Optical Myography-Based Sensing Methodology of Application of Random Loads to Muscles during Hand-Gripping Training
Abstract
:1. Introduction
2. Related Works
3. Objective
4. Materials and Methods
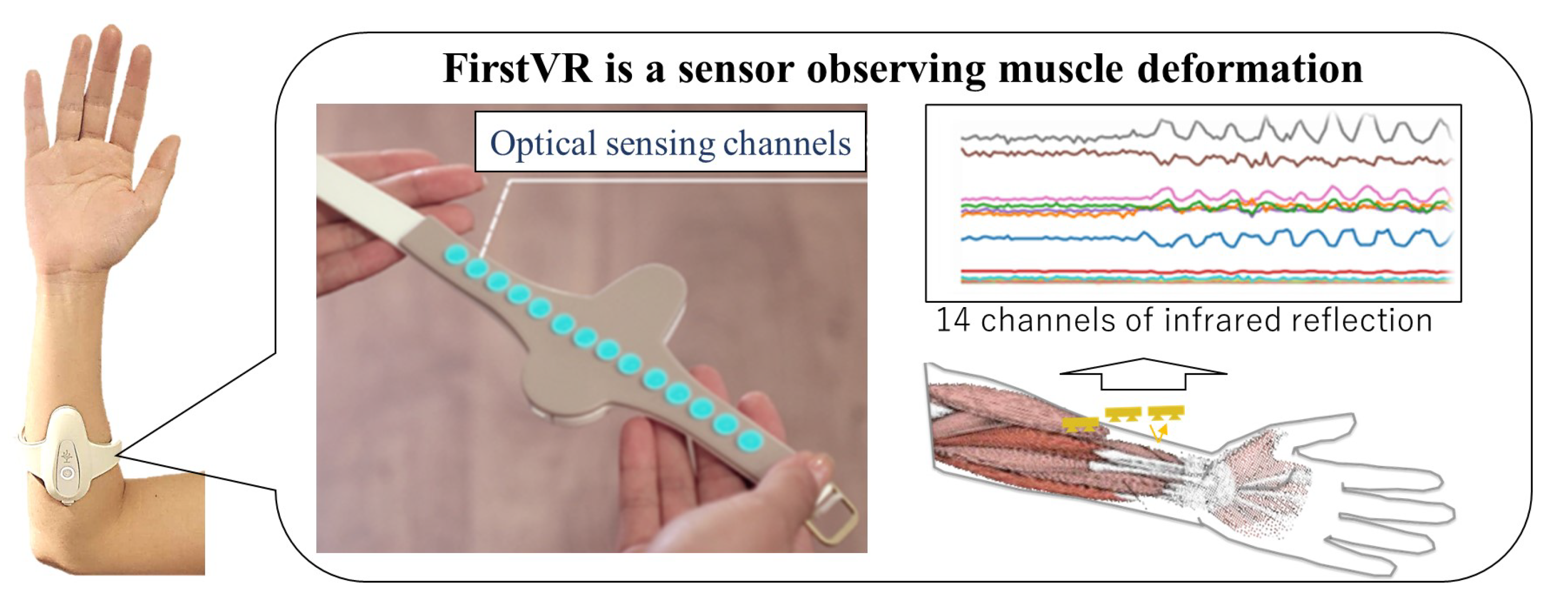
4.1. System
4.2. Experimental Procedures
- Gripper: grip and release a 25 kg hand-gripper approximately 10 times for 10 s.
- Ball: hold a tennis ball and keep exerting gripping force for 10 s.
- Palm clenching (hand): keep clenching the hand in front of the chest for 10 s.
- Balloon: hold a paper balloon in front of the chest for 10 s while applying force to the arms so as not to crush the balloon.
- Paper exercise: Crumple pieces of newspaper for 10 s. The number of paper layers was two.
