Changes in the Activity of the Erector Spinae and Gluteus Medius Muscles with the Presence of Simulated Lower Limb Dysmetria
Abstract
1. Introduction
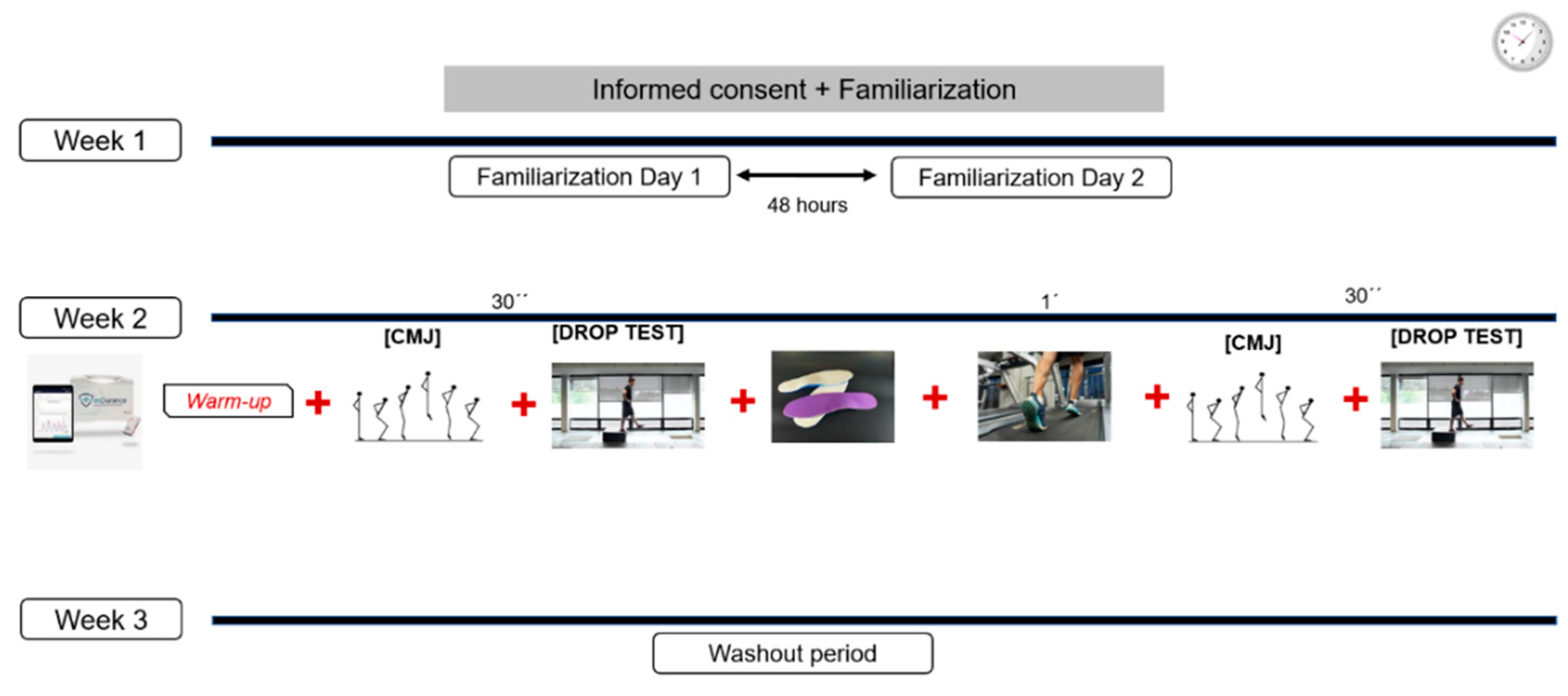
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size Calculation
2.4. Instrumentation and Data Collection
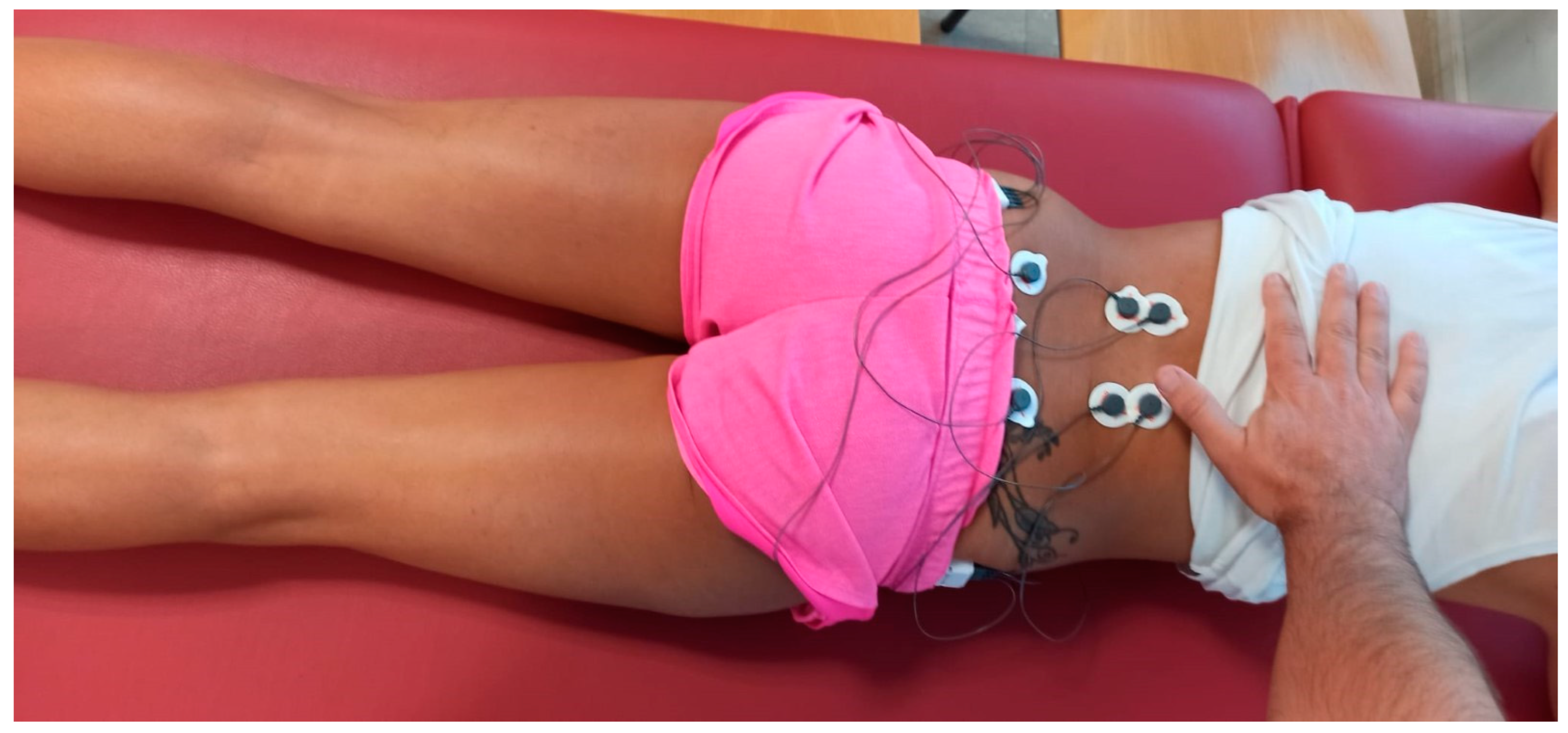
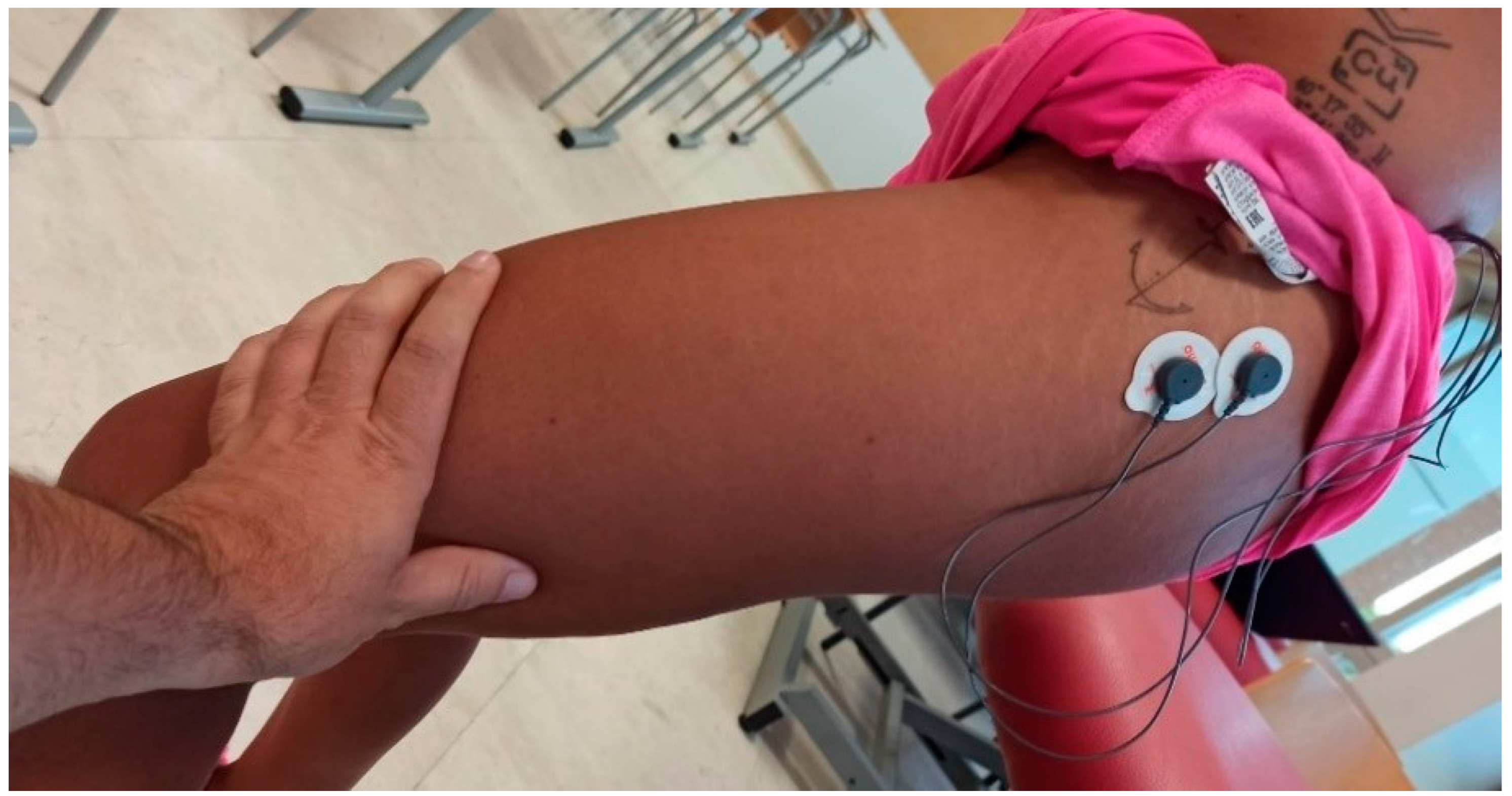
2.4.1. EMG Measurement
MVC Measurement
EMG Recording during Walking
CMJ and Drop Test
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brady, R.J.; Dean, J.B.; Marc Skinner, T.; Gross, M.T. Limb Length Inequality: Clinical Implications for Assessment and Intervention. J. Orthop. Sports Phys. Ther. 2003, 33, 221–234. [Google Scholar] [CrossRef]
- Walsh, M.; Connolly, P.; Jenkinson, A.; O’Brien, T. Leg length discrepancy—An experimental study of compensatory changes in three dimensions using gait analysis. Gait Posture 2000, 12, 156–161. [Google Scholar] [CrossRef]
