225–232. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, H.Q.; Huangfu, B.H.; Tian, W.L.; Sheng, L.Y.; Li, Z.J.; Tan, S.Q.; Li, Q.D. Bimetal nickel-cobalt phosphide directly grown on commercial graphite substrate by the one-step electrodeposition as efficient electrocatalytic electrode. Prog. Nat. Sci.-Mater. 2020, 30, 461–468. [Google Scholar] [CrossRef]
- Samanta, S.; Srivastava, R. CuCo2O4 based economical electrochemical sensor for the nanomolar detection of hydrazine and metol. J. Electroanal. Chem. 2016, 777, 48–57. [Google Scholar] [CrossRef]
- Liu, Q.; Kang, X.Z.; Xing, L.Z.; Ye, Z.X.; Yang, Y.C. A facile synthesis of nanostructured CoFe2O4 for the electrochemical sensing of bisphenol A. RSC Adv. 2020, 10, 6156–6162. [Google Scholar] [CrossRef]
- Chinnapaiyan, S.; Chen, T.W.; Chen, S.M.; Alothman, Z.A.; Ali, M.A.; Wabaidur, S.M.; Al-Hemaid, F.; Lee, S.Y.; Chang, W.H. Ultrasonic-assisted preparation and characterization of magnetic ZnFe2O4/g-C3N4 nanomaterial and their applications towards electrocatalytic reduction of 4-nitrophenol. Ultrason. Sonochem. 2020, 68, 105071. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, S.L.; Hu, H.H.; Li, H.; Jiang, Z.F. Hierarchically grown ZnFe2O4-decorated polyaniline-coupled-graphene nanosheets as a novel electrocatalyst for selective detecting p-nitrophenol. Microchem. J. 2021, 160, 105777. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Fayazi, M.; Nodehi, M.; Esmaeelnia, A. Electrochemical detection of bisphenol a on a MWCNTs/CuFe2O4 nanocomposite modified glassy carbon electrode. Mater. Chem. Phys. 2021, 261, 124247. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Wang, P.; Luo, Z.W.; Li, J.J.; Wang, Y.L.; Li, Z.; Chen, C.; Xie, Y.X.; Zhao, P.C.; Fei, J.J. Enhanced electrocatalytic activity of 2D ordered mesoporous nitrogen-rich carbon nanosheets functional NiFe2O4 nanospheres for ultrasensitive detection of chlorogenic acid in natural samples. Chem. Eng. J. 2023, 468, 143815. [Google Scholar] [CrossRef]
- Yin, A.P.; Wei, X.H.; Cao, Y.X.; Li, H.Q. High-quality molybdenum disulfide nanosheets with 3D structure for electrochemical sensing. Appl. Surf. Sci. 2016, 385, 63–71. [Google Scholar] [CrossRef]
- Kumar, J.V.; Karthik, R.; Chen, S.M.; Saravanakumar, K.; Govindasamy, M.; Muthuraj, V. Novel hydrothermal synthesis of MoS2 nanocluster structure for sensitive electrochemical detection of human and environmental hazardous pollutant 4-aminophenol. RSC Adv. 2016, 6, 40399–40407. [Google Scholar] [CrossRef]
- Mani, V.; Govindasamy, M.; Chen, S.M.; Karthik, R.; Huang, S.T. Determination of dopamine using a glassy carbon electrode modified with a graphene and carbon nanotube hybrid decorated with molybdenum disulfide flowers. Microchim. Acta 2016, 183, 2267–2275. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Li, J.; Liu, Y.M. Electrochemical sensing based on layered MoS2-graphene composites. Sens. Actuators B Chem. 2013, 178, 671–677. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, J.; Asiri, A.M.; Siddiquey, I.A.; Hasnat, M.A. Development of 4-methoxyphenol chemical sensor based on NiS2-CNT nanocomposites. J. Taiwan Inst. Chem. E 2016, 64, 157–165. [Google Scholar] [CrossRef]