4.3. Data Analysis
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cronin, J.; Lawton, T.; Harris, N.; Kilding, A.; McMaster, D.T. A brief review of handgrip strength and sport performance. J. Strength Cond. Res. 2017, 31, 3187–3217. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. Evolution of the human hand: The role of throwing and clubbing. J. Anat. 2003, 202, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Bonitch Góngora, J.G.; Almeida, F.; Padial Puche, P.; Bonitch-Domínguez, J.G.; Feriche Fernández-Castanys, M.B. Maximal isometric handgrip strength and endurance differences between elite and non-elite young judo athletes. Arch. Budo 2013, 9, 239–248. [Google Scholar]
- Peterson, B.J.; Fitzgerald, J.S.; Dietz, C.C.; Ziegler, K.S.; Ingraham, S.J.; Baker, S.E.; Snyder, E.M. Division I hockey players generate more power than division III players during on-and off-ice performance tests. J. Strength Cond. Res. 2015, 29, 1191–1196. [Google Scholar] [CrossRef]
- Marsh, D.W.; Richard, L.A.; Verre, A.B.; Myers, J. Relationships among balance, visual search, and lacrosse-shot accuracy. J. Strength Cond. Res. 2010, 24, 1507–1514. [Google Scholar] [CrossRef]
- Moritz, E.F.; Haake, S.; Schmidt, E.; Roberts, J.; Rothberg, S. Time-resolved measurements of grip force during a golf shot. In The Engineering of Sport 6: Volume 2: Developments for Disciplines; Springer: New York, NY, USA, 2006; pp. 57–62. [Google Scholar]
- Ambike, S.; Paclet, F.; Zatsiorsky, V.M.; Latash, M.L. Factors affecting grip force: Anatomy, mechanics, and referent configurations. Exp. Brain Res. 2014, 232, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Malhotra, V.; Kumar, A.; Dhar, U.; Tripathi, Y. Effect of isometric handgrip exercise training on resting blood pressure in normal healthy adults. J. Clin. Diagn. Res. JCDR 2014, 8, BC08. [Google Scholar] [CrossRef]
- Garcia-Hernandez, N.; Garza-Martinez, K.; Parra-Vega, V.; Alvarez-Sanchez, A.; Conchas-Arteaga, L. Development of an EMG-based exergaming system for isometric muscle training and its effectiveness to enhance motivation, performance and muscle strength. Int. J. Hum. Comput. Stud. 2019, 124, 44–55. [Google Scholar] [CrossRef]
- Shimose, R.; Matsunaga, A.; Muro, M. Effect of submaximal isometric wrist extension training on grip strength. Eur. J. Appl. Physiol. 2011, 111, 557–565. [Google Scholar] [CrossRef]
- Yamauchi, J.; Hargens, A. Effects of dynamic and static handgrip exercises on hand and wrist volume. Eur. J. Appl. Physiol. 2008, 103, 41–45. [Google Scholar] [CrossRef]
- Bernstein, N.A. Dexterity and Its Development, 1st ed.; Latash, M.L., Turvey, M.T., Eds.; Psychology Press: London, UK, 1996. [Google Scholar]
- Watson, A.H. What can studying musicians tell us about motor control of the hand? J. Anat. 2006, 208, 527–542. [Google Scholar] [CrossRef]
- Kimoto, Y.; Oku, T.; Furuya, S. Neuromuscular and biomechanical functions subserving finger dexterity in musicians. Sci. Rep. 2019, 9, 12224. [Google Scholar] [CrossRef]
- Langdown, B.L.; Bridge, M.; Li, F.X. Movement variability in the golf swing. Sport. Biomech. 2012, 11, 273–287. [Google Scholar] [CrossRef]
- Izawa, J.; Shadmehr, R. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comput. Biol. 2011, 7, e1002012. [Google Scholar] [CrossRef]
- Roemmich, R.T.; Bastian, A.J. Two ways to save a newly learned motor pattern. J. Neurophysiol. 2015, 113, 3519–3530. [Google Scholar] [CrossRef]
- Monaco, V.; Tropea, P.; Aprigliano, F.; Martelli, D.; Parri, A.; Cortese, M.; Molino-Lova, R.; Vitiello, N.; Micera, S. An ecologically-controlled exoskeleton can improve balance recovery after slippage. Sci. Rep. 2017, 7, 46721. [Google Scholar] [CrossRef] [PubMed]
- Monaco, V.; Zabban, C.; Miyake, T. Short-Term Effects of the Repeated Exposure to Trip-like Perturbations on Inter-Segment Coordination during Walking: An UCM Analysis. Appl. Sci. 2021, 11, 9663. [Google Scholar] [CrossRef]
