Exploring Novel Sensor Design Ideas through Concentration-Induced Conformational Changes in PEG Single Chains
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
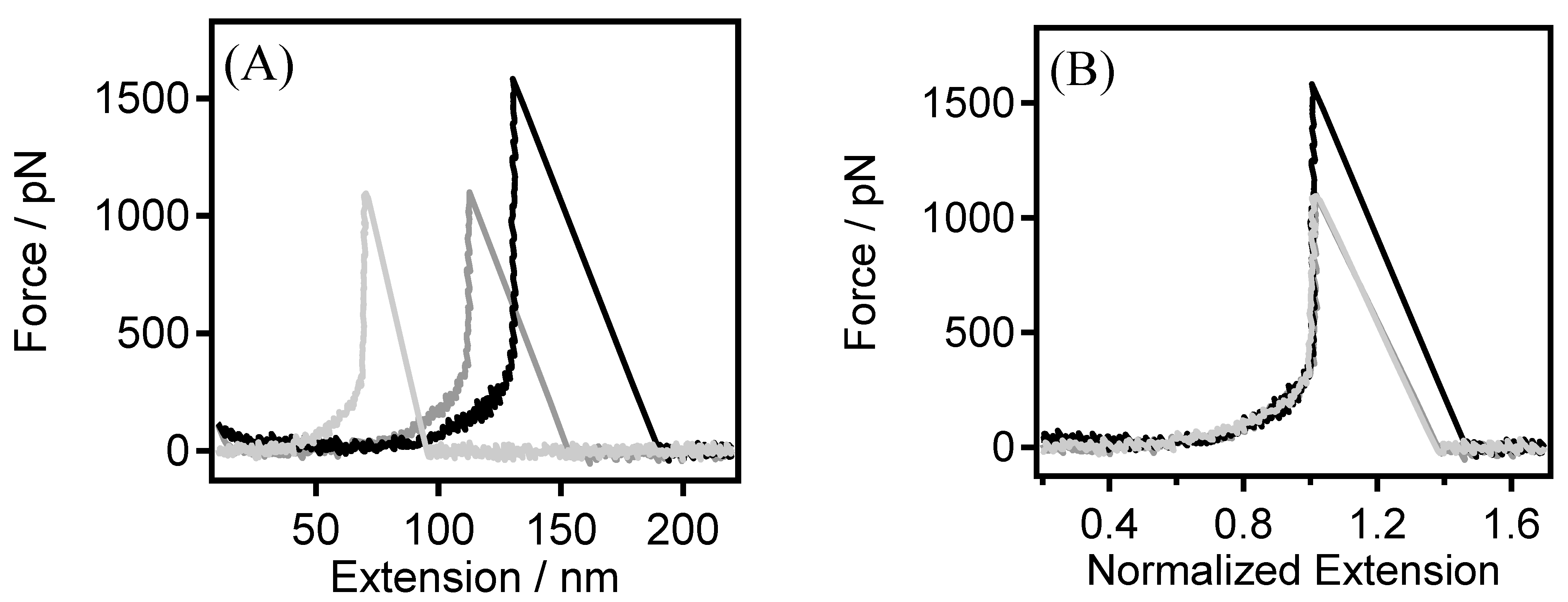
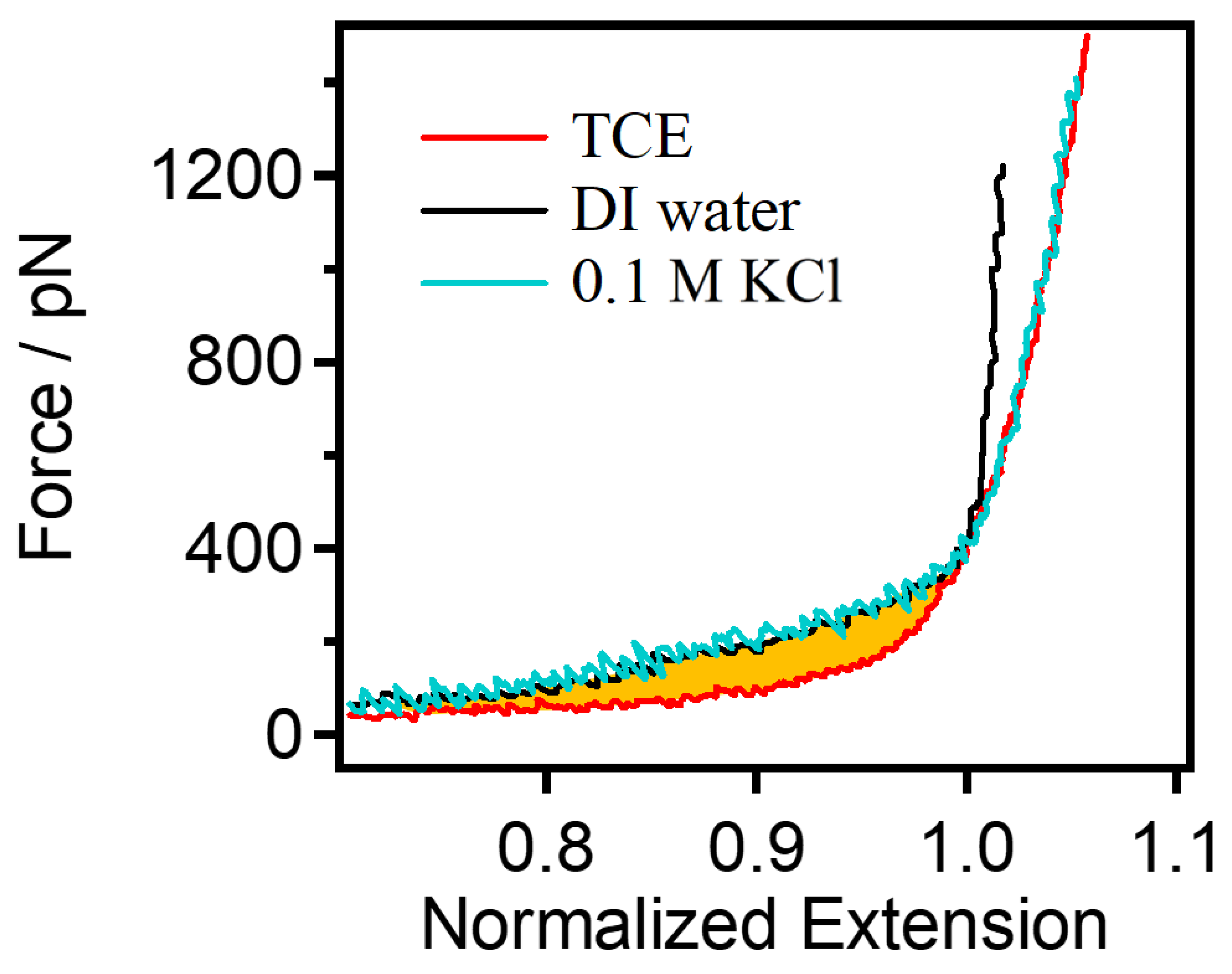
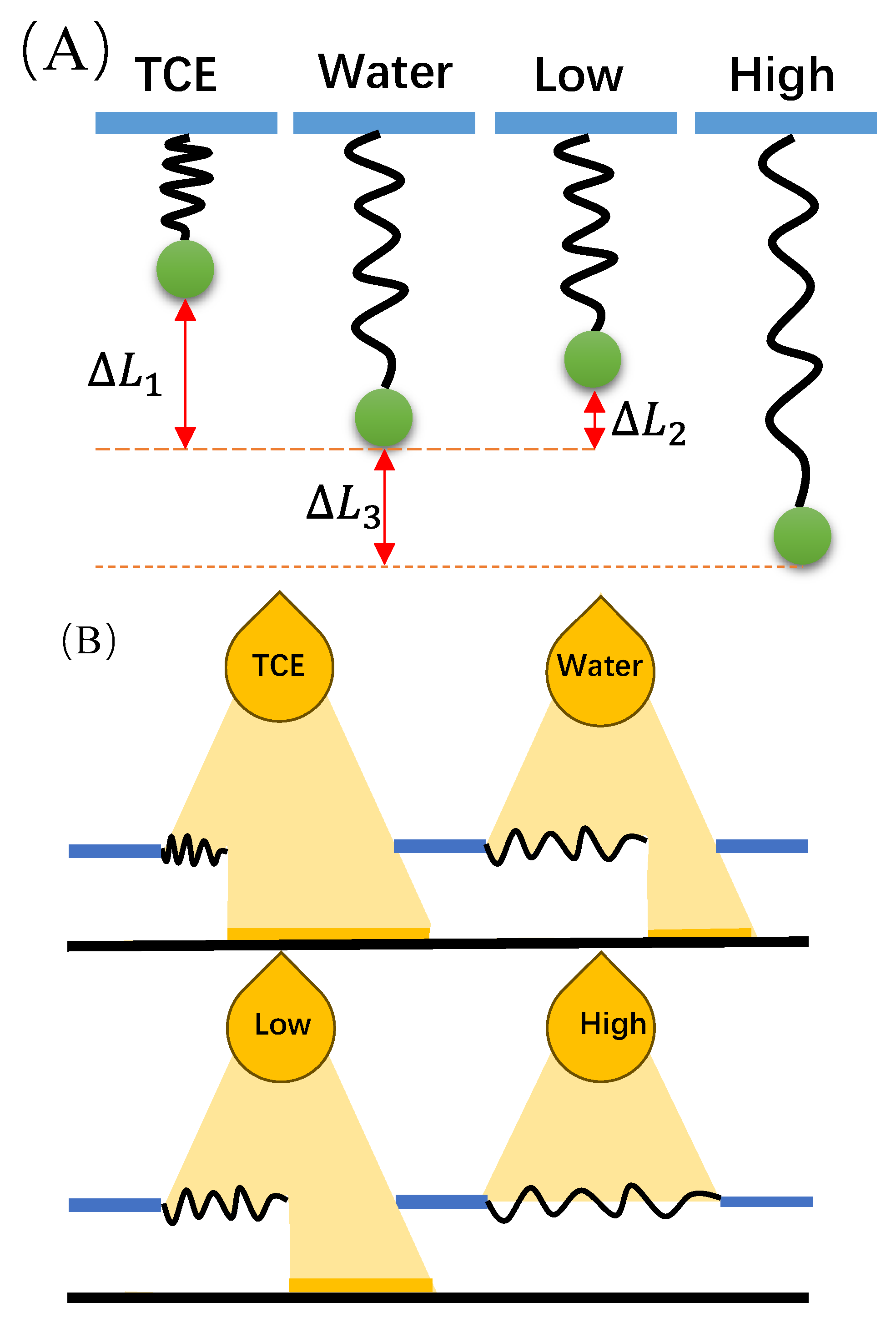
3.1. The Single-Chain Elasticity of PEG in Water
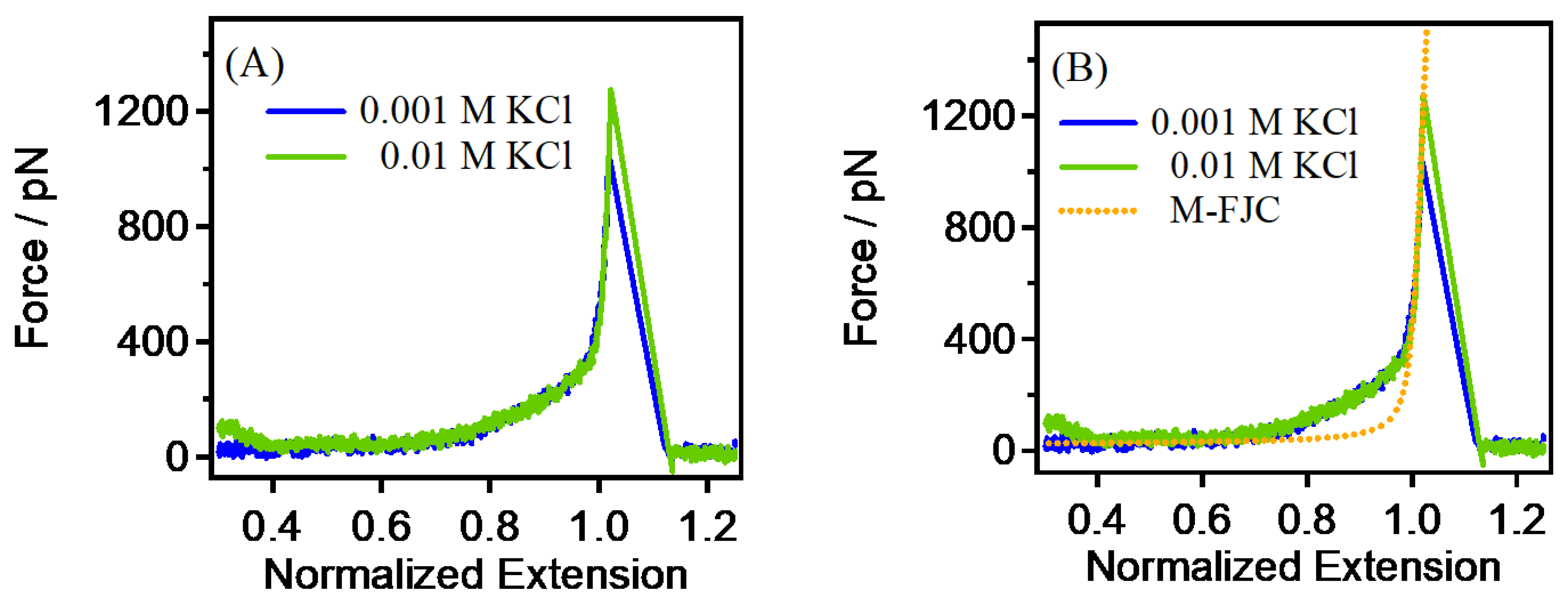
3.2. The Single-Chain Elasticity of PEG at Low Concentrations of KCl Solution
3.3. The Single-Chain Elasticity of PEG at High Concentrations of KCl Solution
3.4. Novel Sensor-Design Ideas
- (1)
- Ion-Concentration-Modulated Sensor: Exploiting the lower rigidity of PEG single chains at low ion concentrations and their higher rigidity at high ion concentrations, a sensor is designed to modulate ion concentration. The sensor can dynamically monitor changes in PEG single-chain rigidity, providing real-time insights into ion concentration, with quantitative information based on different rigidity levels.
- (2)
- Threshold-Type Ion-Concentration Sensor: Leveraging the sharp increase in PEG single-chain rigidity when the ion concentration surpasses a certain threshold, a threshold-type ion-concentration sensor is proposed. This sensor exhibits a rapid rigidity change when detecting ion concentrations exceeding a specific critical level, offering a clear signal indicating the presence of ions beyond a critical threshold.
- (3)
- Ion-Concentration Gradient Sensor: Utilizing the gradient of PEG single-chain rigidity, a sensor is designed to capture ion concentration gradients. By measuring the rigidity of PEG single chains at various ion concentrations, the sensor generates a gradient map, providing detailed and comprehensive ion-concentration information.
- (4)