- Vu, T.D.; Duy, P.K.; Bui, H.T.; Han, S.H.; Chung, H. Reduced graphene oxide-Nickel sulfide (NiS) composited on mechanical pencil lead as a versatile and cost-effective sensor for electrochemical measurements of bisphenol A and mercury (II). Sens. Actuators B Chem. 2019, 281, 320–325. [Google Scholar] [CrossRef]
- Annalakshmi, M.; Balamurugan, T.S.T.; Kumaravel, S.; Chen, S.M.; He, J.L. Facile hydrothermal synthesis of manganese sulfide nanoelectrocatalyst for high sensitive detection of Bisphenol A in food and eco-samples. Food Chem. 2022, 393, 133316. [Google Scholar] [CrossRef] [PubMed]
- Gokulkumar, K.; Priscillal, I.J.D.; Wang, S.F. Deep eutectic solvent-mediated synthesis of PDA coated f-CNF doped ZnS nanoparticles for electrode modification: Innovative sensing platform for determination of pollutant 3-nitrophenol. J. Alloys Compd. 2022, 924, 166561. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Liu, Y.J.; Gan, T.; Liu, Y.M.; Wang, L.L.; Fan, Y. Synthesis and electrochemical performances of layered tungsten sulfide-graphene nanocomposite as a sensing platform for catechol, resorcinol and hydroquinone. Electrochim. Acta 2013, 107, 379–387. [Google Scholar] [CrossRef]
- Xiong, L.W.; Qiu, Y.F.; Peng, X.; Liu, Z.T.; Chu, P.K. Electronic structural engineering of transition metal-based electrocatalysts for the hydrogen evolution reaction. Nano Energy 2022, 104, 107882. [Google Scholar] [CrossRef]
- Yuan, J.J.; Jiang, L.; Che, J.F.; He, G.Y.; Chen, H.Q. Composites of NiS2 Microblocks, MoS2 Nanosheets, and Reduced Graphene Oxide for Energy Storage and Electrochemical Detection of Bisphenol A. ACS Appl. Nano Mater. 2021, 4, 6093–6102. [Google Scholar] [CrossRef]
- Tomy, A.M.; Sathyan, B.; Cyriac, J. Ni(OH)(2)-MoS2 Nanocomposite Modified Glassy Carbon Electrode for the Detection of Dopamine and alpha-Lipoic Acid. J. Electrochem. Soc. 2023, 170, 047506. [Google Scholar] [CrossRef]
- Cheng, X.L.; Xia, X.; Xu, Q.Q.; Wang, J.; Sun, J.C.; Zhang, Y.X.; Li, S.S. Superior conductivity FeSe2 for highly sensitive electrochemical detection of p-nitrophenol and o-nitrophenol based on synergistic effect of adsorption and catalysis. Sens. Actuators B Chem. 2021, 348, 130692. [Google Scholar] [CrossRef]
- Singh, H.; Bernabe, J.; Chern, J.; Nath, M. Copper selenide as multifunctional non-enzymatic glucose and dopamine sensor. J. Mater. Res. 2021, 36, 1413–1424. [Google Scholar] [CrossRef]
- Yang, H.Y.; Driess, M.; Menezes, P.W. Self-Supported Electrocatalysts for Practical Water Electrolysis. Adv. Energy Mater. 2021, 11, 2102074. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhu, D.D.; Zheng, Y.; Vasileff, A.; Qiao, S.Z. Self-Supported Earth-Abundant Nanoarrays as Efficient and Robust Electrocatalysts for Energy-Related Reactions. ACS Catal. 2018, 8, 6707–6732. [Google Scholar] [CrossRef]
- Huang, K.J.; Zhang, J.Z.; Cai, J.L. Preparation of porous layered molybdenum selenide-graphene composites on Ni foam for high-performance supercapacitor and electrochemical sensing. Electrochim. Acta 2015, 180, 770–777. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.M.; Rui, R.; Wan, Z.G.; Chen, F.; Zuo, X.S.; Wang, C.; Wu, H. A Novel Electrochemical Sensor Based on the Synergistic Effect of Trace Platinum and Foliate Co0.85Se for the Determination of Dopamine. Int. J. Electrochem. Sci. 2019, 14, 4327–4337. [Google Scholar] [CrossRef]
