Recent Advances and Current Trends in Transmission Tomographic Diffraction Microscopy
Abstract
:1. Introduction
2. Tomographic Diffraction Microscopy
2.1. General Principles: Helmholtz Equation and First-Order Born Approximation
2.2. General Principles: Helmholtz Equation and Rytov Approximation
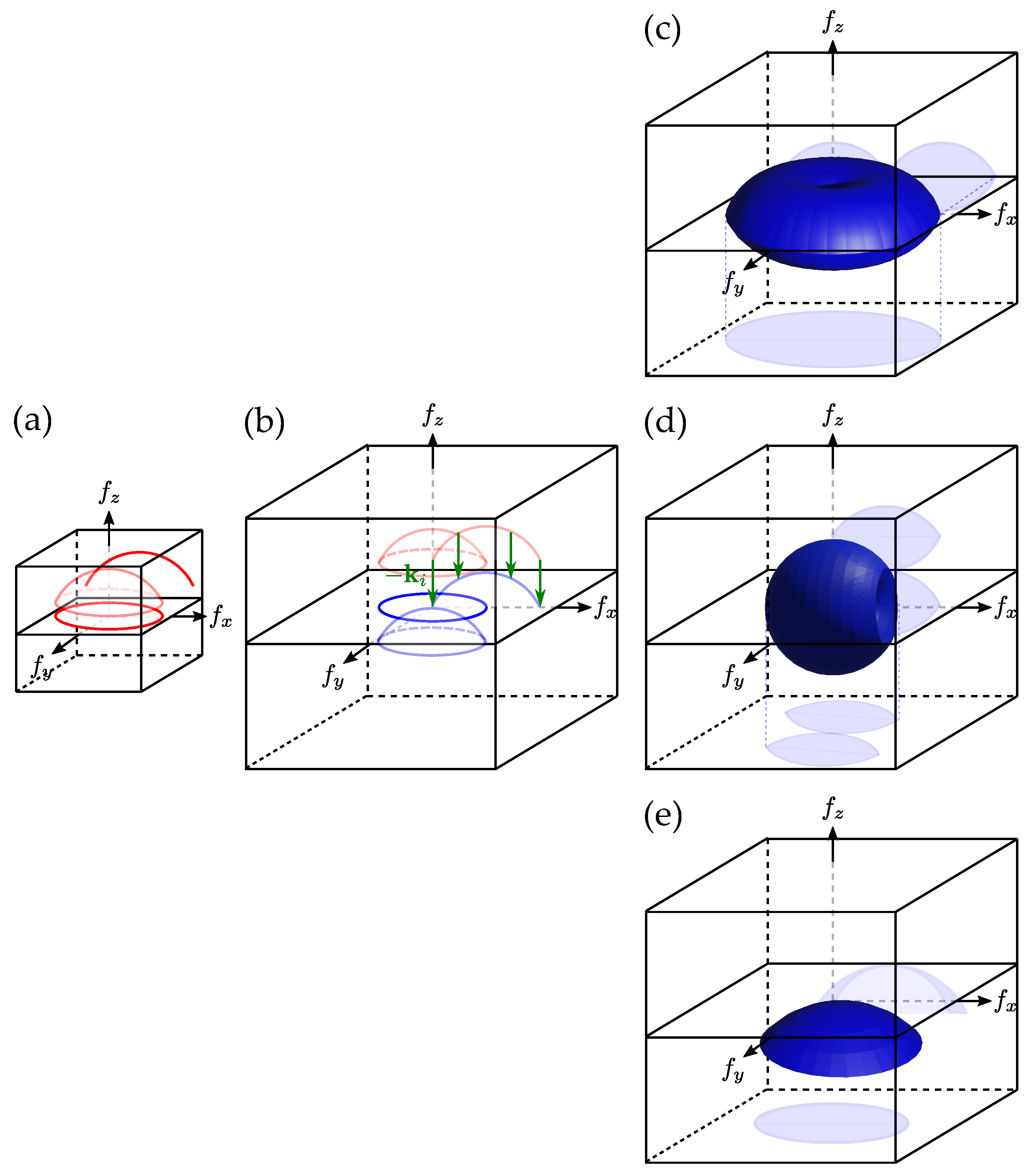
2.3. 3D Aperture Synthesis
- Scanning the illumination over the object;
- Rotating the object within a fixed illumination;
- Varying the illumination wavelength.
2.3.1. 3D Aperture Synthesis with Illumination Sweep
2.3.2. 3D Aperture Synthesis with Sample Rotation
2.3.3. 3D Aperture Synthesis with Illumination Wavelength Variation
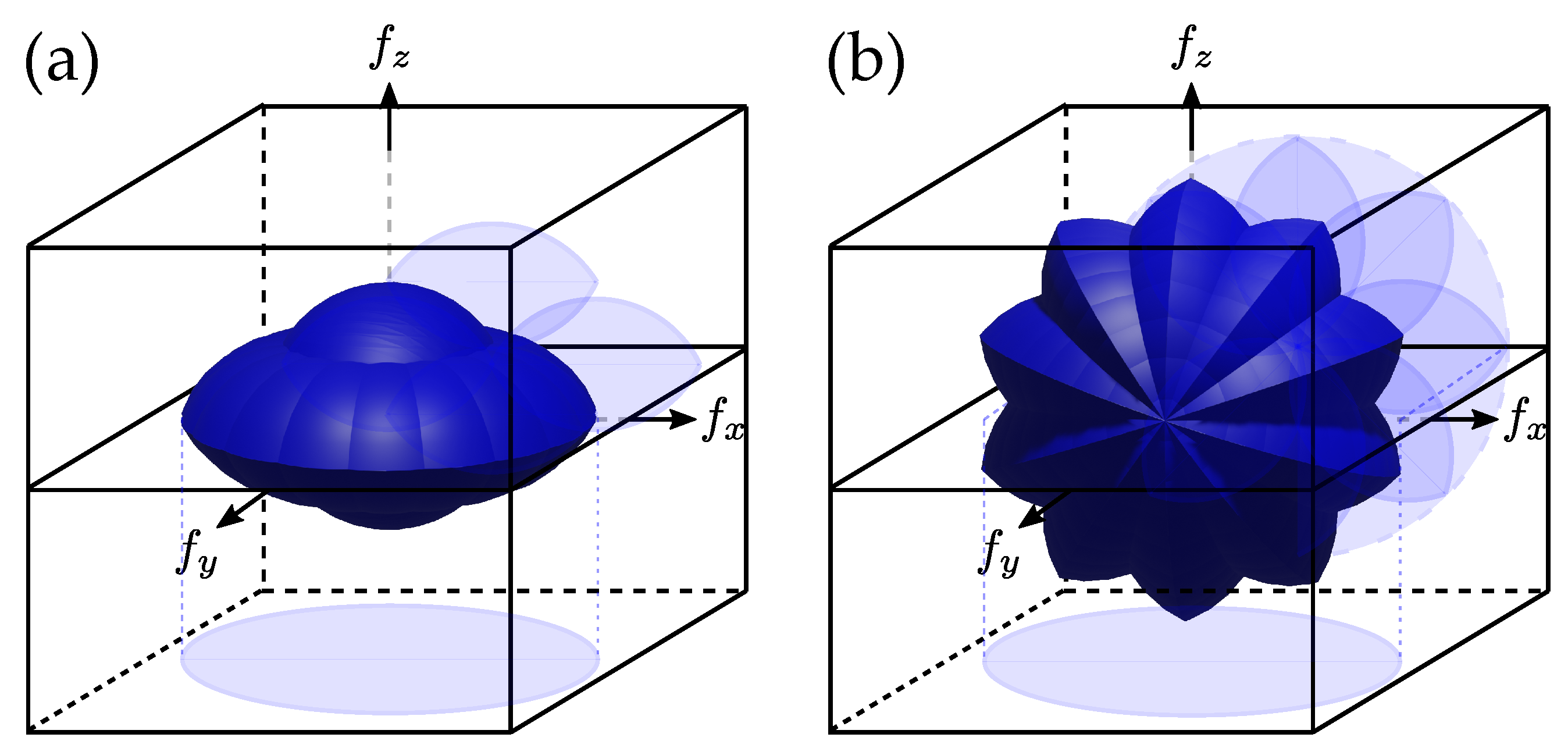
2.3.4. 3D Aperture Synthesis with Combined Approaches
2.3.5. Examples of Achievement
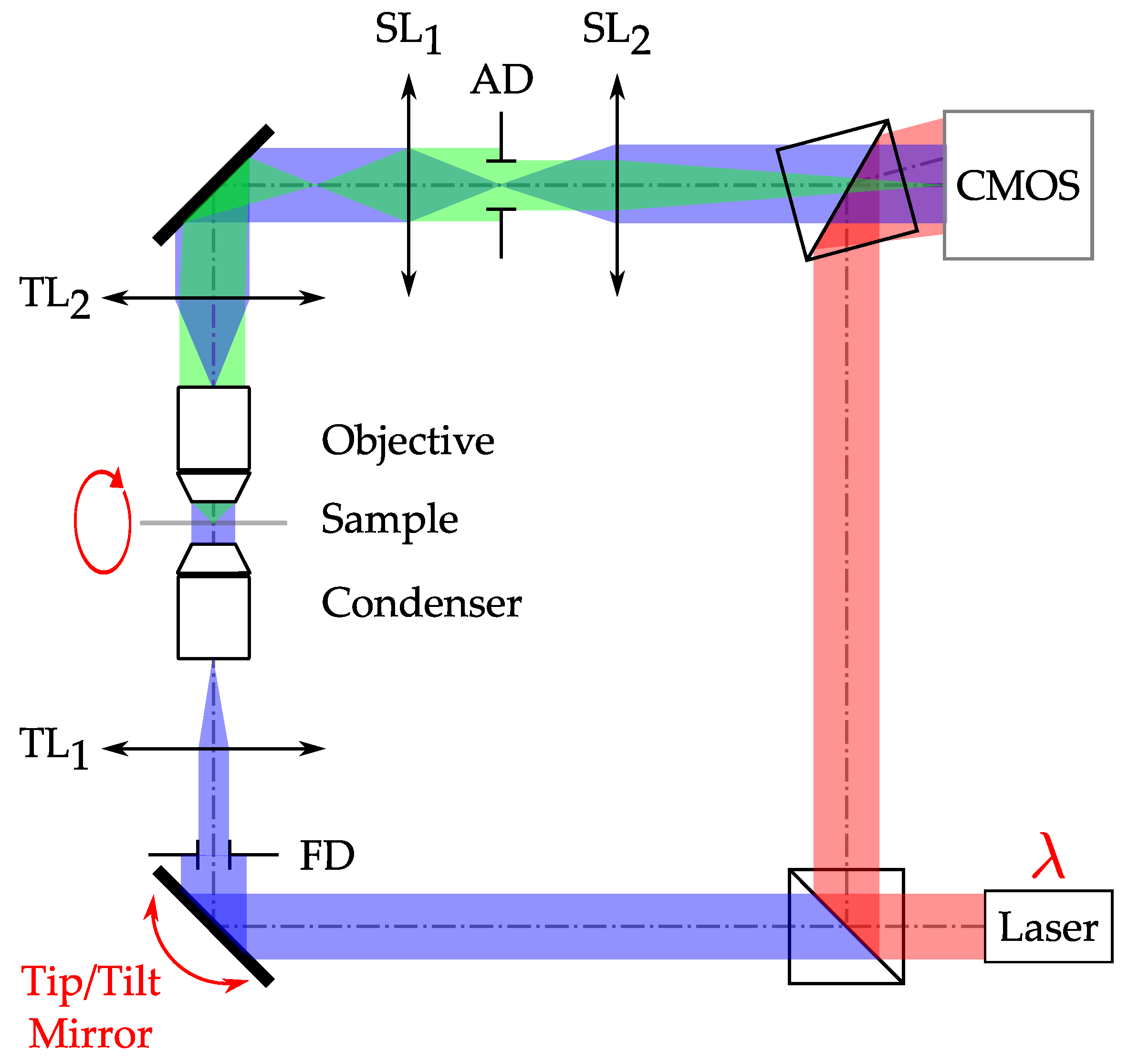
2.4. Implementation
3. Data Reconstruction and Multimodal Imaging
- Holograms that will be used for simulation are selected according to the illumination angle, which is equivalent to the illumination selection performed by the condenser in a conventional microscope.
- Optional spectrum filtering can be envisaged. This is the numerical equivalent of the backfocal plane pupil filter that can be found in some specific microscope objectives. For instance, considering the case of the Zernike phase-contrast microscopy [128], one can multiply the following pupil filter to the