Anodic Stripping Voltammetric Procedure of Thallium(I) Determination by Means of a Bismuth-Plated Gold-Based Microelectrode Array
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Reagents
2.3. Preparation of Real Water Sample
2.4. Standard Procedure of Measurements
3. Results
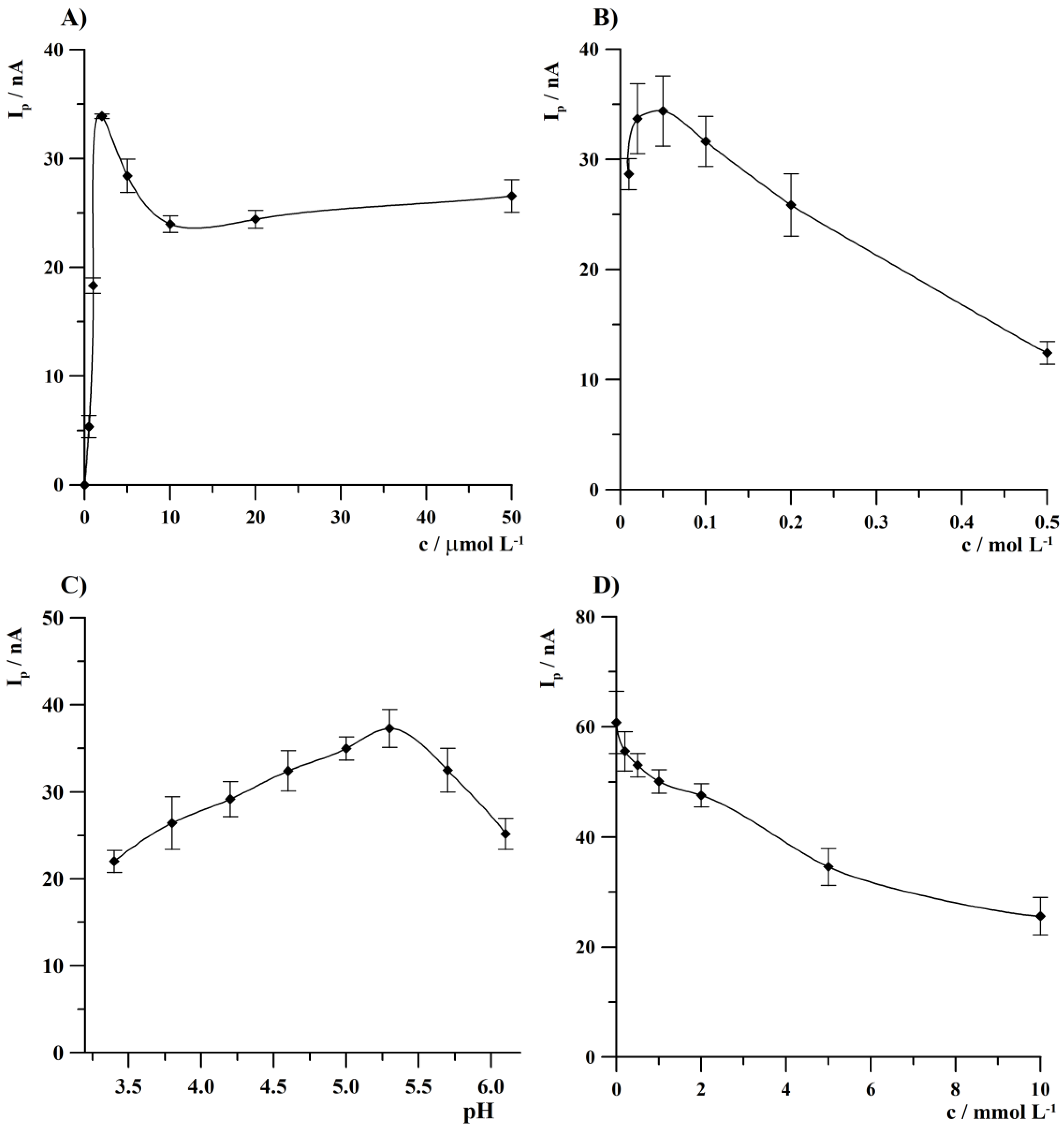
3.1. Optimization of the Acetate Buffer, Bi(III), and Na2EDTA Concentration and a pH of Acetate Buffer
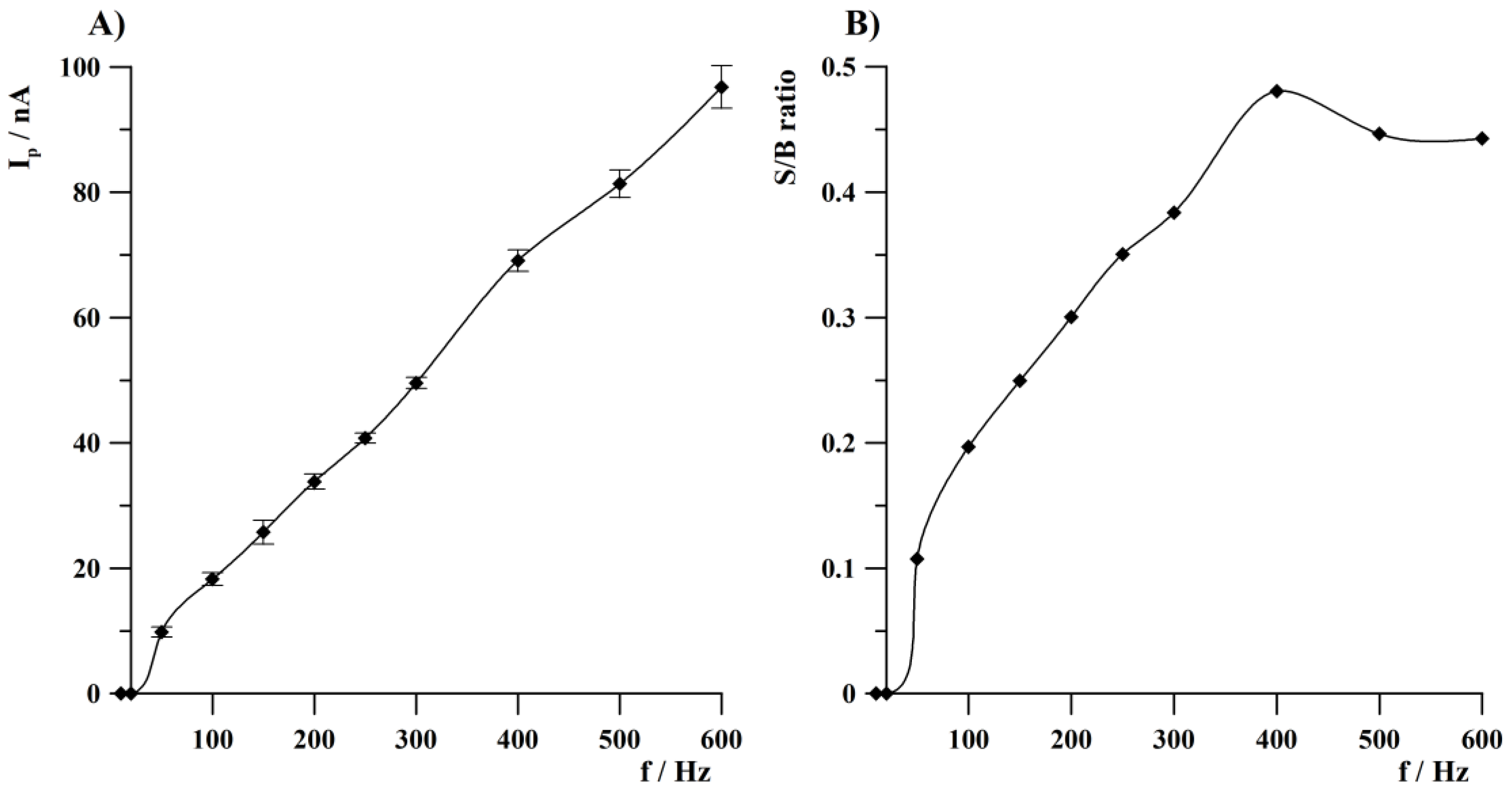
3.2. Square Wave Parameters Optimization
3.3. Optimization of the Measurement Conditions
3.4. Calibration Studies
3.5. Repeatability Studies
3.6. Interferences
3.7. Procedure Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomčík, P. Microelectrode Arrays with Overlapped Diffusion Layers as Electroanalytical Detectors: Theory and Basic Applications. Sensors 2013, 13, 13659–13684. [Google Scholar] [CrossRef]
- Wu, H.P. Fabrication and Characterization of a New Class of Microelectrode Arrays Exhibiting Steady-State Current Behavior. Anal. Chem. 1993, 65, 1643–1646. [Google Scholar] [CrossRef]
- Szunerits, S.; Thouin, L. Microelectrode Arrays. In Handbook of Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2007; pp. 391–428. [Google Scholar] [CrossRef]
- Tanwar, A.; Gandhi, H.A.; Kushwaha, D.; Bhattacharya, J. A Review on Microelectrode Array Fabrication Techniques and Their Applications. Mater. Today Chem. 2022, 26, 101153. [Google Scholar] [CrossRef]
- Lowinsohn, D.; Peres, H.E.M.; Kosminsky, L.; Paixão, T.R.L.C.; Ferreira, T.L.; Ramirez-Fernandez, F.J.; Bertotti, M. Design and Fabrication of a Microelectrode Array for Iodate Quantification in Small Sample Volumes. Sens. Actuators B Chem. 2006, 113, 80–87. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Song, Y.; Luo, J.; Wei, W.; Xu, S.; Cai, X. Highly Sensitive Detection of Quantal Dopamine Secretion from Pheochromocytoma Cells Using Neural Microelectrode Array Electrodeposited with Polypyrrole Graphene. ACS Appl. Mater. Interfaces 2015, 7, 7619–7626. [Google Scholar] [CrossRef] [PubMed]
- Lupu, S.; Lete, C.; Balaure, P.C.; Del Campo, F.J.; Muñoz, F.X.; Lakard, B.; Hihn, J.Y. In Situ Electrodeposition of Biocomposite Materials by Sinusoidal Voltages on Microelectrodes Array for Tyrosinase Based Amperometric Biosensor Development. Sens. Actuators B Chem. 2013, 181, 136–143. [Google Scholar] [CrossRef]