- Zang, Y.J.; Nie, J.; He, B.; Yin, W.; Zheng, J.; Hou, C.J.; Huo, D.Q.; Yang, M.; Liu, F.M.; Sun, Q.Q.; et al. Fabrication of S-MoSe2/NSG/Au/MIPs imprinted composites for electrochemical detection of dopamine based on synergistic effect. Microchem. J. 2020, 156, 104845. [Google Scholar] [CrossRef]
- Wang, S.S.; Zhang, T.H.; Jia, L.P.; Yang, P.L.; He, P.; Xiao, F.; Zhou, P.C.; Wang, Y.; Wang, X.Y. Electrochemical reduction of nickel selenide/reduced graphene oxide nanocomposites: Highly sensitive detection of 4-nitrophenol. Microchem. J. 2023, 186, 108252. [Google Scholar] [CrossRef]
- Hwa, K.Y.; Ganguly, A.; Santhan, A.; Sharma, T.S.K. Vanadium selenide decorated reduced graphene oxide nanocomposite: A co-active catalyst for the detection of 2,4,6-Trichlorophenol. Chemosphere 2021, 282, 130874. [Google Scholar] [CrossRef]
- Ramaraj, S.; Sakthivel, M.; Chen, S.M.; Ho, K.C. Active-Site-Rich 1T-Phase CoMoSe2 Integrated Graphene Oxide Nanocomposite as an Efficient Electrocatalyst for Electrochemical Sensor and Energy Storage Applications. Anal. Chem. 2019, 91, 8358–8365. [Google Scholar] [CrossRef]
- Ramaraj, S.; Sakthivel, M.; Chen, S.M.; Elshikh, M.S.; Chen, T.W.; Yu, M.C.; Ho, K.C. Electrochemical sensing of anti-inflammatory agent in paramedical sample based on FeMoSe2 modified SPCE: Comparison of various preparation methods and morphological effects. Anal. Chim. Acta 2019, 1083, 88–100. [Google Scholar] [CrossRef]
- Sakthivel, M.; Ramaraj, S.; Chen, S.M.; Dinesh, B.; Ramasamy, H.V.; Lee, Y.S. Entrapment of bimetallic CoFeSe2 nanosphere on functionalized carbon nanofiber for selective and sensitive electrochemical detection of caffeic acid in wine samples. Anal. Chim. Acta 2018, 1006, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.H.; Li, S.; Lei, S.; Qiao, J.T.; Zou, L.N.; Ye, B.X. Highly sensitive determination of bisphenol A based on MoCuSe nanoparticles decorated reduced graphene oxide modified electrode. J. Electroanal. Chem. 2018, 827, 137–144. [Google Scholar] [CrossRef]
- Luo, J.L.; Jiang, C.; Zhao, J.H.; Zhao, L.Y.; Zheng, P.Z.; Fang, J. Hierarchical tungsten-doped bimetallic selenides nanosheets arrays/nickel foam composite electrode as efficient gallic acid electrochemical sensor. Microchim. Acta 2023, 190, 165. [Google Scholar] [CrossRef]
- Pradhan, S.; Pramanik, S.; Das, D.K.; Bhar, R.; Bandyopadhyay, R.; Millner, P.; Pramanik, P. Nanosized iron telluride for simultaneous nanomolar voltammetric determination of dopamine, uric acid, guanine and adenine. New J. Chem. 2019, 43, 10590–10600. [Google Scholar] [CrossRef]
- Pradhan, S.; Das, R.; Biswas, S.; Das, D.K.; Bhar, R.; Bandyopadhyay, R.; Pramanik, P. Chemical synthesis of nanoparticles of nickel telluride and cobalt telluride and its electrochemical applications for determination of uric acid and adenine. Electrochim. Acta 2017, 238, 185–193. [Google Scholar] [CrossRef]