hologram’s Fourier transform:which allows for both the phase shifting and attenuation of the specular illumination contribution (part of the illumination beam that does not encounter the sample). In this situation, the attenuation can be defined as follows:where is the radius of the pupil filter, and is the attenuation ratio. Here, the filter is centered on the specular illumination coordinate . The same applies to the phase shift , which can be expressed as follows:It should be noted that, in the case of the Zernike phase contrast, the condenser selects illumination angles along an annulus. Therefore, if we add all the processed holograms, the proposed filter will map an annulus in the Fourier space. This annulus is the perfect equivalent to the one existing in a phase-contrast microscope objective.
- The multiplexing of each processed hologram is finally performed by summing the calculated contrast intensity.
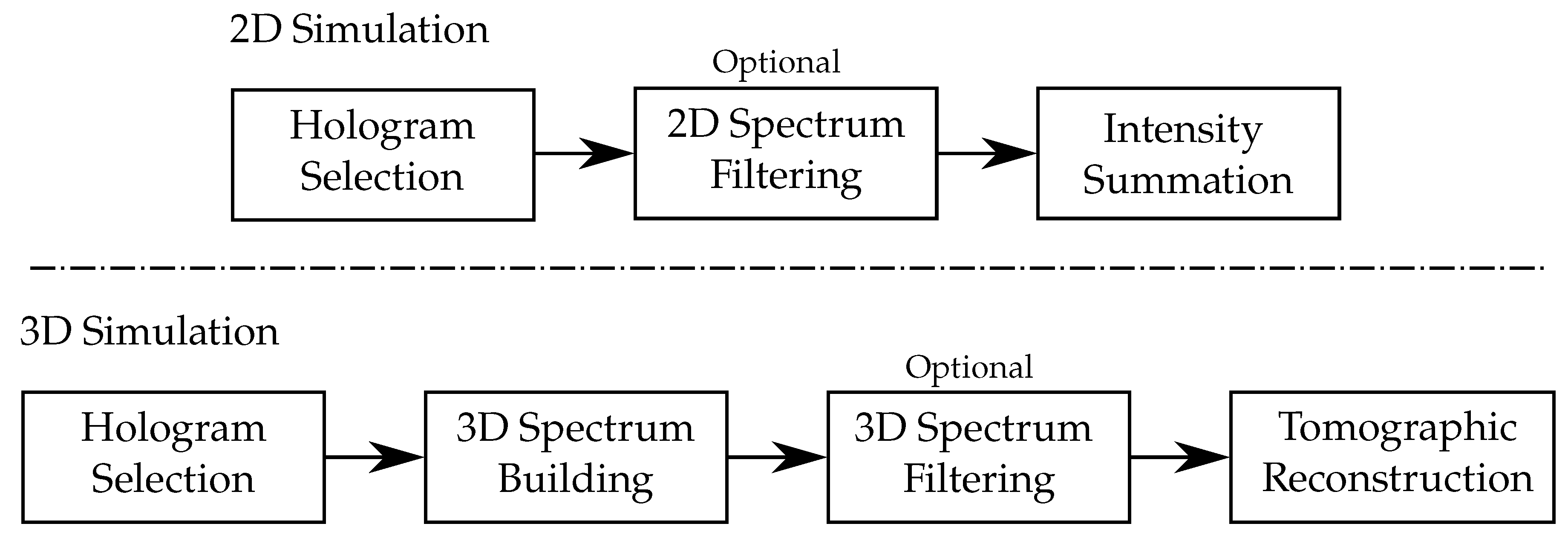
4. Advanced Reconstruction Methods
4.1. Iterative Reconstructions
4.2. Forward Models
4.2.1. Multi-Layer Models
- Propagation of the incident field (total field from the previous layer) with the angular spectrum method [164],
- Calculation of the scattered field under the first Born approximation, i.e., convolution between the modified Green function and the product of the incident field by the potential (Equation (12) on the slice thickness).