- Murrell, P.; Cornwall, M.W.; Doucet, S.K. Leg-length discrepancy: Effect on the amplitude of postural sway. Arch. Phys. Med. Rehab. 1991, 72, 646–648. [Google Scholar]
- Azizan, N.A.; Basaruddin, K.S.; Salleh, A.F.; Sulaiman, A.R.; Safar, M.J.A.; Rusli, W.M.R. Leg Length Discrepancy: Dynamic Balance Response during Gait. J. Healthc. Eng. 2018, 2018, 7815451. [Google Scholar] [CrossRef]
- Khamis, S.; Carmeli, E. Relationship and significance of gait deviations associated with limb length discrepancy: A systematic review. Gait Posture. Gait Posture 2017, 57, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.R. Unequal leg length: An accurate method of detection and some clinical results. Rheumatol. Phys. Med. 1972, 11, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Friberg, O. Clinical symptoms and biomechanics of lumbar spine and hip joint in leg length inequality. Spine (Phila Pa 1976) 1983, 8, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Cooperstein, R. The relationship between pelvic torsion and anatomical leg length inequality: A review of the literature. J. Chiropr. Med. 2010, 9, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Cartwright-Terry, M.; Moorehead, J.D.; Bowey, A.; Scott, S.J. The effect of leg length discrepancy upon load distribution in the static phase (standing). Gait Posture 2014, 40, 561–563. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, J.C. Keys to recognizing and treating limb length discrepancy. Podiatry Today 2014, 27, 66–75. [Google Scholar]
- Queiros, A.F.C.; Costa, F.G.M. Leg Length Discrepancy: A Brief Review. Portuguese Journal of Orthopaedic and Traumatology. 2018. Available online: https://repositorio-aberto.up.pt/bitstream/10216/114362/2/278728.pdf (accessed on 30 December 2023).