- Lupu, S.; del Campo, F.J.; Muñoz, F.X. Development of Microelectrode Arrays Modified with Inorganic–Organic Composite Materials for Dopamine Electroanalysis. J. Electroanal. Chem. 2010, 639, 147–153. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.; Huo, K.; Chu, P.K. Microelectrode Arrays Based on Carbon Nanomaterials: Emerging Electrochemical Sensors for Biological and Environmental Applications. RSC Adv. 2013, 3, 18698–18715. [Google Scholar] [CrossRef]
- Kadara, R.O.; Jenkinson, N.; Banks, C.E. Screen Printed Recessed Microelectrode Arrays. Sens. Actuators B Chem. 2009, 142, 342–346. [Google Scholar] [CrossRef]
- Tan, F.; Metters, J.P.; Banks, C.E. Electroanalytical Applications of Screen Printed Microelectrode Arrays. Sens. Actuators B Chem. 2013, 181, 454–462. [Google Scholar] [CrossRef]
- Schuettler, M.; Stiess, S.; King, B.V.; Suaning, G.J. Fabrication of Implantable Microelectrode Arrays by Laser Cutting of Silicone Rubber and Platinum Foil*. J. Neural Eng. 2005, 2, S121–S128. [Google Scholar] [CrossRef] [PubMed]
- Liébana, S.; Jones, L.J.; Drago, G.A.; Pittson, R.W.; Liu, D.; Perrie, W.; Hart, J.P. Design and Development of Novel Screen-Printed Microelectrode and Microbiosensor Arrays Fabricated Using Ultrafast Pulsed Laser Ablation. Sens. Actuators B Chem. 2016, 231, 384–392. [Google Scholar] [CrossRef]
- Kemnic, T.R.; Coleman, M. Thallium Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Liu, J.; Wei, X.; Zhou, Y.; Tsang, D.C.W.; Bao, Z.; Yin, M.; Lippold, H.; Yuan, W.; Wang, J.; Feng, Y.; et al. Thallium Contamination, Health Risk Assessment and Source Apportionment in Common Vegetables. Sci. Total Environ. 2020, 703, 135547. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Song, J. Thallium in Aquatic Environments and the Factors Controlling Tl Behavior. Environ. Sci. Pollut. Res. 2021, 28, 35472–35487. [Google Scholar] [CrossRef] [PubMed]
- Meeravali, N.N.; Madhavi, K.; Kumar, S.J. Microwave Assisted Aqua Regia Extraction of Thallium from Sediment and Coal Fly Ash Samples and Interference Free Determination by Continuum Source ETAAS after Cloud Point Extraction. Talanta 2013, 104, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Fayazi, M.; Ghanei-Motlagh, M.; Taher, M.A.; Ghanei-Motlagh, R.; Salavati, M.R. Synthesis and Application of a Novel Nanostructured Ion-Imprinted Polymer for the Preconcentration and Determination of Thallium(I) Ions in Water Samples. J. Hazard. Mater. 