- Ion-Concentration Trend-Monitoring Sensor: Exploiting the sensitivity of PEG single-chain rigidity to trends in ion-concentration changes, a sensor is designed to monitor ion-concentration trends. The sensor can continuously monitor and record changes in PEG single chain rigidity, offering insights into trends in ion-concentration fluctuations.
- (5)
- Controllable Drug-Release Sensor: Integrating PEG single chains with drugs, a sensor is designed for controllable drug release based on the modulation of PEG single-chain rigidity. Changes in the ion concentration can influence PEG single-chain rigidity, thereby adjusting the rate of drug release.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, S.J.; Han, Y.L.; Hwang, S.H.; Kang, S.J.; Lee, J.M.; Bae, B.S. Integrated Water-Level Sensor Using Thin-Film Transistor Technology. Acs Omega 2023, 8, 36868–36875. [Google Scholar] [CrossRef] [PubMed]
- Erçarikci, E.; Dagci Kiransan, K.; Topçu, E. A Flexible Graphene Paper Electrochemical Sensor with Electrodeposited Ag and Ni Nanoparticles for H2O2 Detection. IEEE Sens. J. 2023, 23, 7087–7094. [Google Scholar] [CrossRef]
- Ko, Y.; Jeong, H.Y.; Kwon, G.; Kim, D.; Lee, C.; You, J. pH-responsive polyaniline/polyethylene glycol composite arrays for colorimetric sensor application. Sens. Actuators B Chem. 2020, 305, 127447. [Google Scholar] [CrossRef]
- Cao, Y.; Weng, M.M.; Mahmoud, M.H.H.; Elnaggar, A.Y.; Zhang, L.; El Azab, I.H.; Chen, Y.; Huang, M.N.; Huang, J.T.; Sheng, X.X. Flame-retardant and leakage-proof phase change composites based on MXene/polyimide aerogels toward solar thermal energy harvesting. Adv. Compos. Hybrid Mater. 2022, 5, 1253–1267. [Google Scholar] [CrossRef]
- Redij, R.; Kaur, A.; Muddaloor, P.; Sethi, A.K.; Aedma, K.; Rajagopal, A.; Gopalakrishnan, K.; Yadav, A.; Damani, D.N.; Chedid, V.G.; et al. Practicing Digital Gastroenterology through Phonoenterography Leveraging Artificial Intelligence: Future Perspectives Using Microwave Systems. Sensors 2023, 23, 2302. [Google Scholar] [CrossRef] [PubMed]