- Triano, J.J. Objective electromyographic evidence form the use and effects of lift therapy. J. Manip. Physiol. Ther. 1983, 6, 13–16. [Google Scholar]
- Knutson, G.A.; Owens, E. Erector spinae and quadratus lumborum muscle endurance tests and supine leg-length alignment asymmetry: An observational study. J. Manip. Physiol. Ther. 2005, 28, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.H. Leg length discrepancy in marathon runners. Am. J. Sports Med. 1983, 11, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Hellsing, A.L. Leg Length Inequality. Upsala J. Med. Sci. 1988, 93, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Khamis, S.; Carmeli, E. The effect of simulated leg length discrepancy on lower limb biomechanics during gait. Gait Posture 2018, 61, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Betsch, M.; Wild, M.; Große, B.; Rapp, W.; Horstmann, T. The effect of simulating leg length inequality on spinal posture and pelvic position: A dynamic rasterstereographic analysis. Eur. Spine J. 2012, 21, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Young, R.S.; Andrew, P.D.; Cummings, G.S. Effect of simulating leg length inequality on pelvic torsion and trunk mobility. Gait Posture 2000, 11, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.; Baldry, P. Regional myofascial pain: Diagnosis and management. Best Pract. Res. Clin. Rheumatol. 2007, 21, 367–387. [Google Scholar] [CrossRef]
- Bennell, K.; Matheson, G.; Meeuwisse, W.; Brukner, P. Risk Factors for Stress Fractures. Sports Med. 1999, 28, 91–122. [Google Scholar] [CrossRef]
- Harvey, W.F.; Yang, M.; Cooke, T.D.V.; Segal, N.A.; Lane, N.; Lewis, C.E.; Felson, D.T. Association of leg-length inequality with knee osteoarthritis a cohort study. Ann. Intern Med. 2010, 152, 287–295. [Google Scholar] [CrossRef]
- Sanhudo, J.A.V.; Gomes, J.L.E. Association between Leg Length Discrepancy and Posterior Tibial Tendon Dysfunction. Foot Ankle Spec. 2014, 7, 119–126. [Google Scholar] [CrossRef]
- Kaljumae, U.; Martson, A.; Haviko, T.; Hanninen, O. The effect of lengthening of the femur on the extensors of the knee. An electromyographic study. J. Bone Jt. Surg. Ser. A 1995, 77, 247–250. [Google Scholar] [CrossRef]
- Abate, M.; Di Carlo, L.; Di Romualdo, S.; Ionta, S.; Ferretti, A.; Romani, G.L.; Merla, A. Postural adjustment in experimental leg length difference evaluated by means of thermal infrared imaging. Physiol. Meas. 2010, 31, 35–43. [Google Scholar] [CrossRef]
- Gurney, B.; Mermier, C.; Robergs, R.; Gibson, A.; Rivero, D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J. Bone Jt. Surg. Am. 2001, 83, 907–915. [Google Scholar] [CrossRef]
- Vink, P.; Huson, A. Lumbar back muscle activity during walking with a leg inequlity. Acta Morphol. Neerl. Scand. 1987, 25, 261–271. [Google Scholar]
- Brinten Brinke, A.; van der Aa, H.E.; van der Palen, J.; Oosterveld, F. Is leg length discrepancy associated with the side of radiating pain in patients with a lumbar herniated disc? Spine (Phila Pa 1976) 1999, 24, 684–686. [Google Scholar] [CrossRef]