2016, 309, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, H.; Xu, B.; Xiao, L.; Chigan, Y.; Zhangluo, Y. In-Situ Pre-Concentration through Repeated Sampling and Pyrolysis for Ultrasensitive Determination of Thallium in Drinking Water by Electrothermal Atomic Absorption Spectrometry. Talanta 2018, 179, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Shams, A.; Ashraf, N.; Arbab-Zavar, M.H.; Masrournia, M. Ultra-Trace Determination of Thallium by Electrochemical Hydride Generation Using Efficient Tungsten Electrodes Followed by in Situ Trapping on a Graphite Tube and Detection by Electrothermal Atomic Absorption Spectrometry. J. Anal. At. Spectrom. 2017, 32, 2173–2181. [Google Scholar] [CrossRef]
- Oliveira, L.A.; Santos, J.L.O.; Teixeira, L.S.G. Determination of Thallium in Water Samples via Solid Sampling HR-CS GF AAS after Preconcentration on Chromatographic Paper. Talanta 2024, 266, 124945. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, R.; Madhavi, K.; Meeravali, N.N.; Kumar, S.J. Determination of Thallium at Trace Levels by Electrolyte Cathode Discharge Atomic Emission Spectrometry with Improved Sensitivity. Anal. Methods 2014, 6, 732–740. [Google Scholar] [CrossRef]
- Biata, N.R.; Dimpe, K.M.; Ramontja, J.; Mketo, N.; Nomngongo, P.N. Determination of Thallium in Water Samples Using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) after Ultrasonic Assisted-Dispersive Solid Phase Microextraction. Microchem. J. 2018, 137, 214–222. [Google Scholar] [CrossRef]
- Zu, W.; Wang, Y.; Yang, X.; Liu, C. A Portable Solution Cathode Glow Discharge-Atomic Emission Spectrometer for the Rapid Determination of Thallium in Water Samples. Talanta 2017, 173, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.M.; Poitras, E.P.; Weber, F.X.; Fernando, R.A.; Liyanapatirana, C.; Robinson, V.G.; Levine, K.E.; Waidyanatha, S. Validation of Analytical Method for Determination of Thallium in Rodent Plasma and Tissues by Inductively Coupled Plasma–Mass Spectrometry (ICP-MS). Anal. Lett. 2022, 55, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Drwal, A.; Tutaj, K.; Kovshun, L.; Krasnodębska-Ostręga, B. On Site Separation of Inorganic Forms of Thallium and Arsenic in Sea Water Systems Followed by ICP-MS Determination. Anal. Methods 2023, 15, 6082–6087. [Google Scholar] [CrossRef]