4.2.2. Full 3D Models
5. Accounting for the Vectorial Nature of Light
6. Present and Future Trends
6.1. Hardware Simplification
6.2. Functionalization of Tomography
6.3. Metrological Approaches
6.4. Promising Applications
6.5. Current Trends and Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–796. [Google Scholar] [CrossRef]
- Balzarotti, F.; Eilers, Y.; Gwosch, K.C.; Gynnå, A.H.; Westphal, V.; Stefani, F.D.; Elf, J.; Hell, S.W. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 2017, 355, 606–612. [Google Scholar] [CrossRef]
- Icha, J.; Weber, M.; Waters, J.C.; Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays 2017, 39, 1700003. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kesarwani, S.; Javoor, M.G.; Vinothkumar, K.R.; Sirajuddin, M. Structural insights into actin filament recognition by commonly used cellular actin markers. EMBO J. 2020, 39, e104006. [Google Scholar] [CrossRef] [PubMed]
- Pospich, S.; Merino, F.; Raunser, S. Structural Effects and Functional Implications of Phalloidin and Jasplakinolide Binding to Actin Filaments. Structure 2020, 28, 437–449.e5. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Depeursinge, C.; Popescu, G. Quantitative phase imaging in biomedicine. Nat. Photonics 2018, 12, 578–589. [Google Scholar] [CrossRef]
- Ou, X.; Horstmeyer, R.; Yang, C.; Zheng, G. Quantitative phase imaging via Fourier ptychographic microscopy. Opt. Lett. 2013, 38, 4845–4848. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, Y. Quantitative phase imaging unit. Opt. Lett. 2014, 39, 3630–3633. [Google Scholar] [CrossRef] [PubMed]
- Bokemeyer, A.; Tepasse, P.R.; Quill, L.; Lenz, P.; Rijcken, E.; Vieth, M.; Ding, N.; Ketelhut, S.; Rieder, F.; Kemper, B.; et al. Quantitative Phase Imaging Using Digital Holographic Microscopy Reliably Assesses Morphology and Reflects Elastic Properties of Fibrotic Intestinal Tissue. Sci. Rep. 2019, 9, 19388. [Google Scholar] [CrossRef]
- Gabor, D. A new microscopic principle. Nature 1948, 161, 777–778. [Google Scholar] [CrossRef]
- Schnars, U.; Jüptner, W. Direct recording of holograms by a CCD target and numerical reconstruction. Appl. Opt. 1994, 33, 179–181. [Google Scholar] [CrossRef]
- Schnars, U.; Jüptner, W.P.O. Digital recording and numerical reconstruction of holograms. Meas. Sci. Technol. 2002, 13, R85. [Google Scholar] [CrossRef]
- Verrier, N.; Atlan, M. Off-axis digital hologram reconstruction: Some practical considerations. Appl. Opt. 2011, 50, H136–H146. [Google Scholar] [CrossRef]
- Verrier, N.; Alexandre, D.; Tessier, G.; Gross, M. Holographic microscopy reconstruction in both object and image half-spaces with an undistorted three-dimensional grid. Appl. Opt. 2015, 54, 4672–4677. [Google Scholar] [CrossRef]
- Leith, E.; Upatnieks, J. Reconstructed wavefronts and communication theory. J. Opt. Soc. Am. 1962, 52, 1123–1128. [Google Scholar] [CrossRef]
- Cuche, E.; Marquet, P.; Depeursinge, C. Spatial filtering for zero-order and twin-image elimination in digital off-axis holography. Appl. Opt. 2000, 39, 4070–4075. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, I.; Zhang, T. Phase-shifting digital holography. Opt. Lett. 1997, 22, 1268–1270. [Google Scholar] [CrossRef]
- Verrier, N.; Donnarumma, D.; Tessier, G.; Gross, M. High numerical aperture holographic microscopy reconstruction with extended z range. Appl. Opt. 2015, 54, 9540–9547. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E. Three-dimensional structure determination of semi-transparent objects from holographic data. Opt. Commun. 1969, 1, 153–156. [Google Scholar] [CrossRef]
- Kawata, S.; Touki, Y.; Minami, S. Optical Microscopic Tomography. In Inverse Optics II; Bates, R.H., Devaney, A.J., Eds.; International Society for Optics and Photonics, SPIE: Philadelphia, PA, USA, 1985; Volume 0558, pp. 15–20. [Google Scholar] [CrossRef]
- Kawata, S.; Nakamura, O.; Minami, S. Optical microscope tomography. I. Support constraint. J. Opt. Soc. Am. A 1987, 4, 292–297. [Google Scholar] [CrossRef]
- Nakamura, O.; Kawata, S.; Minami, S. Optical microscope tomography. II. Nonnegative constraint by a gradient-projection method. J. Opt. Soc. Am. A 1988, 5, 554–561. [Google Scholar] [CrossRef]
- Lauer, V. New approach to optical diffraction tomography yielding a vector equation of diffraction tomography and a novel tomographic microscope. J. Microsc. 2002, 205, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Charrière, F.; Marian, A.; Montfort, F.; Kuehn, J.; Colomb, T.; Cuche, E.; Marquet, P.; Depeursinge, C. Cell refractive index tomography by digital holographic microscopy. Opt. Lett. 2006, 31, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Fang-Yen, C.; Badizadegan, K.; Oh, S.; Lue, N.; Dasari, R.R.; Feld, M.S. Tomographic phase microscopy. Nat. Methods 2007, 4, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Ralston, T.S.; Marks, D.L.; Scott Carney, P.; Boppart, S.A. Interferometric synthetic aperture microscopy. Nat. Phys. 2007, 3, 129–134. [Google Scholar] [CrossRef]
- Davis, B.J.; Marks, D.L.; Ralston, T.S.; Carney, P.S.; Boppart, S.A. Interferometric Synthetic Aperture Microscopy: Computed Imaging for Scanned Coherent Microscopy. Sensors 2008, 8, 3903–3931. [Google Scholar] [CrossRef]
- Debailleul, M.; Simon, B.; Georges, V.; Haeberlé, O.; Lauer, V. Holographic microscopy and diffractive microtomography of transparent samples. Meas. Sci. Technol. 2008, 19, 074009. [Google Scholar] [CrossRef]
- Debailleul, M.; Georges, V.; Simon, B.; Morin, R.; Haeberlé, O. High-resolution three-dimensional tomographic diffractive microscopy of transparent inorganic and biological samples. Opt. Lett. 2009, 34, 79–81. [Google Scholar] [CrossRef]
- Cotte, Y.; Toy, F.; Jourdain, P.; Pavillon, N.; Boss, D.; Magistretti, P.; Marquet, P.; Depeursinge, C. Marker-free phase nanoscopy. Nat. Photonics 2013, 7, 113–117. [Google Scholar] [CrossRef]
- Agard, D.A. Optical Sectioning Microscopy: Cellular Architecture in Three Dimensions. Annu. Rev. Biophys. Bioeng. 1984, 13, 191–219. [Google Scholar] [CrossRef]
- Vertu, S.; Delaunay, J.J.; Yamada, I.; Haeberlé, O. Diffraction microtomography with sample rotation: Influence of a missing apple core in the recorded frequency space. Cent. Eur. J. Phys. 2009, 7, 22–31. [Google Scholar] [CrossRef]
- Vertu, S.; Flügge, J.; Delaunay, J.J.; Haeberlé, O. Improved and isotropic resolution in tomographic diffractive microscopy combining sample and illumination rotation. Cent. Eur. J. Phys. 2011, 9, 969–974. [Google Scholar] [CrossRef]
- Vinoth, B.; Lai, X.J.; Lin, Y.C.; Tu, H.Y.; Cheng, C.J. Integrated dual-tomography for refractive index analysis of free-floating single living cell with isotropic superresolution. Sci. Rep. 2018, 8, 5943. [Google Scholar] [CrossRef]
- Simon, B.; Debailleul, M.; Houkal, M.; Ecoffet, C.; Bailleul, J.; Lambert, J.; Spangenberg, A.; Liu, H.; Soppera, O.; Haeberlé, O. Tomographic diffractive microscopy with isotropic resolution. Optica 2017, 4, 460–463. [Google Scholar] [CrossRef]
- Available online: https://www.nanolive.ch/ (accessed on 20 February 2024).
- Available online: https://www.tomocube.com/ (accessed on 20 February 2024).
- Available online: https://phioptics.com/ (accessed on 20 February 2024).