- Goor, O.; Brouns, J.E.P.; Dankers, P.Y.W. Introduction of anti-fouling coatings at the surface of supramolecular elastomeric materials via post-modification of reactive supramolecular additives. Polym. Chem. 2017, 8, 5228–5238. [Google Scholar] [CrossRef]
- Lilly, J.L.; Romero, G.; Xu, W.J.; Shin, H.Y.; Berron, B.J. Characterization of Molecular Transport in Ultrathin Hydrogel Coatings for Cellular Immunoprotection. Biomacromolecules 2015, 16, 541–549. [Google Scholar] [CrossRef]
- Liu, S.; Tian, X.Y.; Zhang, X.S.; Xu, C.Z.; Wang, L.L.; Xia, Y.Z. A green MXene-based organohydrogel with tunable mechanics and freezing tolerance for wearable strain sensors. Chin. Chem. Lett. 2022, 33, 2205–2211. [Google Scholar] [CrossRef]
- Seifert, A.; Göpfrich, K.; Burns, J.R.; Fertig, N.; Keyser, U.F.; Howorka, S. Bilayer-Spanning DNA Nanopores with Voltage-Switching between Open and Closed State. Acs Nano 2015, 9, 1117–1126. [Google Scholar] [CrossRef]
- Zhen, G.L.; Falconnet, D.; Kuennemann, E.; Vörös, J.; Spencer, N.D.; Textor, M.; Zürcher, S. Nitrilotriacetic acid functionalized graft copolymers:: A polymeric interface for selective and reversible binding of histidine-tagged proteins. Adv. Funct. Mater. 2006, 16, 243–251. [Google Scholar] [CrossRef]
- Sharma, S.; Johnson, R.W.; Desai, T.A. XPS and AFM analysis of antifouling PEG interfaces for microfabricated silicon biosensors. Biosens. Bioelectron. 2004, 20, 227–239. [Google Scholar] [CrossRef]
- Vu, C.A.; Su, Y.T.; Wang, J.S.; Chang, C.Y.; Hu, W.P.; Huang, C.J.; Chan, H.W.H.; Chen, W.Y. Comparing surface modification methods for silicon nanowire field-effect transistor biosensors for diagnosis applications: A case study of cardiac troponin I. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132146. [Google Scholar] [CrossRef]
- Tehrani, A.G.; Makki, H.; Anbaran, S.R.G.; Vakili, H.; Ghermezcheshme, H.; Zandi, N. Superior anti-biofouling properties of mPEG-modified polyurethane networks via incorporation of a hydrophobic dangling chain. Prog. Org. Coat. 2021, 158, 106358. [Google Scholar] [CrossRef]