- Bonelli, J.E.; Taylor, H.E.; Skogerboe, R.K. A Direct Differential Pulse Anodic Stripping Voltammetric Method for the Determination of Thallium in Natural Waters. Anal. Chim. Acta 1980, 118, 243–256. [Google Scholar] [CrossRef]
- Lu, T.H.; Yang, H.Y.; Sun, I.W. Square-Wave Anodic Stripping Voltammetric Determination of Thallium(I) at a Nafion/Mercury Film Modified Electrode. Talanta 1999, 49, 59–68. [Google Scholar] [CrossRef]
- Mahamane, A.A.; Guel, B.; Fabre, P.L. Anodic Stripping Voltammetric Determination of Thallium at a Mercury Film/Glassy Carbon Electrode: Optimization of the Method and Application to Environmental Waters: Science Publishing Group. Sci. J. Anal. Chem. 2022, 10, 74–79. [Google Scholar]
- Jorge, E.O.; Neto, M.M.M.; Rocha, M.M. A Mercury-Free Electrochemical Sensor for the Determination of Thallium(I) Based on the Rotating-Disc Bismuth Film Electrode. Talanta 2007, 72, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Rutyna, I.; Korolczuk, M. Determination of Ultratrace Thallium(I) by Anodic Stripping Voltammetry at Bismuth Film Electrodes Following Double Deposition and Stripping Steps. Electroanalysis 2014, 26, 2639–2643. [Google Scholar] [CrossRef]
- Tarasova, V.A. Voltammetric Determination of Thallium(I) at a Mechanically Renewed Bi-Graphite Electrode. J. Anal. Chem. 2007, 62, 157–160. [Google Scholar] [CrossRef]
- Wegiel, K.; Jedlińska, K.; Baś, B. Application of Bismuth Bulk Annular Band Electrode for Determination of Ultratrace Concentrations of Thallium(I) Using Stripping Voltammetry. J. Hazard. Mater. 2016, 310, 199–206. [Google Scholar] [CrossRef]
- Lee, G.J.; Lee, H.M.; Uhm, Y.R.; Lee, M.K.; Rhee, C.K. Square-Wave Voltammetric Determination of Thallium Using Surface Modified Thick-Film Graphite Electrode with Bi Nanopowder. Electrochem. Commun. 2008, 10, 1920–1923. [Google Scholar] [CrossRef]
- Domańska, K.; Tyszczuk-Rotko, K. Integrated Three-Electrode Screen-Printed Sensor Modified with Bismuth Film for Voltammetric Determination of Thallium(I) at the Ultratrace Level. Anal. Chim. Acta 2018, 1036, 16–25. [Google Scholar] [CrossRef]
- Kozak, J.; Tyszczuk-Rotko, K.; Rotko, M. Voltammetric Screen-Printed Carbon Sensor Modified with Multiwalled Carbon Nanotubes and Bismuth Film for Trace Analysis of Thallium(I). Physicochem. Probl. Miner. Process. 2019, 55, 1422–1428. [Google Scholar] [CrossRef]
- Gęca, I.; Ochab, M.; Korolczuk, M. Anodic Stripping Voltammetry of Tl(I) Determination with the Use of a Solid Bismuth Microelectrode. J. Electrochem. Soc. 2020, 167, 086506. [Google Scholar] [CrossRef]