- Defrin, R.; Benyamin, S.B.; Aldubi, R.D.; Pick, C.G. Conservative correction of leg-length discrepancies of 10 mm or less for the relief of chronic low back pain. Arch. Phys. Med. Rehabil. 2005, 86, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Raczkowski, J.W.; Daniszewska, B.; Zolynski, K. Functional scoliosis caused by leg length discrepancy. Arch. Med. Sci. 2010, 6, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.Y.; Puffer, J.C.; Schmalzried, T.P. Lower extremity alignment and risk of overuse injuries in runners. Med. Sci. Sports Exerc. 1997, 29, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- MuMurray, K.J.; Molyneux, T.; Le Grande, M.R.; Mendez, A.C.; Fuss, F.K.; Azari, M.F. Association of Mild Leg Length Discrepancy and Degenerative Changes in the Hip Joint and Lumbar Spine. J. Manip. Physiol. Ther. 2017, 40, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Descarreaux, M.; Lalonde, C.; Normand, M.C. Isometric Force Parameters and Trunk Muscle Recruitment Strategies in a Population With Low Back Pain. J. Manip. Physiol. Ther. 2007, 30, 91–97. [Google Scholar] [CrossRef]
- Kendall, J.C.; Bird, A.R.; Azari, M.F. Foot posture, leg length discrepancy and low back pain—Their relationship and clinical management using foot orthoses—An overview. Foot 2014, 24, 75–80. [Google Scholar] [CrossRef]
- Ekşi, M.Ş.; Özcan-Ekşi, E.E. Fatty infiltration of the erector spinae at the upper lumbar spine could be a landmark for low back pain. Pain Pract. 2023. Early View. [Google Scholar] [CrossRef]
- Bird, A.R.; Bendrups, A.P.; Payne, C.B. The effect of foot wedging on electromyographic activity in the erector spinae and gluteus medius muscles during walking. Gait Posture 2003, 18, 81–91. [Google Scholar] [CrossRef]
- Smith, N. Gluteus medius function and low back pain: Is there a relationship? Phys. Ther. Rev. 1999, 4, 283–288. [Google Scholar] [CrossRef]
- Lee, D. The Pelvic Girdle: An Approach to the Examination and Treatment of the Lumbo-Pelvic-Hip Region, 2nd ed. Churchill Livingstone: London UK, 1999.
- Fredericson, M.; Cookingham, C.L.; Chaudhari, M.; Dowdell, B.C.; Oestreicher, N.; Sahrmann, S.A. Hip Abductor Weakness in Distance Runners with Iliotibial Band Syndrome. Clin. J. Sport Med. 2000, 10, 169–175. [Google Scholar] [CrossRef]
- Arab, A.M.; Nourbakhsh, M.R. The relationship between hip abductor muscle strength and iliotibial band tightness in individuals with low back pain. Chiropr. Osteopat. 2010, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Kanchanomai, S.; Janwantanakul, P.; Pensri, P.; Jiamjarasrangsi, W. A prospective study of incidence and risk factors for the onset and persistence of low back pain in Thai university students. Asia Pac. J. Public Health 2015, 27, NP106–NP115. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Medina, L.; González-Badillo, J.J. Velocity Loss as an indicator of neuromuscular fatigue during resistance training. Med. Sci. Sports Exerc. 2011, 43, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Castaño, M.V.; Domínguez, R.; Ruiz-Solano, P.; Maté-Muñoz, J.L. Acute physiological and mechanical responses during resistance exercise executed at the lactate threshold workload. J. Strength Cond. Res. 2015, 29, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Dousset, E.; Avela, J.; Kyröläinen, H.; Kallio, J.; Linnamo, V.; Kuitunen, S.; Nicol, C.; Komi, P.V. Changes in the soleus muscle architecture after exhausting stretch-shortening cycle exercise in humans. Eur. J. Appl. Physiol. 2006, 97, 298–306. [Google Scholar] [CrossRef]