- Wawro, A.M.; Muraoka, T.; Kinbara, K. Chromatography-free synthesis of monodisperse oligo(ethylene glycol) mono-p-toluenesulfonates and quantitative analysis of oligomer purity. Polym. Chem. 2016, 7, 2389–2394. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, K.; Kim, S.; Park, H.H.; Shin, H.; Joo, J. Tailored polyethylene glycol grafting on porous nanoparticles for enhanced targeting and intracellular siRNA delivery. Nanoscale 2022, 14, 14482–14490. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Hui, N. Electrochemical functionalization of polypyrrole nanowires for the development of ultrasensitive biosensors for detecting microRNA. Sens. Actuators B Chem. 2019, 281, 478–485. [Google Scholar] [CrossRef]
- Gaikwad, H.; Li, Y.; Gifford, G.; Groman, E.; Banda, N.K.; Saba, L.; Scheinman, R.; Wang, G.K.; Simberg, D. Complement Inhibitors Block Complement C3 Opsonization and Improve Targeting Selectivity of Nanoparticles in Blood. Bioconjug. Chem. 2020, 31, 1844–1856. [Google Scholar] [CrossRef] [PubMed]
- Shahvandi, S.K.; Ahmar, H.; Rezaei, S.J.T. Palladium nanoparticles immobilized on polymer-functionalized magnetic nanoparticles for the determination of hydrogen peroxide. Surf. Interfaces 2018, 12, 71–77. [Google Scholar] [CrossRef]
- Qian, W.H.; Song, T.; Ye, M.; Huang, X.Y.; Li, Y.J.; Hao, B.J. Functionalized nanographene oxide/PEG/rhodamine B/gold nanocomposite for electrochemical determination of glucose. J. Mater. Sci. Technol. 2022, 122, 141–147. [Google Scholar] [CrossRef]
- O’Neal, D.P.; Meledeo, M.A.; Davis, J.R.; Ibey, B.L.; Gant, V.A.; Pishko, M.V.; Coté, G.L. Oxygen sensor based on the fluorescence quenching of a ruthenium complex immobilized in a biocompatible poly(ethylene glycol) hydrogel. IEEE Sens. J. 2004, 4, 728–734. [Google Scholar] [CrossRef]