- Spano, N.; Panzanelli, A.; Piu, P.C.; Pilo, M.I.; Sanna, G.; Seeber, R.; Tapparo, A. Anodic Stripping Voltammetric Determination of Traces and Ultratraces of Thallium at a Graphite Microelectrode: Method Development and Application to Environmental Waters. Anal. Chim. Acta 2005, 553, 201–207. [Google Scholar] [CrossRef]
- Nasiri-Majd, M.; Taher, M.A.; Fazelirad, H. Synthesis and Application of Nano-Sized Ionic Imprinted Polymer for the Selective Voltammetric Determination of Thallium. Talanta 2015, 144, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Mnyipika, S.H.; Nomngongo, P.N. Square Wave Anodic Stripping Voltammetry for Simultaneous Determination of Trace Hg (II) and Tl(I) in Surface Water Samples Using SnO 2 @MWCNTs Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2017, 12, 4811–4827. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, Y.; Ma, J.; Xie, L.; Du, X. Simultaneous Determination of Indium and Thallium Ions by Anodic Stripping Voltammetry Using Antimony Film Electrode. Sens. Lett. 2009, 7, 605–608. [Google Scholar] [CrossRef]
- Cao, G.X.; Jimenez, O.; Zhou, F.; Xu, M.; Zhou, F. Nafion-Coated Bismuth Film and Nafion-Coated Mercury Film Electrodes for Anodic Stripping Voltammetry Combined On-Line with ICP-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2006, 17, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Afkhami, A.; Khoshsafar, H.; Rezaei, M.; Sabounchei, S.J.; Sarlakifar, M. Simultaneous Electrochemical Sensing of Thallium, Lead and Mercury Using a Novel Ionic Liquid/Graphene Modified Electrode. Anal. Chim. Acta 2015, 870, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zheng, H.; Lin, L.; Ye, B. Determination of Thallium and Cadmium on a Chemically Modified Electrode with Langmuir–Blodgett Film of p-Allylcalix[4]Arene. Sens. Actuators B Chem. 2006, 115, 303–308. [Google Scholar] [CrossRef]
- Cheraghi, S.; Taher, M.A.; Fazelirad, H. Voltammetric Sensing of Thallium at a Carbon Paste Electrode Modified with a Crown Ether. Microchim. Acta 2013, 180, 1157–1163. [Google Scholar] [CrossRef]
- Shah, A.; Nisar, A.; Khan, K.; Nisar, J.; Niaz, A.; Ashiq, M.N.; Akhter, M.S. Amino Acid Functionalized Glassy Carbon Electrode for the Simultaneous Detection of Thallium and Mercuric Ions. Electrochim. Acta 2019, 321, 134658. [Google Scholar] [CrossRef]