- Dändliker, R.; Weiss, K. Reconstruction of the three-dimensional refractive index from scattered waves. Opt. Commun. 1970, 1, 323–328. [Google Scholar] [CrossRef]
- Coupland, J.M.; Lobera, J. Holography, tomography and 3D microscopy as linear filtering operations. Meas. Sci. Technol. 2008, 19, 074012. [Google Scholar] [CrossRef]
- McCutchen, C.W. Generalized Aperture and the Three-Dimensional Diffraction Image. J. Opt. Soc. Am. 1964, 54, 240–244. [Google Scholar] [CrossRef]
- Devaney, A.J. Inverse-scattering theory within the Rytov approximation. Opt. Lett. 1981, 6, 374–376. [Google Scholar] [CrossRef]
- Chen, B.; Stamnes, J.J. Validity of diffraction tomography based on the first Born and the first Rytov approximations. Appl. Opt. 1998, 37, 2996–3006. [Google Scholar] [CrossRef]
- Sentenac, A.; Mertz, J. Unified description of three-dimensional optical diffraction microscopy: From transmission microscopy to optical coherence tomography: Tutorial. J. Opt. Soc. Am. A 2018, 35, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Streibl, N. Three-dimensional imaging by a microscope. J. Opt. Soc. Am. A 1985, 2, 121–127. [Google Scholar] [CrossRef]
- Stallinga, S.; Radmacher, N.; Delon, A.; Enderlein, J. Optimal transfer functions for bandwidth-limited imaging. Phys. Rev. Res. 2022, 4, 023003. [Google Scholar] [CrossRef]
- Lin, Y.C.; Cheng, C.J. Sectional imaging of spatially refractive index distribution using coaxial rotation digital holographic microtomography. J. Opt. 2014, 16, 065401. [Google Scholar] [CrossRef]
- Bachelot, R.; Ecoffet, C.; Deloeil, D.; Royer, P.; Lougnot, D.J. Integration of micrometer-sized polymer elements at the end of optical fibers by free-radical photopolymerization. Appl. Opt. 2001, 40, 5860–5871. [Google Scholar] [CrossRef] [PubMed]
- Kozacki, T.; Krajewski, R.; Kujawińska, M. Reconstruction of refractive-index distribution in off-axis digital holography optical diffraction tomographic system. Opt. Express 2009, 17, 13758–13767. [Google Scholar] [CrossRef] [PubMed]
- Kostencka, J.; Kozacki, T.; Kuś, A.; Kujawińska, M. Accurate approach to capillary-supported optical diffraction tomography. Opt. Express 2015, 23, 7908–7923. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, J.; Park, Y. Simultaneous 3D visualization and position tracking of optically trapped particles using optical diffraction tomography. Optica 2015, 2, 343–346. [Google Scholar] [CrossRef]
- Vouldis, A.T.; Kechribaris, C.N.; Maniatis, T.A.; Nikita, K.S.; Uzunoglu, N.K. Investigating the enhancement of three-dimensional diffraction tomography by using multiple illumination planes. J. Opt. Soc. Am. A 2005, 22, 1251–1262. [Google Scholar] [CrossRef]
- Vouldis, A.T.; Kechribaris, C.N.; Maniatis, T.A.; Nikita, K.S.; Uzunoglu, N.K. Three-Dimensional Diffraction Tomography Using Filtered Backpropagation and Multiple Illumination Planes. IEEE Trans. Instrum. Meas. 2006, 55, 1975–1984. [Google Scholar] [CrossRef]
- Vertu, S.; Ochiai, M.; Shuzo, M.; Yamada, I.; Delaunay, J.J.; Haeberlé, O.; Okamoto, Y. Optical projection microtomography of transparent objects. In Optical Coherence Tomography and Coherence Techniques III; Optica Publishing Group: Washington, DC, USA, 2007; p. 6627. [Google Scholar]
- Kühn, J.; Montfort, F.; Colomb, T.; Rappaz, B.; Moratal, C.; Pavillon, N.; Marquet, P.; Depeursinge, C. Submicrometer tomography of cells by multiple-wavelength digital holographic microscopy in reflection. Opt. Lett. 2009, 34, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K. Wavelength-scanning digital interference holography for optical section imaging. Opt. Lett. 1999, 24, 1693–1695. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Zhou, R.; Mir, M.; Babacan, S.D.; Carney, P.S.; Goddard, L.L.; Popescu, G. White-light diffraction tomography of unlabelled live cells. Nat. Photonics 2014, 8, 256–263. [Google Scholar] [CrossRef]
- Wang, Z.; Millet, L.; Mir, M.; Ding, H.; Unarunotai, S.; Rogers, J.; Gillette, M.U.; Popescu, G. Spatial light interference microscopy (SLIM). Opt. Express 2011, 19, 1016–1026. [Google Scholar] [CrossRef]
- Wang, Z.; Marks, D.L.; Carney, P.S.; Millet, L.J.; Gillette, M.U.; Mihi, A.; Braun, P.V.; Shen, Z.; Prasanth, S.G.; Popescu, G. Spatial light interference tomography (SLIT). Opt. Express 2011, 19, 19907–19918. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, K.; Oh, J.; Park, Y. Isotropically resolved label-free tomographic imaging based on tomographic moulds for optical trapping. Light. Sci. Appl. 2021, 10, 102. [Google Scholar] [CrossRef]
- Haeberlé, O.; Belkebir, K.; Giovaninni, H.; Sentenac, A. Tomographic diffractive microscopy: Basics, techniques and perspectives. J. Mod. Opt. 2010, 57, 686–699. [Google Scholar] [CrossRef]
- South, F.A.; Liu, Y.Z.; Carney, P.S.; Boppart, S.A. Computed Optical Interferometric Imaging: Methods, Achievements, and Challenges. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 186–196. [Google Scholar] [CrossRef]
- Jin, D.; Zhou, R.; Yaqoob, Z.; So, P.T.C. Tomographic phase microscopy: Principles and applications in bioimaging [Invited]. J. Opt. Soc. Am. B 2017, 34, B64–B77. [Google Scholar] [CrossRef]
- Kuś, A.; Krauze, W.; Makowski, P.L.; Kujawińska, M. Holographic tomography: Hardware and software solutions for 3D quantitative biomedical imaging (Invited paper). ETRI J. 2019, 41, 61–72. [Google Scholar] [CrossRef]
- Balasubramani, V.; Kuś, A.; Tu, H.Y.; Cheng, C.J.; Baczewska, M.; Krauze, W.; Kujawińska, M. Holographic tomography: Techniques and biomedical applications [Invited]. Appl. Opt. 2021, 60, B65–B80. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kandel, M.E.; Popescu, G. Spatial light interference microscopy: Principle and applications to biomedicine. Adv. Opt. Photon. 2021, 13, 353–425. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.; Haeberle, O. Unconventional Optical Imaging for Biology; ISTE-Wiley: London, UK, 2024. [Google Scholar]
- Kuś, A.; Dudek, M.; Kemper, B.; Kujawińska, M.; Vollmer, A. Tomographic phase microscopy of living three-dimensional cell cultures. J. Biomed. Opt. 2014, 19, 046009. [Google Scholar] [CrossRef] [PubMed]
- Fienup, J.R. Phase retrieval algorithms: A comparison. Appl. Opt. 1982, 21, 2758–2769. [Google Scholar] [CrossRef] [PubMed]
- Taddese, A.M.; Verrier, N.; Debailleul, M.; Courbot, J.B.; Haeberlé, O. Optimizing sample illumination scanning in transmission tomographic diffractive microscopy. Appl. Opt. 2021, 60, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Taddese, A.M.; Lo, M.; Verrier, N.; Debailleul, M.; Haeberlé, O. Jones tomographic diffractive microscopy with a polarized array sensor. Opt. Express 2023, 31, 9034–9051. [Google Scholar] [CrossRef]
- Clerc, F.L.; Gross, M.; Collot, L. Synthetic-aperture experiment in the visible with on-axis digital heterodyne holography. Opt. Lett. 2001, 26, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.; Quiroga, J.A.; Belenguer, T. Phase-shifting interferometry based on principal component analysis. Opt. Lett. 2011, 36, 1326–1328. [Google Scholar] [CrossRef]
- Vargas, J.; Wang, S.; Gómez-Pedrero, J.A.; Estrada, J.C. Robust weighted principal components analysis demodulation algorithm for phase-shifting interferometry. Opt. Express 2021, 29, 16534–16546. [Google Scholar] [CrossRef]