- Carroll, M.; Ellis, R.; Kohut, S.; Garrett, N.; Fernández-de-Las-Peñas, C. Associations Between Gluteus Medius Trigger Points With Hip Passive Range of Movement and Muscle Strength in Adults With Chronic Nonspecific Low Back Pain: A Cross-Sectional Study. J. Manip. Physiol. Ther. 2022, 45, 641–651. [Google Scholar] [CrossRef]
- Frącz, W.; Matuska, J.; Szyszka, J.; Dobrakowski, P.; Szopka, W.; Skorupska, E. The Cross-Sectional Area Assessment of Pelvic Muscles Using the MRI Manual Segmentation among Patients with Low Back Pain and Healthy Subjects. J. Imaging 2023, 9, 155. [Google Scholar] [CrossRef]
- Amaro, A.; Amado, F.; Duarte, J.A.; Appell, H.J. Gluteus medius muscle atrophy is related to contralateral and ipsilateral hip joint osteoarthritis. Int. J. Sports Med. 2007, 28, 1035–1039. [Google Scholar] [CrossRef]
- Nelson-Wong, E.; Gregory, D.E.; Winter, D.A.; Callaghan, J.P. Gluteus medius muscle activation patterns as a predictor of low back pain during standing. Clin. Biomech. 2008, 23, 545–553. [Google Scholar] [CrossRef]
- Gordon, J.E.; Davis, L.E. Leg Length Discrepancy: The Natural History (And What Do We Really Know). J. Pediatr. Orthop. 2019, 39, S10–S13. [Google Scholar] [CrossRef]
- Beattie, P.; Isaacson, K.; Riddle, D.L.; Rothstein, J.M. Validity of derived measurements of leg-length differences obtained by use of a tape measure. Phys. Ther. 1990, 70, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, A.; Ruiz-Malagón, E.J.; Carrillo-Pérez, F.; Roche-Seruendo, L.E.; Damas, M.; Banos, O.; García-Pinillos, F. Validation of mDurance, A Wearable Surface Electromyography System for Muscle Activity Assessment. Front. Physiol. 2020, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, K.; Banzer, W. Motor performance in different dynamic tests in knee rehabilitation. Scand. J. Med. Sci. Sports 1999, 9, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carnero, J.; Ge, H.Y.; Kimura, Y.; Fernández-de-Las-Peñas, C.; Arendt-Nielsen, L. Increased spontaneous electrical activity at a latent myofascial trigger point after nociceptive stimulation of another latent trigger point. Clin. J. Pain 2010, 26, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Cram, J.R.; Kasman, G.S. Introduction to Surface Electromyography; Aspen Publishers: Gaithersburg, MD, USA, 1998; pp. 352–353. [Google Scholar]
- Plamondon, A.; Marceau, C.; Stainton, S.; Oesjardins, P. Toward a better prescription of the prone back extension exercise to strengthen the back muscles. Scand. J. Med. Sci. Sports 1999, 9, 226–232. [Google Scholar] [CrossRef]
- Williams, R.; Binkley, J.; Bloch, R.; Goldsmith, C.H.; Minuk, T. Reliability of the modified-modified Schober and double inclinometer methods for measuring lumbar flexion and extension. Phys. Ther. 1993, 73, 26–37. [Google Scholar] [CrossRef]
- Murley, G.S.; Bird, A.R. The effect of three levels of foot orthotic wedging on the surface electromyographic activity of selected lower limb muscles during gait. Clin. Biomech. 2006, 21, 1074–1080. [Google Scholar] [CrossRef]