- Cai, Q.; Khoo, S.B. Differential Pulse Stripping Voltammetric Determination of Thallium with an 8-Hydroxyquinoline-Modified Carbon Paste Electrode. Electroanalysis 1995, 7, 379–385. [Google Scholar] [CrossRef]
- Gęca, I.; Ochab, M.; Robak, A.; Mergo, P.; Korolczuk, M. Anodic Stripping Voltammetric Determination of Se(IV) by Means of a Novel Reusable Gold Microelectrodes Array. Desalin. Water Treat. 2023, 286, 248–256. [Google Scholar] [CrossRef]
- Lee, H.W.; Schmidt, M.A.; Russell, R.F.; Joly, N.Y.; Tyagi, H.K.; Uebel, P.; Russell, P.S.J. Pressure-Assisted Melt-Filling and Optical Characterization of Au Nano-Wires in Microstructured Fibers. Opt. Express 2011, 19, 12189. [Google Scholar] [CrossRef] [PubMed]
- Kacanovska, A.; Rong, Z.; Schmidt, M.; Russell, P.S.J.; Vadgama, P. Bio-Sensing Using Recessed Gold-Filled Capillary Amperometric Electrodes. Anal. Bioanal. Chem. 2010, 398, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.A.; Prill Sempere, L.N.; Tyagi, H.K.; Poulton, C.G.; Russell, P.S.J. Waveguiding and Plasmon Resonances in Two-Dimensional Photonic Lattices of Gold and Silver Nanowires. Phys. Rev. B 2008, 77, 033417. [Google Scholar] [CrossRef]
- Obata, H.; Yoshida, T.; Ogawa, H. Determination of Picomolar Levels of Platinum in Estuarine Waters: A Comparison of Cathodic Stripping Voltammetry and Isotope Dilution-Inductively Coupled Plasma Mass Spectrometry. Anal. Chim. Acta 2006, 580, 32–38. [Google Scholar] [CrossRef]
- Kapturski, P.; Bobrowski, A. The Silver Amalgam Film Electrode in Catalytic Adsorptive Stripping Voltammetric Determination of Cobalt and Nickel. J. Electroanal. Chem. 2008, 617, 1–6. [Google Scholar] [CrossRef]
- Guo, J.; Lindner, E. Cyclic Voltammograms at Coplanar and Shallow Recessed Microdisk Electrode Arrays: Guidelines for Design and Experiment. Anal. Chem. 2009, 81, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M.; Ogorevc, B. Bismuth-Coated Carbon Electrodes for Anodic Stripping Voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Karapa, A.; Kokkinos, C.; Peter, R.F.; Baldock, S.J.; Goddard, N.J.; Economou, A.; Mamas, I.P. Eco-Friendly Voltammetric Platform for Trace Metal Determination Using a Conductive Polymer Sensor Modified with Bismuth Nanoparticles Generated by Spark Discharge. Microchim. Acta 2023, 190, 376. [Google Scholar] [CrossRef]




| Analytical Parameter | Value | Unit | |
|---|---|---|---|
| 120 s | 180 s | ||