- Simon, B.; Debailleul, M.; Georges, V.; Lauer, V.; Haeberlé, O. Tomographic diffractive microscopy of transparent samples. Eur. Phys. J.-Appl. Phys. 2008, 44, 29–35. [Google Scholar] [CrossRef]
- Simon, B.; Debailleul, M.; Beghin, A.; Tourneur, Y.; Haeberlé, O. High-resolution tomographic diffractive microscopy of biological samples. J. Biophotonics 2010, 3, 462–467. [Google Scholar] [CrossRef]
- Liu, F.; Chen, C.H. Electrohydrodynamic cone-jet bridges: Stability diagram and operating modes. J. Electrost. 2014, 72, 330–335. [Google Scholar] [CrossRef]
- Foucault, L.; Verrier, N.; Debailleul, M.; Courbot, J.B.; Colicchio, B.; Simon, B.; Vonna, L.; Haeberlé, O. Versatile transmission/reflection tomographic diffractive microscopy approach. J. Opt. Soc. Am. A 2019, 36, C18–C27. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Choi, W.; Lue, N.; Dasari, R.R.; Yaqoob, Z. Stain-free quantification of chromosomes in live cells using regularized tomographic phase microscopy. PLoS ONE 2012, 7, e49502. [Google Scholar] [CrossRef] [PubMed]
- Kostencka, J.; Kozacki, T.; Kuś, A.; Kemper, B.; Kujawińska, M. Holographic tomography with scanning of illumination: Space-domain reconstruction for spatially invariant accuracy. Biomed. Opt. Express 2016, 7, 4086–4101. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Kawata, S.; Minami, S. Three-dimensional phase-contrast imaging by a computed-tomography microscope. Appl. Opt. 1992, 31, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, Y.; Fang-Yen, C.; Sung, Y.; Dasari, R.R.; Feld, M.S.; Choi, W. High-speed synthetic aperture microscopy for live cell imaging. Opt. Lett. 2011, 36, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Berdeu, A.; Laperrousaz, B.; Bordy, T.; Mandula, O.; Morales, S.; Gidrol, X.; Picollet-D’hahan, N.; Allier, C. Lens-free microscopy for 3D+ time acquisitions of 3D cell culture. Sci. Rep. 2018, 8, 16135. [Google Scholar] [CrossRef] [PubMed]
- Zdańkowski, P.; Winnik, J.; Patorski, K.; Gocłowski, P.; Ziemczonok, M.; Józwik, M.; Kujawińska, M.; Trusiak, M. Common-path intrinsically achromatic optical diffraction tomography. Biomed. Opt. Express 2021, 12, 4219–4234. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.S.; Sheppard, C.J.R. Image formation in holographic tomography: High-aperture imaging conditions. Appl. Opt. 2009, 48, H168–H175. [Google Scholar] [CrossRef]
- Momey, F.; Berdeu, A.; Bordy, T.; Dinten, J.M.; Marcel, F.K.; Picollet-D’Hahan, N.; Gidrol, X.; Allier, C. Lensfree diffractive tomography for the imaging of 3D cell cultures. Biomed. Opt. Express 2016, 7, 949–962. [Google Scholar] [CrossRef]
- Kuś, A.; Krauze, W.; Kujawińska, M. Active limited-angle tomographic phase microscope. J. Biomed. Opt. 2015, 20, 111216. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Eldridge, W.J.; Wax, A.; Izatt, J. Refractive index tomography with structured illumination. Optica 2017, 4, 537–545. [Google Scholar] [CrossRef]
- Balasubramani, V.; Tu, H.Y.; Lai, X.J.; Cheng, C.J. Adaptive wavefront correction structured illumination holographic tomography. Sci. Rep. 2019, 9, 10489. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, C.H.; Vinoth, B.; Chu, D.; Lai, X.J.; Cheng, C.J. A compact synthetic aperture digital holographic microscope with mechanical movement-free beam scanning and optimized active aberration compensation for isotropic resolution enhancement. Opt. Lasers Eng. 2020, 134, 106251. [Google Scholar] [CrossRef]
- Park, C.; Lee, K.; Baek, Y.; Park, Y. Low-coherence optical diffraction tomography using a ferroelectric liquid crystal spatial light modulator. Opt. Express 2020, 28, 39649–39659. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Popescu, G. Spatial Light Interference Microscopy (SLIM) using twisted-nematic liquid-crystal modulation. Biomed. Opt. Express 2013, 4, 1571–1583. [Google Scholar] [CrossRef]
- Shin, S.; Kim, K.; Yoon, J.; Park, Y. Active illumination using a digital micromirror device for quantitative phase imaging. Opt. Lett. 2015, 40, 5407–5410. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, K.; Kim, T.; Yoon, J.; Hong, K.; Park, J.; Park, Y. Optical diffraction tomography using a digital micromirror device for stable measurements of 4D refractive index tomography of cells. In Proceedings of the Quantitative Phase Imaging II. International Society for Optics and Photonics, San Francisco, CA, USA, 17 June 2016; Volume 9718, p. 971814. [Google Scholar]
- Lee, K.; Kim, K.; Kim, G.; Shin, S.; Park, Y. Time-multiplexed structured illumination using a DMD for optical diffraction tomography. Opt. Lett. 2017, 42, 999–1002. [Google Scholar] [CrossRef]
- Jin, D.; Zhou, R.; Yaqoob, Z.; So, P.T.C. Dynamic spatial filtering using a digital micromirror device for high-speed optical diffraction tomography. Opt. Express 2018, 26, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Dhalla, A.H.; Izatt, J. Structured oblique illumination microscopy for enhanced resolution imaging of non-fluorescent, coherently scattering samples. Biomed. Opt. Express 2012, 3, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wen, K.; Li, J.; Ma, Y.; Zheng, J.; An, S.; Min, J.; Zalevsky, Z.; Yao, B.; Gao, P. Multi-harmonic structured illumination-based optical diffraction tomography. Appl. Opt. 2023, 62, 9199–9206. [Google Scholar] [CrossRef] [PubMed]
- Wicker, K.; Heintzmann, R. Resolving a misconception about structured illumination. Nat. Photonics 2014, 8, 342–344. [Google Scholar] [CrossRef]
- Isikman, S.O.; Bishara, W.; Mavandadi, S.; Yu, F.W.; Feng, S.; Lau, R.; Ozcan, A. Lens-free optical tomographic microscope with a large imaging volume on a chip. Proc. Natl. Acad. Sci. USA 2011, 108, 7296–7301. [Google Scholar] [CrossRef]
- Horstmeyer, R.; Chung, J.; Ou, X.; Zheng, G.; Yang, C. Diffraction tomography with Fourier ptychography. Optica 2016, 3, 827–835. [Google Scholar] [CrossRef]
- Gorski, W.; Osten, W. Tomographic imaging of photonic crystal fibers. Opt. Lett. 2007, 32, 1977–1979. [Google Scholar] [CrossRef]
- Dragomir, N.; Goh, X.M.; Roberts, A. Three-dimensional refractive index reconstruction with quantitative phase tomography. Microsc. Res. Tech. 2008, 71, 5–10. [Google Scholar] [CrossRef]
- Kostencka, J.; Kozacki, T.; Józwik, M. Holographic tomography with object rotation and two-directional off-axis illumination. Opt. Express 2017, 25, 23920–23934. [Google Scholar] [CrossRef]
- Wedberg, T.C.; Wedberg, W.C. Tomographic reconstruction of the cross-sectional refractive index distribution in semi-transparent, birefringent fibres. J. Microsc. 1995, 177, 53–67. [Google Scholar] [CrossRef]
- Malek, M.; Khelfa, H.; Picart, P.; Mounier, D.; Poilâne, C. Microtomography imaging of an isolated plant fiber: A digital holographic approach. Appl. Opt. 2016, 55, A111–A121. [Google Scholar] [CrossRef] [PubMed]
- Lue, N.; Choi, W.; Popescu, G.; Badizadegan, K.; Dasari, R.R.; Feld, M.S. Synthetic aperture tomographic phase microscopy for 3D imaging of live cells in translational motion. Opt. Express 2008, 16, 16240–16246. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Habaza, M.; Gilboa, B.; Roichman, Y.; Shaked, N.T. Tomographic phase microscopy with 180° rotation of live cells in suspension by holographic optical tweezers. Opt. Lett. 2015, 40, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-c.; Chen, H.-C.; Tu, H.-Y.; Liu, C.-Y.; Cheng, C.-J. Optically driven full-angle sample rotation for tomographic imaging in digital holographic microscopy. Opt. Lett. 2017, 42, 1321–1324. [Google Scholar] [CrossRef]