- Popat, K.C.; Mor, G.; Grimes, C.A.; Desai, T.A. Surface modification of nanoporous alumina surfaces with poly(ethylene glycol). Langmuir 2004, 20, 8035–8041. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.; Boden, A.; Al Kobaisi, M.; Pingle, H.; Wang, P.Y.; Kingshott, P. The influence of PEG-thiol derivatives on controlling cellular and bacterial interactions with gold surfaces. Appl. Surf. Sci. 2018, 462, 980–990. [Google Scholar] [CrossRef]
- Huang, M.J.; Zhu, M.L.; Feng, X.W.; Zhang, Z.X.; Tang, T.Y.; Guo, X.E.; Chen, T.; Liu, H.C.; Sun, L.N.; Lee, C.K. Intelligent Cubic-Designed Piezoelectric Node (iCUPE) with Simultaneous Sensing and Energy Harvesting Ability toward Self- Sustained Artificial Intelligence of Things (AIoT). Acs Nano 2023, 17, 6435–6451. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Zhu, D.D.; Jia, H.Y.; Yang, C.C.; Zheng, Z.; Wang, X.L. Bio-based visual optical pressure-responsive sensor. Carbohydr. Polym. 2021, 260, 117823. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.J.; Blondel, J.A.K.; Lu, J.R.; Thomas, R.K.; Wang, Y.L.; Han, B.X.; Yan, H.K.; Penfold, J. Interaction between poly(ethylene oxide) and monovalent dodecyl sulfates studied by neutron reflection. Langmuir 1998, 14, 1990–1995. [Google Scholar] [CrossRef]
- Ren, C.L.; Tian, W.D.; Szleifer, I.; Ma, Y.Q. Specific Salt Effects on Poly(ethylene oxide) Electrolyte Solutions. Macromolecules 2011, 44, 1719–1727. [Google Scholar] [CrossRef]
- Buzzi, O.; Yuan, S.Y.; Routley, B. Development and Validation of a New Near-Infrared Sensor to Measure Polyethylene Glycol (PEG) Concentration in Water. Sensors 2017, 17, 1354. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Otsuka, H.; Kaneko, M.; Kataoka, K.; Nagasaki, Y. A reactive poly(ethylene glycol) layer to achieve specific surface plasmon resonance sensing with a high S/N ratio: The substantial role of a short underbrushed PEG layer in minimizing nonspecific adsorption. Anal. Chem. 2005, 77, 1075–1080. [Google Scholar] [CrossRef]