| Slope | 3.95 | 7.14 | nA/nmol L−1 |
| Intercept | 18.71 | 4.14 | nA |
| R 1 | 0.9989 | 0.9988 | - |
| Linear range | 0.5–500 | 0.2–200 | nmol L−1 |
| LOD 2 | 0.22 | 0.08 | nmol L−1 |
| Working Electrode | Linear Range [nmol L−1] | Detection Limit [nmol L−1] | Remarks | Ref. |
|---|---|---|---|---|
| BiFE | 12–150 | 10.8 | Rotating disc | [30] |
| BiFEs | 0.05–5 | 0.021 | Two BiFE electrodes | [31] |
| Bi-graphite electrode | 49–4900 | 4.9 | Mechanically renewed electrode | [32] |
| BiABE | 0.5–49 | 0.005 | - | [33] |
| GrE/Bi nanopowder | 4.9–2446 | 0.15 | Surface-modified electrode | [34] |
| BiF/SPE | 5–1000 | 0.847 | Integrated three-electrode sensor | [35] |
| 0.05–1 | 0.00671 | |||
| SPCE/MWCNTs/BiF | 10–1000 | 2.8 | - | [36] |
| BiµE | 2–200 | 0.83 | Solid metal microelectrode | [37] |
| SbFE | 100–490 | 10 | - | [41] |
| BiF/AuµE | 0.5–500 | 0.22 | - | [this work] |
| 0.2–200 | 0.08 |
| Interfering Ions | Molar Excess of Interfering Ions | Relative Signal of Tl(I) (I/I0) |
|---|---|---|
| Cu(II) | 20 | 0.82 |
| 50 | 0.59 | |
| Pb(II) | 50 | 0.96 |
| 100 | 0.78 | |
| Fe(III) | 100 | 0.95 |
| 250 | 0.89 | |
| Zn(II) | 100 | 0.92 |
| 250 | 0.86 | |
| Cd(II) | 100 | 0.98 |
| 250 | 0.91 | |
| Mn(II) | 100 | 1.03 |
| 250 | 1.07 |
| Sample | Measured Value ± SD (n = 5) [μg L−1] | Certified Value ± SD [μg L−1] | Recovery [%] |
|---|---|---|---|
| CRM (TM 25.5) | 29.0 ± 1.0 | 30.0 ± 2.8 | 96.7 |
| Sample | Added [nmol L−1] | Found± SD (n = 3) [nmol L−1] | Recovery [%] |
| Bystrzyca River | 0 | 0 | - |
| 10.0 | 9.8 ± 0.2 | 98.0 | |
| 20.0 | 19.8 ± 0.4 | 99.0 | |
| 30.0 | 29.6 ± 0.6 | 98.7 | |
| 40.0 | 40.7 ± 1.1 | 101.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korolczuk, M.; Ochab, M.; Gęca, I. Anodic Stripping Voltammetric Procedure of Thallium(I) Determination by Means of a Bismuth-Plated Gold-Based Microelectrode Array. Sensors 2024, 24, 1206. https://doi.org/10.3390/s24041206
Korolczuk M, Ochab M, Gęca I. Anodic Stripping Voltammetric Procedure of Thallium(I) Determination by Means of a Bismuth-Plated Gold-Based Microelectrode Array. Sensors. 2024; 24(4):1206. https://doi.org/10.3390/s24041206
Chicago/Turabian StyleKorolczuk, Mieczyslaw, Mateusz Ochab, and Iwona Gęca. 2024. "Anodic Stripping Voltammetric Procedure of Thallium(I) Determination by Means of a Bismuth-Plated Gold-Based Microelectrode Array" Sensors 24, no. 4: 1206. https://doi.org/10.3390/s24041206