- Padmanaban, A.; Padmanathan, N.; Dhanasekaran, T.; Manigandan, R.; Srinandhini, S.; Sivaprakash, P.; Arumugam, S.; Narayanan, V. Hexagonal phase Pt-doped cobalt telluride magnetic semiconductor nanoflakes for electrochemical sensing of dopamine. J. Electroanal. Chem. 2020, 877, 114658. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Asadpour-Zeynali, K.; Ozkan, S.A. Preparation of porous Cu metal organic framework/ZnTe nanorods/Au nanoparticles hybrid platform for nonenzymatic determination of catechol. J. Electroanal. Chem. 2020, 856, 113672. [Google Scholar] [CrossRef]
- Ulbrich, K.D.; Winiarski, J.P.; Jost, C.L.; de Campos, C.E.M. Green and facile solvent-free synthesis of NiTe2 nanocrystalline material applied to voltammetric determination of antioxidant morin. Mater. Today Commun. 2020, 25, 101251. [Google Scholar] [CrossRef]
- Ulbrich, K.D.; Winiarski, J.P.; Jost, C.L.; de Campos, C.E.M. Mechanochemical synthesis of a Ni3-xTe2 nanocrystalline composite and its application for simultaneous electrochemical detection of dopamine and adrenaline. Compos. Part B Eng. 2020, 183, 107649. [Google Scholar] [CrossRef]
- Malagutti, M.A.; Ulbrich, K.D.; Winiarski, J.P.; Paes, V.Z.C.; Geshev, J.; Jost, C.L.; de Campos, C.E.M. Mechanochemical synthesis of gamma-CoTe2 nanocrystals and their application for determination of ferulic acid. Mater. Today Commun. 2022, 31, 103481. [Google Scholar] [CrossRef]
- Xiao, L.L.; Xu, R.Y.; Wang, F. Facile synthesis of CoxP decorated porous carbon microspheres for ultrasensitive detection of 4-nitrophenol. Talanta 2018, 179, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.F.; Cui, M.L.; Zhao, Y.; Wang, C.; Song, Q.J. Preparation of Nitrogen and FeP Doped Carbon Nanotubes for Selective and Simultaneous Electrochemical Detection of Dihydroxybenzoic Acid Isomers. Electrochim. Acta 2017, 242, 107–116. [Google Scholar] [CrossRef]
- Wang, K.D.; Wu, C.; Wang, F.; Jiang, G.Q. MOF-Derived CoPx Nanoparticles Embedded in Nitrogen-Doped Porous Carbon Polyhedrons for Nanomolar Sensing of p-Nitrophenol. ACS Appl. Nano Mater. 2018, 1, 5843–5853. [Google Scholar] [CrossRef]
- Liu, X.B.; He, F.Y.; Bai, L.W.; Cao, X.W.; Liu, C.; Lu, W.B. A two-dimensional G-CoP/N,P-co-doped carbon nanowire electrode for the simultaneous determination of hydroquinone and catechol in domestic wastewater. Anal. Chim. Acta 2022, 1210, 339871. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Lu, W.B.; Liu, G.Q.; Jiang, Y.M.; Liu, X.B.; Bai, L.W.; Cao, X.W.; Jia, J.F.; Wu, H.S. Ni2P Nanosheets: A High Catalytic Activity Platform for Electrochemical Detection of Acetaminophen. Chin. J. Chem. 2021, 39, 1849–1854. [Google Scholar] [CrossRef]
- Karuppusamy, N.; Mariyappan, V.; Chen, S.M.; Thangamuthu, M.D.; Li, R.H. Unveiling electrocatalytic performance of MnCo-P on sulfur-doped reduced graphene oxide for electrochemical detection of acetaminophen. Surf. Interfaces 2023, 37, 102681. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Kang, K.; Jia, J.; Wang, S.; Wang, J. Insights into the enhanced electrochemical sensing behavior of hydroquinone and catechol simultaneously enabled by ultrafine layer CoP-NiCoP heterostructure on graphene frameworks. Nanoscale 2023, 15, 9823–9834. [Google Scholar] [CrossRef]