- Wu, B.; Xu, L.; Wang, S.F.; Wang, Y.; Zhang, W.A. A PEGylated colorimetric and turn-on fluorescent sensor based on BODIPY for Hg(II) detection in water. Polym. Chem. 2015, 6, 4279–4289. [Google Scholar] [CrossRef]
- Han, D.; Liu, L.L.; Liu, Z.H.; Li, D.H.; Chen, Y.; Duan, Q.; Wang, Y.; Sang, S.B. Polyethyleneimine/polyethylene-glycol/Ti3C2Tx composites for ultrasensitive room-temperature CO2 sensing. Sens. Actuators B Chem. 2023, 393, 134221. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, Y.F.; Li, X.F.; Zhao, C.J. A Novel Polymer-Salt Complex Based on LiCl Doped SPEEK/Poly(Ether Ether Ketone)-Co-Poly(Ethylene Glycol) for Humidity Sensors. IEEE Sens. J. 2021, 21, 8886–8895. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liu, Y.Q.; Zou, F.; Jiang, C.; Mou, C.B.; Wang, T.Y. Humidity Sensor Based on a Long-Period Fiber Grating Coated with Polymer Composite Film. Sensors 2019, 19, 2263. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Shi, L.Q.; Zhang, W.Q.; An, Y.L.; Zhu, X.X. Adjustable temperature sensor with double thermoresponsiveness based on the aggregation property of binary diblock copolymers. J. Appl. Polym. Sci. 2006, 102, 3144–3148. [Google Scholar] [CrossRef]
- Baba, M.; Nemoto, K.; Onuki, C.; Yamazawa, T.; Wakakuwa, S.; Tanaka, H.; Sekino, T.; Nakayama, T.; Yamada, N.; Takeda, M. Demonstration of pyroelectric generation for self-powered wireless sensor nodes. Sens. Actuators A Phys. 2023, 352, 114199. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, K.; Guo, X.; Qian, L. Effects of the Degree of Deacetylation on the Single-Molecule Mechanics of Chitosans. J. Phys. Chem. B 2023, 127, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Guo, X.; Zhang, K.; Yu, M. Effects of hydrogen bonds on the single-chain mechanics of chitin. Phys. Chem. Chem. Phys. 2022, 24, 24535–24541. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.C.B.; Min, D.Y. Single-Molecule Force Spectroscopy of Membrane Protein Folding. J. Mol. Biol. 2023, 435, 167975. [Google Scholar] [CrossRef]