- Cunningham, C.B.; Schilling, N.; Anders, C.; Carrier, D.R. The influence of foot posture on the cost of transport in humans. J. Exp. Biol. 2010, 213, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Fleming, N.; Walters, J.; Grounds, J.; Fife, L.; Finch, A. Acute response to barefoot running in habitually shod males. Hum. Mov. Sci. 2015, 42, 27–37. [Google Scholar] [CrossRef]
- Gorostiaga, E.M.; Asiáin, X.; Izquierdo, M.; Postigo, A.; Aguado, R.; Alonso, J.M.; Ibáñez, J. Vertical jump performance and blood ammonia and lactate levels during typical training sessions in elite 400-m runners. J. Strength Cond. Res. 2010, 24, 1138–1149. [Google Scholar] [CrossRef]
- Murray, K.J.; Azari, M.F. Leg length discrepancy and osteoarthritis in the knee, hip and lumbar spine. J. Can. Chiropr. Assoc. 2015, 59, 226–237. [Google Scholar]
- Coates, J.E.; McGregor, A.H.; Beith, I.D.; Hughes, S.P.F. The influence of initial resting posture on range of motion of the lumbar spine. Man. Ther. 2001, 6, 139–144. [Google Scholar] [CrossRef]
- Popovich, J.M.; Welcher, J.B.; Hedman, T.P.; Tawackoli, W.; Anand, N.; Chen, T.C.; Kulig, K. Lumbar facet joint and intervertebral disc loading during simulated pelvic obliquity. Spine J. 2013, 13, 1581–1589. [Google Scholar] [CrossRef]
- Kakushima, M.; Miyamoto, K.; Shimizu, K. The Effect of Leg Length Discrepancy on Spinal Motion during Gait: Three-Dimensional Analysis in Healthy Volunteers. Spine (Phila Pa 1976) 2003, 28, 2472–2476. [Google Scholar] [CrossRef]
- Nelson-Wong, E.; Callaghan, J.P. Is muscle co-activation a predisposing factor for low back pain development during standing? A multifactorial approach for early identification of at-risk individuals. J. Electromyogr. Kinesiol. 2010, 20, 256–263. [Google Scholar] [CrossRef]
- Vink, P.; Karssemeijer, N. Low back muscle activity and pelvic rotation during walking. Anat. Embryol. 1988, 178, 455–460. [Google Scholar] [CrossRef]
- Solomonow, M.; Zhou, B.H.; Harris, M.; Lu, Y.; Baratta, R.V. The ligamento-muscular stabilizing system of the spine. Spine (Phila Pa 1976) 1998, 23, 2552–2562. [Google Scholar] [CrossRef]
- HHultman, G.; Nordin, M.; Saraste, H.; Ohlsen, H. Body composition, Endurance, Strenght, Cross-Sectional area, and density of MM Erector Spinae in Men With and Without Low Back Pain. J. Spinal Disord. 1991, 6, 114–123. [Google Scholar]
- Sung, P.S.; Lammers, A.R.; Danial, P. Different parts of erector spinae muscle fatigability in subjects with and without low back pain. Spine J. 2009, 9, 115–120. [Google Scholar] [CrossRef]
- Lariviè, C.; Gagnon, D.; Loisel, P. The comparison of trunk muscles EMG activation between subjects with and without chronic low back pain during flexion-extension and lateral bending tasks. J. Electromyogr. Kinesiol. 2000, 10, 79–91. [Google Scholar] [CrossRef]
- Sutherlin, M.A.; Hart, J.M. Hip Function and Predictors of Subjective Outcomes in Individuals With a History of Low Back Pain. Athl. Train. Sports Health Care 2015, 7, 108–116. [Google Scholar] [CrossRef]
- McGill, S.; Juker, D.; Kropf, P. Appropriately placed surface EMG electrodes reflect deep muscle activity (psoas, quadratus lumborum, abdominal wall) in the lumbar spine. J. Biomech. 1996, 29, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Maté-Muñoz, J.L.; Lougedo, J.H.; Barba, M.; Cañuelo-Márquez, A.M.; Guodemar-Pérez, J.; García-Fernández, P.; Lozano-Estevan, M.d.C.; Alonso-Melero, R.; Sánchez-Calabuig, M.A.; Ruíz-López, M.; et al. Cardiometabolic and Muscular Fatigue Responses to Different CrossFit® Workouts. J. Sports Sci. Med. 2018, 17, 668–679. [Google Scholar] [PubMed]