- Kim, K.; Park, Y. Tomographic active optical trapping of arbitrarily shaped objects by exploiting 3D refractive index maps. Nat. Commun. 2017, 8, 15340. [Google Scholar] [CrossRef]
- Kreysing, M.K.; Kießling, T.; Fritsch, A.; Dietrich, C.; Guck, J.R.; Käs, J.A. The optical cell rotator. Opt. Express 2008, 16, 16984–16992. [Google Scholar] [CrossRef]
- Sun, J.; Yang, B.; Koukourakis, N.; Guck, J.; Czarske, J.W. AI-driven projection tomography with multicore fibre-optic cell rotation. Nat. Commun. 2024, 15, 147. [Google Scholar] [CrossRef]
- Jones, T.B. Basic theory of dielectrophoresis and electrorotation. IEEE Eng. Med. Biol. Mag. 2003, 22, 33–42. [Google Scholar] [CrossRef]
- Kelbauskas, L.; Shetty, R.; Cao, B.; Wang, K.C.; Smith, D.; Wang, H.; Chao, S.H.; Gangaraju, S.; Ashcroft, B.; Kritzer, M.; et al. Optical computed tomography for spatially isotropic four-dimensional imaging of live single cells. Sci. Adv. 2017, 3, e1602580. [Google Scholar] [CrossRef] [PubMed]
- Habaza, M.; Kirschbaum, M.; Guernth-Marschner, C.; Dardikman, G.; Barnea, I.; Korenstein, R.; Duschl, C.; Shaked, N.T. Rapid 3D refractive-index imaging of live cells in suspension without labeling using dielectrophoretic cell rotation. Adv. Sci. 2017, 4, 1600205. [Google Scholar] [CrossRef]
- Ahmed, D.; Ozcelik, A.; Bojanala, N.; Nama, N.; Upadhyay, A.; Chen, Y.; Hanna-Rose, W.; Huang, T.J. Rotational manipulation of single cells and organisms using acoustic waves. Nat. Commun. 2016, 7, 11085. [Google Scholar] [CrossRef]
- Cacace, T.; Memmolo, P.; Villone, M.M.; De Corato, M.; Mugnano, M.; Paturzo, M.; Ferraro, P.; Maffettone, P.L. Assembling and rotating erythrocyte aggregates by acoustofluidic pressure enabling full phase-contrast tomography. Lab Chip 2019, 19, 3123–3132. [Google Scholar] [CrossRef]
- Abbessi, R.; Verrier, N.; Taddese, A.M.; Laroche, S.; Debailleul, M.; Lo, M.; Courbot, J.B.; Haeberlé, O. Multimodal image reconstruction from tomographic diffraction microscopy data. J. Microsc. 2022, 288, 193–206. [Google Scholar] [CrossRef]
- Samson, E.C.; Blanca, C.M. Dynamic contrast enhancement in widefield microscopy using projector-generated illumination patterns. New J. Phys. 2007, 9, 363. [Google Scholar] [CrossRef]
- Fan, X.; Healy, J.J.; O’Dwyer, K.; Hennelly, B.M. Label-free color staining of quantitative phase images of biological cells by simulated Rheinberg illumination. Appl. Opt. 2019, 58, 3104–3114. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakov, G.; Levin, G.; Minaev, V.; Latushko, M.; Nekrasov, N.; Pickalov, V. Differential interference contrast tomography. Opt. Lett. 2016, 41, 3037–3040. [Google Scholar] [CrossRef] [PubMed]
- Bailleul, J.; Simon, B.; Debailleul, M.; Haeberlé, O. An Introduction to Tomographic Diffractive Microscopy. In Micro- and Nanophotonic Technologies; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2017; Chapter 18; pp. 425–442. [Google Scholar] [CrossRef]
- Chang, T.; Shin, S.; Lee, M.; Park, Y. Computational approach to dark-field optical diffraction tomography. Apl. Photonics 2020, 5, 040804. [Google Scholar] [CrossRef]
- Rasedujjaman, M.; Affannoukoué, K.; Garcia-Seyda, N.; Robert, P.; Giovannini, H.; Chaumet, P.C.; Theodoly, O.; Valignat, M.P.; Belkebir, K.; Sentenac, A.; et al. Three-dimensional imaging with reflection synthetic confocal microscopy. Opt. Lett. 2020, 45, 3721–3724. [Google Scholar] [CrossRef] [PubMed]
- von, F. Zernike. Beugungstheorie des schneidenver-fahrens und seiner verbesserten form, der phasenkontrastmethode. Physica 1934, 1, 689–704. [Google Scholar]
- Available online: https://github.com/madeba/MTD_transmission/blob/master/Python_Tomo/PretraitementMulti.py (accessed on 20 February 2024).
- Ralston, T.S.; Marks, D.L.; Carney, P.S.; Boppart, S.A. Real-time interferometric synthetic aperture microscopy. Opt. Express 2008, 16, 2555–2569. [Google Scholar] [CrossRef]
- Bailleul, J.; Simon, B.; Debailleul, M.; Liu, H.; Haeberlé, O. GPU acceleration towards real-time image reconstruction in 3D tomographic diffractive microscopy. In Proceedings of the Real-Time Image and Video Processing 2012, SPIE, Brussels, Belgium, 19 April 2012; Volume 8437, pp. 65–79. [Google Scholar]
- Kim, K.; Kim, K.S.; Park, H.; Ye, J.C.; Park, Y. Real-time visualization of 3-D dynamic microscopic objects using optical diffraction tomography. Opt. Express 2013, 21, 32269–32278. [Google Scholar] [CrossRef]
- Ahmad, A.; Shemonski, N.D.; Adie, S.G.; Kim, H.S.; Hwu, W.M.W.; Carney, P.S.; Boppart, S.A. Real-time in vivo computed optical interferometric tomography. Nat. Photonics 2013, 7, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Backoach, O.; Kariv, S.; Girshovitz, P.; Shaked, N.T. Fast phase processing in off-axis holography by CUDA including parallel phase unwrapping. Opt. Express 2016, 24, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Bailleul, J.; Simon, B.; Debailleul, M.; Foucault, L.; Verrier, N.; Haeberlé, O. Tomographic diffractive microscopy: Towards high-resolution 3-D real-time data acquisition, image reconstruction and display of unlabeled samples. Opt. Commun. 2018, 422, 28–37. [Google Scholar] [CrossRef]
- Yasuhiko, O.; Takeuchi, K.; Yamada, H.; Ueda, Y. Multiple-scattering suppressive refractive index tomography for the label-free quantitative assessment of multicellular spheroids. Biomed. Opt. Express 2022, 13, 962–979. [Google Scholar] [CrossRef] [PubMed]
- Yasuhiko, O.; Takeuchi, K. In-silico clearing approach for deep refractive index tomography by partial reconstruction and wave-backpropagation. Light Sci. Appl. 2023, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.S.; Lichtman, J.W. Clarifying tissue clearing. Cell 2015, 162, 246–257. [Google Scholar] [CrossRef]
- Simonetti, F. Multiple scattering: The key to unravel the subwavelength world from the far-field pattern of a scattered wave. Phys. Rev. E 2006, 73, 036619. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.C.; Chew, W.C. Experimental verification of super resolution in nonlinear inverse scattering. Appl. Phys. Lett. 1998, 72, 3080–3082. [Google Scholar] [CrossRef]
- Sentenac, A.; Guérin, C.A.; Chaumet, P.C.; Drsek, F.; Giovannini, H.; Bertaux, N.; Holschneider, M. Influence of multiple scattering on the resolution of an imaging system: A Cramér-Rao analysis. Opt. Express 2007, 15, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Kamilov, U.S.; Papadopoulos, I.N.; Shoreh, M.H.; Goy, A.; Vonesch, C.; Unser, M.; Psaltis, D. Optical Tomographic Image Reconstruction Based on Beam Propagation and Sparse Regularization. IEEE Trans. Comput. Imaging 2016, 2, 59–70. [Google Scholar] [CrossRef]
- Nesterov, Y. Introductory Lectures on Convex Optimization: A Basic Course; Springer Science & Business Media: New York, NY, USA, 2013; Volume 87. [Google Scholar]
- Bertero, M.; Boccacci, P.; De Mol, C. Introduction to Inverse Problems in Imaging; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Chen, M.; Ren, D.; Liu, H.Y.; Chowdhury, S.; Waller, L. Multi-layer Born multiple-scattering model for 3D phase microscopy. Optica 2020, 7, 394–403. [Google Scholar] [CrossRef]