- Miyake, T.; Aprigliano, F.; Sugano, S.; Micera, S.; Monaco, V. Repeated exposure to tripping like perturbations elicits more precise control and lower toe clearance of the swinging foot during steady walking. Hum. Mov. Sci. 2021, 76, 102775. [Google Scholar] [CrossRef] [PubMed]
- Modchalingam, S.; Vachon, C.M.; ‘t Hart, B.M.; Henriques, D.Y. The effects of awareness of the perturbation during motor adaptation on hand localization. PLoS ONE 2019, 14, e0220884. [Google Scholar] [CrossRef] [PubMed]
- Saeterbakken, A.H.; Solstad, T.E.J.; Stien, N.; Shaw, M.P.; Pedersen, H.; Andersen, V. Muscle activation with swinging loads in bench press. PLoS ONE 2020, 15, e0239202. [Google Scholar] [CrossRef] [PubMed]
- Murofushi, K.; Oshikawa, T.; Kaneoka, K.; Akuzawa, H.; Yamaguchi, D.; Mitomo, S.; Furuya, H.; Hirohata, K.; Yagishita, K. Differences in trunk and lower extremity muscle activity during squatting exercise with and without hammer swing. Sci. Rep. 2022, 12, 13387. [Google Scholar] [CrossRef]
- Holmes, M.W.; Tat, J.; Keir, P.J. Neuromechanical control of the forearm muscles during gripping with sudden flexion and extension wrist perturbations. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Forman, G.N.; Forman, D.A.; Avila-Mireles, E.J.; Zenzeri, J.; Holmes, M.W. Investigating the muscular and kinematic responses to sudden wrist perturbations during a dynamic tracking task. Sci. Rep. 2020, 10, 4161. [Google Scholar] [CrossRef] [PubMed]
- Brændvik, S.M.; Roeleveld, K. The role of co-activation in strength and force modulation in the elbow of children with unilateral cerebral palsy. J. Electromyogr. Kinesiol. 2012, 22, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Potvin, J.; Norman, R.; McGill, S. Mechanically corrected EMG for the continuous estimation of erector spinae muscle loading during repetitive lifting. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Tomatis, L.; Läubli, T. Muscular load and performance compared between a pen and a computer mouse as input devices. Int. J. Ind. Ergon. 2010, 40, 607–617. [Google Scholar] [CrossRef]
- Vanrenterghem, J.; Nedergaard, N.J.; Robinson, M.A.; Drust, B. Training load monitoring in team sports: A novel framework separating physiological and biomechanical load-adaptation pathways. Sport. Med. 2017, 47, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Beato, M.; Drust, B. Acceleration intensity is an important contributor to the external and internal training load demands of repeated sprint exercises in soccer players. Res. Sport. Med. 2021, 29, 67–76. [Google Scholar] [CrossRef]
- Yoon, W.; Choi, S.; Han, H.; Shin, G. Neck muscular load when using a smartphone while sitting, standing, and walking. Hum. Factors 2021, 63, 868–879. [Google Scholar] [CrossRef]
- Farina, D.; Jiang, N.; Rehbaum, H.; Holobar, A.; Graimann, B.; Dietl, H.; Aszmann, O.C. The extraction of neural information from the surface EMG for the control of upper-limb prostheses: Emerging avenues and challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 797–809. [Google Scholar] [CrossRef]
- Merbah, J.; Caré, B.R.; Gorce, P.; Gadea, F.; Prince, F. A New Approach to Quantifying Muscular Fatigue Using Wearable EMG Sensors during Surgery: An Ergonomic Case Study. Sensors 2023, 23, 1686. [Google Scholar] [CrossRef]
- Bartuzi, P.; Roman-Liu, D. Assessment of muscle load and fatigue with the usage of frequency and time-frequency analysis of the EMG signal. Acta Bioeng. Biomech. 2014, 16, 31–39. [Google Scholar]
- Cifrek, M.; Medved, V.; Tonković, S.; Ostojić, S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin. Biomech. 2009, 24, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, D.; Jiang, L.; Liu, H.; Cai, H. Combined use of FSR sensor array and SVM classifier for finger motion recognition based on pressure distribution map. J. Bionic Eng. 2012, 9, 39–47. [Google Scholar] [CrossRef]
- Wu, Y.T.; Fujiwara, E.; Suzuki, C.K. Evaluation of optical myography sensor as predictor of hand postures. IEEE Sens. J. 2019, 19, 5299–5306. [Google Scholar] [CrossRef]