- Zhang, X.M.; Li, Y.K.; Ge, W.Y. Porous NiCoP Nanowire Arrays on the Surface of Ti3C2Tx-Modified Carbon Cloth for Sensitive Determination of Dopamine. ACS Appl. Nano Mater. 2023, 6, 2035–2047. [Google Scholar] [CrossRef]
- Thakur, N.; Chaturvedi, A.; Mandal, D.; Nagaiah, T.C. Ultrasensitive and highly selective detection of dopamine by a NiFeP based flexible electrochemical sensor. Chem. Commun. 2020, 56, 8448–8451. [Google Scholar] [CrossRef]
- Wei, M.; Lu, W.B.; Zhu, M.; Zhang, R.; Hu, W.L.; Cao, X.W.; Jia, J.F.; Wu, H.S. Highly sensitive and selective dopamine sensor uses three-dimensional cobalt phosphide nanowire array. J. Mater. Sci. 2021, 56, 6401–6410. [Google Scholar] [CrossRef]
- Tao, Y.; Kong, Q.; Tao, Z.M.; Duan, J.X.; Guan, H.T.; Chen, G.; Dong, C.J. A nickel foam modified with electrodeposited cobalt and phosphor for amperometric determination of dopamine. Microchim. Acta 2019, 186, 602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.S.; Lin, T.; Xu, Y.; Zhang, W.G.; Asif, M.; Sun, Y.M.; Xiao, F. Integrated electrochemical microfluidic sensor with hierarchically porous nanoarrays modified graphene fiber microelectrode for bioassay. Biosens. Bioelectron. 2022, 205, 114095. [Google Scholar] [CrossRef] [PubMed]
- Rezapasand, S.; Abbasi, S.; Rahmati, Z.; Hosseini, H.; Roushani, M. Metal-organic frameworks-derived Zn-Ni-P nanostructures as high performance electrode materials for electrochemical sensing. J. Electroanal. Chem. 2022, 918, 116441. [Google Scholar] [CrossRef]
- Farokhi, S.; Roushani, M.; Hosseini, H. Advanced core-shell nanostructures based on porous NiCo-P nanodiscs shelled with NiCo-LDH nanosheets as a high-performance electrochemical sensing platform. Electrochim. Acta 2020, 362, 137218. [Google Scholar] [CrossRef]
- Wu, X.X.; Lao, C.F.; Li, Y.C.; Yuan, Q.H.; Gan, W. Tunable Synthesis of CoP and CoP2 Decorated 3D Carbon Nanohybrids and the Application of CoP2 Decorated One in Electrochemical Detection of Chloramphenicol in Milk and Honey. J. Electrochem. Soc. 2018, 165, B916–B923. [Google Scholar] [CrossRef]
- Amorim, I.; Yu, Z.; Bento, F.; Liu, L. Towards an Improved Electrocatalytic Material for Detection of Polyphenols Based on Transition Metal Phosphides Anchored on Reduced Graphene Oxide. J. Electrochem. Soc. 2023, 170, 027506. [Google Scholar] [CrossRef]
- Amorim, I.; Yu, Z.; Liu, L.; Bento, F. Cobalt-nickel phosphide supported on reduced graphene oxide for sensitive electrochemical detection of bisphenol A. Heliyon 2024, 10, e24070. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim, I.; Bento, F. Electrochemical Sensors Based on Transition Metal Materials for Phenolic Compound Detection. Sensors 2024, 24, 756. https://doi.org/10.3390/s24030756
Amorim I, Bento F. Electrochemical Sensors Based on Transition Metal Materials for Phenolic Compound Detection. Sensors. 2024; 24(3):756. https://doi.org/10.3390/s24030756
Chicago/Turabian StyleAmorim, Isilda, and Fátima Bento. 2024. "Electrochemical Sensors Based on Transition Metal Materials for Phenolic Compound Detection" Sensors 24, no. 3: 756. https://doi.org/10.3390/s24030756
APA StyleAmorim, I., & Bento, F. (2024). Electrochemical Sensors Based on Transition Metal Materials for Phenolic Compound Detection. Sensors, 24(3), 756. https://doi.org/10.3390/s24030756