- Unger, K.D.; Chaumet, P.C.; Maire, G.; Sentenac, A.; Belkebir, K. Versatile inversion tool for phaseless optical diffraction tomography. J. Opt. Soc. Am. A 2019, 36, C1–C8. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.A.; Soubies, E.; Goy, A.; Lim, J.; Soulez, F.; Psaltis, D.; Unser, M. Versatile reconstruction framework for diffraction tomography with intensity measurements and multiple scattering. Opt. Express 2018, 26, 2749–2763. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chen, M.; Eckert, R.; Ren, D.; Wu, F.; Repina, N.; Waller, L. High-resolution 3D refractive index microscopy of multiple-scattering samples from intensity images. Optica 2019, 6, 1211–1219. [Google Scholar] [CrossRef]
- Lee, M.; Hugonnet, H.; Park, Y. Inverse problem solver for multiple light scattering using modified Born series. Optica 2022, 9, 177–182. [Google Scholar] [CrossRef]
- Saba, A.; Lim, J.; Ayoub, A.B.; Antoine, E.E.; Psaltis, D. Polarization-sensitive optical diffraction tomography. Optica 2021, 8, 402–408. [Google Scholar] [CrossRef]
- Müller, P.; Schürmann, M.; Guck, J. ODTbrain: A Python library for full-view, dense diffraction tomography. BMC Bioinform. 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Belkebir, K.; Chaumet, P.C.; Sentenac, A. Superresolution in total internal reflection tomography. J. Opt. Soc. Am. A 2005, 22, 1889–1897. [Google Scholar] [CrossRef]
- Maire, G.; Drsek, F.; Girard, J.; Giovannini, H.; Talneau, A.; Konan, D.; Belkebir, K.; Chaumet, P.C.; Sentenac, A. Experimental demonstration of quantitative imaging beyond Abbe’s limit with optical diffraction tomography. Phys. Rev. Lett. 2009, 102, 213905. [Google Scholar] [CrossRef] [PubMed]
- Feit, M.D.; Fleck, J.A. Light propagation in graded-index optical fibers. Appl. Opt. 1978, 17, 3990–3998. [Google Scholar] [CrossRef]
- Feit, M.; Fleck, J. Beam nonparaxiality, filament formation, and beam breakup in the self-focusing of optical beams. JOSA B 1988, 5, 633–640. [Google Scholar] [CrossRef]
- Fan, S.; Smith-Dryden, S.; Li, G.; Saleh, B. Iterative optical diffraction tomography for illumination scanning configuration. Opt. Express 2020, 28, 39904–39915. [Google Scholar] [CrossRef] [PubMed]
- Denneulin, L.; Momey, F.; Brault, D.; Debailleul, M.; Taddese, A.M.; Verrier, N.; Haeberlé, O. GSURE criterion for unsupervised regularized reconstruction in tomographic diffractive microscopy. J. Opt. Soc. Am. A 2022, 39, A52–A61. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, W.; Pan, F. Optical tomographic reconstruction based on multi-slice wave propagation method. Opt. Express 2017, 25, 22595–22607. [Google Scholar] [CrossRef]
- Suski, D.; Winnik, J.; Kozacki, T. Fast multiple-scattering holographic tomography based on the wave propagation method. Appl. Opt. 2020, 59, 1397–1403. [Google Scholar] [CrossRef]
- Moser, S.; Jesacher, A.; Ritsch-Marte, M. Efficient and accurate intensity diffraction tomography of multiple-scattering samples. Opt. Express 2023, 31, 18274–18289. [Google Scholar] [CrossRef]
- Brenner, K.H.; Singer, W. Light propagation through microlenses: A new simulation method. Appl. Opt. 1993, 32, 4984–4988. [Google Scholar] [CrossRef]
- Bao, Y.; Gaylord, T.K. Clarification and unification of the obliquity factor in diffraction and scattering theories: Discussion. JOSA A 2017, 34, 1738–1745. [Google Scholar] [CrossRef]
- Tong, Z.; Ren, X.; Zhang, Z.; Wang, B.; Miao, Y.; Meng, G. Three-dimensional refractive index microscopy based on the multi-layer propagation model with obliquity factor correction. Opt. Lasers Eng. 2024, 174, 107966. [Google Scholar] [CrossRef]
- Goodman, J.W. Introduction to Fourier Optics; Roberts and Company Publishers: Englewood, CO, USA, 2005. [Google Scholar]
- Liu, H.Y.; Liu, D.; Mansour, H.; Boufounos, P.T.; Waller, L.; Kamilov, U.S. SEAGLE: Sparsity-driven image reconstruction under multiple scattering. IEEE Trans. Comput. Imaging 2017, 4, 73–86. [Google Scholar] [CrossRef]
- Pham, T.a.; Soubies, E.; Ayoub, A.; Lim, J.; Psaltis, D.; Unser, M. Three-Dimensional Optical Diffraction Tomography With Lippmann-Schwinger Model. IEEE Trans. Comput. Imaging 2020, 6, 727–738. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light; Cambridge University: Cambridge, UK, 1999. [Google Scholar]
- Kamilov, U.S.; Liu, D.; Mansour, H.; Boufounos, P.T. A Recursive Born Approach to Nonlinear Inverse Scattering. IEEE Signal Process. Lett. 2016, 23, 1052–1056. [Google Scholar] [CrossRef]
- Osnabrugge, G.; Leedumrongwatthanakun, S.; Vellekoop, I.M. A convergent Born series for solving the inhomogeneous Helmholtz equation in arbitrarily large media. J. Comput. Phys. 2016, 322, 113–124. [Google Scholar] [CrossRef]
- Krüger, B.; Brenner, T.; Kienle, A. Solution of the inhomogeneous Maxwell’s equations using a Born series. Opt. Express 2017, 25, 25165–25182. [Google Scholar] [CrossRef] [PubMed]
- Ciattoni, A.; Porto, P.D.; Crosignani, B.; Yariv, A. Vectorial nonparaxial propagation equation in the presence of a tensorial refractive-index perturbation. J. Opt. Soc. Am. B 2000, 17, 809–819. [Google Scholar] [CrossRef]
- van Rooij, J.; Kalkman, J. Large-scale high-sensitivity optical diffraction tomography of zebrafish. Biomed. Opt. Express 2019, 10, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, J.; Kalkman, J. Polarization contrast optical diffraction tomography. Biomed. Opt. Express 2020, 11, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- South, F.A.; Liu, Y.Z.; Xu, Y.; Shemonski, N.D.; Carney, P.S.; Boppart, S.A. Polarization-sensitive interferometric synthetic aperture microscopy. Appl. Phys. Lett. 2015, 107, 211106. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Yang, T.D.; Seo, D.; Hyeon, M.G.; Kong, T.; Kim, B.M.; Choi, Y.; Choi, W.; Choi, Y. Jones matrix microscopy for living eukaryotic cells. ACS Photonics 2021, 8, 3042–3050. [Google Scholar] [CrossRef]
- Shin, S.; Eun, J.; Lee, S.S.; Lee, C.; Hugonnet, H.; Yoon, D.K.; Kim, S.H.; Jeong, J.; Park, Y. Tomographic measurement of dielectric tensors at optical frequency. Nat. Mater. 2022, 21, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Mihoubi, S.; Lapray, P.J.; Bigué, L. Survey of Demosaicking Methods for Polarization Filter Array Images. Sensors 2018, 18, 3688. [Google Scholar] [CrossRef] [PubMed]
- Verrier, N.; Taddese, A.M.; Abbessi, R.; Debailleul, M.; Haeberlé, O. 3D differential interference contrast microscopy using polarisation-sensitive tomographic diffraction microscopy. J. Microsc. 2023, 289, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortiga, E.; Doblas, A.; Saavedra, G.; Martinez-Corral, M.; Garcia-Sucerquia, J. Off-axis digital holographic microscopy: Practical design parameters for operating at diffraction limit. Appl. Opt. 2014, 53, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Choi, W.; Fang-Yen, C.; Badizadegan, K.; Dasari, R.R.; Feld, M.S. Optical diffraction tomography for high resolution live cell imaging. Opt. Express 2009, 17, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Fiolka, R.; Wicker, K.; Heintzmann, R.; Stemmer, A. Simplified approach to diffraction tomography in optical microscopy. Opt. Express 2009, 17, 12407–12417. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lee, K.; Jin, K.H.; Shin, S.; Lee, S.; Park, Y.; Ye, J.C. Comparative study of iterative reconstruction algorithms for missing cone problems in optical diffraction tomography. Opt. Express 2015, 23, 16933–16948. [Google Scholar] [CrossRef] [PubMed]