- Xia, J.H.; Zuo, J.C.; Li, H.B. Molecular homogeneity of GB1 revealed by single molecule force spectroscopy. Nanoscale 2022, 14, 9925–9931. [Google Scholar] [CrossRef]
- Yu, M.; Kang, X.M.; Qian, L. Interactions between monovalent cations and polyethylene glycol: A study at micro level. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132731. [Google Scholar] [CrossRef]
- Cao, N.P.; Zhao, Y.H.; Chen, H.B.; Huang, J.Y.; Yu, M.; Bao, Y.; Wang, D.P.; Cui, S.X. Poly(ethylene glycol) Becomes a Supra-Polyelectrolyte by Capturing Hydronium Ions in Water. Macromolecules 2022, 55, 4656–4664. [Google Scholar] [CrossRef]
- Oesterhelt, F.; Rief, M.; Gaub, H.E. Single molecule force spectroscopy by AFM indicates helical structure of poly(ethylene-glycol) in water. New J. Phys. 1999, 1, 1367–2630. [Google Scholar] [CrossRef]
- Smith, S.B.; Cui, Y.J.; Bustamante, C. Overstretching B-DNA: The elastic response of individual double-stranded and single-stranded DNA molecules. Science 1996, 271, 795–799. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Jiang, C.; Lai, B.; Zhang, K. Exploring Novel Sensor Design Ideas through Concentration-Induced Conformational Changes in PEG Single Chains. Sensors 2024, 24, 883. https://doi.org/10.3390/s24030883
Yu M, Jiang C, Lai B, Zhang K. Exploring Novel Sensor Design Ideas through Concentration-Induced Conformational Changes in PEG Single Chains. Sensors. 2024; 24(3):883. https://doi.org/10.3390/s24030883
Chicago/Turabian StyleYu, Miao, Chong Jiang, Bing Lai, and Kai Zhang. 2024. "Exploring Novel Sensor Design Ideas through Concentration-Induced Conformational Changes in PEG Single Chains" Sensors 24, no. 3: 883. https://doi.org/10.3390/s24030883