- Sharma, N.; Prakash, A.; Sharma, S. An optoelectronic muscle contraction sensor for prosthetic hand application. Rev. Sci. Instrum. 2023, 94, 035009. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Paszkiel, S. Muscle activity measurement using visible light and infrared. IFAC-PapersOnLine 2019, 52, 329–334. [Google Scholar] [CrossRef]
- Kauppi, K.; Korhonen, V.; Ferdinando, H.; Kallio, M.; Myllylä, T. Combined surface electromyography, near-infrared spectroscopy and acceleration recordings of muscle contraction: The effect of motion. J. Innov. Opt. Health Sci. 2017, 10, 1650056. [Google Scholar] [CrossRef]
- Herrmann, S.; Attenberger, A.; Buchenrieder, K. Prostheses control with combined near-infrared and myoelectric signals. In Proceedings of the Computer Aided Systems Theory–EUROCAST 2011: 13th International Conference, Las Palmas de Gran Canaria, Spain, 6–11 February 2011; Revised Selected Papers, Part II 13. pp. 601–608. [Google Scholar]
- McIntosh, J.; Marzo, A.; Fraser, M. Sensir: Detecting hand gestures with a wearable bracelet using infrared transmission and reflection. In Proceedings of the 30th Annual ACM Symposium on User Interface Software and Technology, Québec City, QC, Canada, 22–25 October 2017; pp. 593–597. [Google Scholar]
- Nitzan, M.; Nitzan, I.; Arieli, Y. The various oximetric techniques used for the evaluation of blood oxygenation. Sensors 2020, 20, 4844. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Chiesa, S.T.; Chaturvedi, N.; Hughes, A.D. Recent developments in near-infrared spectroscopy (NIRS) for the assessment of local skeletal muscle microvascular function and capacity to utilise oxygen. Artery Res. 2016, 16, 25–33. [Google Scholar] [CrossRef]
- Barstow, T.J. Understanding near infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Sugiura, Y.; Ogata, M.; Inami, M. Tangential force sensing system on forearm. In Proceedings of the 4th Augmented Human International Conference, Stuttgart, Germany, 7–8 March 2013; pp. 29–34. [Google Scholar]
- Hosono, S.; Miyake, T.; Tamaki, E. PondusHand: Estimation Method of Fingertips Force by User’s Forearm Muscle Deformation based on Calibration with Mobile Phone’s Touch Screen. In Proceedings of the 2022 9th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), Seoul, Republic of Korea, 21–24 August 2022; pp. 1–6. [Google Scholar]
- Kozakai, R.; Ando, F.; Kim, H.Y.; Yuki, A.; Otsuka, R.; Shimokata, H. Sex-differences in age-related grip strength decline: A 10-year longitudinal study of community-living middle-aged and older Japanese. J. Phys. Fit. Sport. Med. 2016, 5, 87–94. [Google Scholar] [CrossRef]
- Miyake, T.; Ito, H.; Okamura, N.; Kobayashi, Y.; Fujie, M.G.; Sugano, S. EMG-Based Detection of Minimum Effective Load With Robotic-Resistance Leg Extensor Training. IEEE Trans. Hum. Mach. Syst. 2024, 54, 34–43. [Google Scholar] [CrossRef]
- Castellini, C.; van der Smagt, P. Evidence of muscle synergies during human grasping. Biol. Cybern. 2013, 107, 233–245. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyake, T.; Minakuchi, T.; Sato, S.; Okubo, C.; Yanagihara, D.; Tamaki, E. Optical Myography-Based Sensing Methodology of Application of Random Loads to Muscles during Hand-Gripping Training. Sensors 2024, 24, 1108. https://doi.org/10.3390/s24041108
Miyake T, Minakuchi T, Sato S, Okubo C, Yanagihara D, Tamaki E. Optical Myography-Based Sensing Methodology of Application of Random Loads to Muscles during Hand-Gripping Training. Sensors. 2024; 24(4):1108. https://doi.org/10.3390/s24041108
Chicago/Turabian StyleMiyake, Tamon, Tomohito Minakuchi, Suguru Sato, Chihiro Okubo, Dai Yanagihara, and Emi Tamaki. 2024. "Optical Myography-Based Sensing Methodology of Application of Random Loads to Muscles during Hand-Gripping Training" Sensors 24, no. 4: 1108. https://doi.org/10.3390/s24041108
APA StyleMiyake, T., Minakuchi, T., Sato, S., Okubo, C., Yanagihara, D., & Tamaki, E. (2024). Optical Myography-Based Sensing Methodology of Application of Random Loads to Muscles during Hand-Gripping Training. Sensors, 24(4), 1108. https://doi.org/10.3390/s24041108