- Bobbert, M.F.; van Soest, A.J. Why do people jump the way they do? Exerc. Sport Sci. Rev. 2001, 3, 95–102. [Google Scholar] [CrossRef] [PubMed]
| Variable | Without Insole (M ± SD, 95% IC) | 5 mm Insole (M ± SD, 95% IC) | 10 mm Insole (M ± SD, 95% IC) | 15 mm Insole (M ± SD, 95% IC) | ηp2 | PS | p Time |
|---|---|---|---|---|---|---|---|
| Height CMJ (cm) | 27.75 ± 4.80 (25.50–29.99) | 29.20 ± 5.22 * (26.76–31.64) | 29.24 ± 6.17 (26.35–32.13) | 29.27 ± 6.63 (26.17–32.73) | 0.198 | 0.848 | 0.007 |
| Poser CMJ (watts·kg−1) | 840.90 ± 289.09 (705.60–979.19) | 821.79 ± 129.77 (761.05–882.52) | 821.06 ± 136.18 (757.03–885.09) | 821.67 ± 152.54 (750.27–893.06) | 0.013 | 0.078 | 0.660 |
| Height DJ left (cm) | 11.55 ± 3.81 ‡ (9.76–13.33) | 11.63 ± 4.07 ‡ (9.73–13.54) | 12.31 ± 4.77 (10.07–14.54) | 12.80 ± 3.81 (11.01–14.58) | 0.183 | 0.812 | 0.011 |
| Height DJ right (cm) | 11.53 ± 3.25 (10.01–13.05) | 12.21 ± 4.04 (10.32–14.12) | 12.79 ± 2.79 (11.48–14.10) | 12.86 ± 3.55 (11.19–14.52) | 0.101 | 0.485 | 0.114 |
| Variable | Limbs | 5 mm Insole (M ± SD, 95% IC) | 10 mm Insole (M ± SD, 95% IC) | 15 mm Insole (M ± SD, 95% IC) | p Time ηp2 SP | p Group ηp2 SP | p Time × Group ηp2 SP |
|---|---|---|---|---|---|---|---|
| Gluteus medius (mV) | Left | 35.46 ± 43.11 (17.67–53.24) | 30.36 ± 30.31 (17.89–42.83) | 28.97 ± 26.98 (16.47–41.47) | 0.027 * 0.059 0.643 | 0.690 0.002 0.068 | 0.814 0.002 0.069 |
| Right | 38.80 ± 59.47 (21.02–56.59) | 33.67 ± 41.64 (21.20–46.14) | 34.25 ± 44.00 (21.76–46.75) | ||||
| Erector spinae (%) | Left | 39.21 ± 40.20 (27.47–50.95) | 40.50 ± 48.96 (25.68–55.32) | 35.74 ± 47.62 (21.04–50.43) | 0.987 <0.001 0.052 | 0.354 0.013 0.151 | 0.706 0.005 0.105 |
| Right | 30.35 ± 27.13 (18.61–42.10) | 29.87 ± 36.70 (15.05–44.68) | 33.04 ± 37.63 (18.34–47.73) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benito de Pedro, M.; Benito de Pedro, A.I.; Aguilera Rubio, Á.; Maté Muñoz, J.L.; Hernández Lougedo, J. Changes in the Activity of the Erector Spinae and Gluteus Medius Muscles with the Presence of Simulated Lower Limb Dysmetria. Sensors 2024, 24, 1223. https://doi.org/10.3390/s24041223
Benito de Pedro M, Benito de Pedro AI, Aguilera Rubio Á, Maté Muñoz JL, Hernández Lougedo J. Changes in the Activity of the Erector Spinae and Gluteus Medius Muscles with the Presence of Simulated Lower Limb Dysmetria. Sensors. 2024; 24(4):1223. https://doi.org/10.3390/s24041223
Chicago/Turabian StyleBenito de Pedro, María, Ana Isabel Benito de Pedro, Ángela Aguilera Rubio, Jose Luis Maté Muñoz, and Juan Hernández Lougedo. 2024. "Changes in the Activity of the Erector Spinae and Gluteus Medius Muscles with the Presence of Simulated Lower Limb Dysmetria" Sensors 24, no. 4: 1223. https://doi.org/10.3390/s24041223
APA StyleBenito de Pedro, M., Benito de Pedro, A. I., Aguilera Rubio, Á., Maté Muñoz, J. L., & Hernández Lougedo, J. (2024). Changes in the Activity of the Erector Spinae and Gluteus Medius Muscles with the Presence of Simulated Lower Limb Dysmetria. Sensors, 24(4), 1223. https://doi.org/10.3390/s24041223