- Taddese, A.M.; Verrier, N.; Debailleul, M.; Courbot, J.B.; Haeberlé, O. Optimizing sample illumination scanning for reflection and 4Pi tomographic diffractive microscopy. Appl. Opt. 2021, 60, 7745–7753. [Google Scholar] [CrossRef]
- Aziz, J.A.B.; Smith-Dryden, S.; Saleh, B.E.A.; Li, G. Selection of Illumination Angles for Object Rotation and Illumination Scanning Configurations in Optical Diffraction Tomography. In Proceedings of the 2023 IEEE Photonics Conference (IPC), Orlando, FL, USA, 12–16 November 2023; pp. 1–2. [Google Scholar] [CrossRef]
- Aziz, J.A.B.; Smith-Dryden, S.; Li, G.; Saleh, B.E.A. Voronoi Weighting for Optical Diffraction Tomography using Nonuniform Illumination Angles. In Proceedings of the 2023 IEEE Photonics Conference (IPC), Orlando, FL, USA, 12–16 November 2023; pp. 1–2. [Google Scholar] [CrossRef]
- Liu, H.; Bailleul, J.; Simon, B.; Debailleul, M.; Colicchio, B.; Haeberlé, O. Tomographic diffractive microscopy and multiview profilometry with flexible aberration correction. Appl. Opt. 2014, 53, 748–755. [Google Scholar] [CrossRef]
- Machnio, P.; Ziemczonok, M.; Kujawińska, M. Reconstruction enhancement via projection screening in holographic tomography. Photonics Lett. Pol. 2021, 13, 37–39. [Google Scholar] [CrossRef]
- Ryu, D.; Jo, Y.; Yoo, J.; Chang, T.; Ahn, D.; Kim, Y.S.; Kim, G.; Min, H.S.; Park, Y. Deep learning-based optical field screening for robust optical diffraction tomography. Sci. Rep. 2019, 9, 15239. [Google Scholar] [CrossRef] [PubMed]
- Girshovitz, P.; Shaked, N.T. Real-time quantitative phase reconstruction in off-axis digital holography using multiplexing. Opt. Lett. 2014, 39, 2262–2265. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.; Dardikman, G.; Mirsky, S.K.; Turko, N.A.; Shaked, N.T. Six-pack off-axis holography. Opt. Lett. 2017, 42, 4611–4614. [Google Scholar] [CrossRef] [PubMed]
- Mirsky, S.K.; Barnea, I.; Shaked, N.T. Dynamic Tomographic Phase Microscopy by Double Six-Pack Holography. ACS Photonics 2022, 9, 1295–1303. [Google Scholar] [CrossRef]
- Foucault, L.; Verrier, N.; Debailleul, M.; Simon, B.; Haeberlé, O. Simplified tomographic diffractive microscopy for axisymmetric samples. OSA Contin. 2019, 2, 1039–1055. [Google Scholar] [CrossRef]
- Sung, Y. Snapshot Holographic Optical Tomography. Phys. Rev. Appl. 2019, 11, 014039. [Google Scholar] [CrossRef]
- Sung, Y. Snapshot Three-Dimensional Absorption Imaging of Microscopic Specimens. Phys. Rev. Appl. 2021, 15, 064065. [Google Scholar] [CrossRef]
- Kus, A. Real-time, multiplexed holographic tomography. Opt. Lasers Eng. 2021, 149, 106783. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wang, Y.; Li, D. Quantitative real-time phase microscopy for extended depth-of-field imaging based on the 3D single-shot differential phase contrast (ssDPC) imaging method. Opt. Express 2024, 32, 2081–2096. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, S.; Ma, C.; Xi, T.; Di, J.; Zhao, J. A review of common-path off-axis digital holography: Towards high stable optical instrument manufacturing. Light Adv. Manuf. 2021, 2, 333–349. [Google Scholar] [CrossRef]
- Kim, Y.; Shim, H.; Kim, K.; Park, H.; Heo, J.H.; Yoon, J.; Choi, C.; Jang, S.; Park, Y. Common-path diffraction optical tomography for investigation of three-dimensional structures and dynamics of biological cells. Opt. Express 2014, 22, 10398–10407. [Google Scholar] [CrossRef]
- Bianchi, S.; Brasili, F.; Saglimbeni, F.; Cortese, B.; Leonardo, R.D. Optical diffraction tomography of 3D microstructures using a low coherence source. Opt. Express 2022, 30, 22321–22332. [Google Scholar] [CrossRef]
- Hsu, W.C.; Su, J.W.; Tseng, T.Y.; Sung, K.B. Tomographic diffractive microscopy of living cells based on a common-path configuration. Opt. Lett. 2014, 39, 2210–2213. [Google Scholar] [CrossRef]
- Kim, K.; Yaqoob, Z.; Lee, K.; Kang, J.W.; Choi, Y.; Hosseini, P.; So, P.T.C.; Park, Y. Diffraction optical tomography using a quantitative phase imaging unit. Opt. Lett. 2014, 39, 6935–6938. [Google Scholar] [CrossRef] [PubMed]
- Bon, P.; Maucort, G.; Wattellier, B.; Monneret, S. Quadriwave lateral shearing interferometry for quantitative phase microscopy of living cells. Opt. Express 2009, 17, 13080–13094. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Bon, P.; Mudry, E.; Maire, G.; Chaumet, P.C.; Giovannini, H.; Belkebir, K.; Talneau, A.; Wattellier, B.; Monneret, S.; et al. Tomographic diffractive microscopy with a wavefront sensor. Opt. Lett. 2012, 37, 1631–1633. [Google Scholar] [CrossRef]
- Kak, A.C.; Slaney, M. Principles of Computerized Tomographic Imaging; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2001. [Google Scholar] [CrossRef]
- Maleki, M.H.; Devaney, A.J. Phase-retrieval and intensity-only reconstruction algorithms for optical diffraction tomography. J. Opt. Soc. Am. A 1993, 10, 1086–1092. [Google Scholar] [CrossRef]
- Tian, L.; Waller, L. 3D intensity and phase imaging from light field measurements in an LED array microscope. Optica 2015, 2, 104–111. [Google Scholar] [CrossRef]
- Soto, J.M.; Rodrigo, J.A.; Alieva, T. Label-free quantitative 3D tomographic imaging for partially coherent light microscopy. Opt. Express 2017, 25, 15699–15712. [Google Scholar] [CrossRef]
- Li, J.; Matlock, A.; Li, Y.; Chen, Q.; Tian, L.; Zuo, C. Resolution-enhanced intensity diffraction tomography in high numerical aperture label-free microscopy. Photonics Res. 2020, 8, 1818–1826. [Google Scholar] [CrossRef]
- Ayoub, A.B.; Roy, A.; Psaltis, D. Optical Diffraction Tomography Using Nearly In-Line Holography with a Broadband LED Source. Appl. Sci. 2022, 12, 951. [Google Scholar] [CrossRef]
- Ling, R.; Tahir, W.; Lin, H.Y.; Lee, H.; Tian, L. High-throughput intensity diffraction tomography with a computational microscope. Biomed. Opt. Express 2018, 9, 2130–2141. [Google Scholar] [CrossRef]
- Matlock, A.; Tian, L. High-throughput, volumetric quantitative phase imaging with multiplexed intensity diffraction tomography. Biomed. Opt. Express 2019, 10, 6432–6448. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Matlock, A.; Li, Y.; Chen, Q.; Zuo, C.; Tian, L. High-speed in vitro intensity diffraction tomography. Adv. Photonics 2019, 1, 066004. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Zhang, J.; Zhang, Z.; Zhang, Y.; Zuo, C. Optical diffraction tomography microscopy with transport of intensity equation using a light-emitting diode array. Opt. Lasers Eng. 2017, 95, 26–34. [Google Scholar] [CrossRef]
- Li, J.; Zhou, N.; Sun, J.; Zhou, S.; Bai, Z.; Lu, L.; Chen, Q.; Zuo, C. Transport of intensity diffraction tomography with non-interferometric synthetic aperture for three-dimensional label-free microscopy. Light Sci. Appl. 2022, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Chen, Q.; Ullah, H.; Lu, L.; Zhou, N.; Zhou, S.; Li, J.; Zuo, C. Absorption and phase decoupling in transport of intensity diffraction tomography. Opt. Lasers Eng. 2022, 156, 107082. [Google Scholar] [CrossRef]
- Ullah, H.; Li, J.; Zhou, S.; Bai, Z.; Ye, R.; Chen, Q.; Zuo, C. Parallel synthetic aperture transport-of-intensity diffraction tomography with annular illumination. Opt. Lett. 2023, 48, 1638–1641. [Google Scholar] [CrossRef]
- Zuo, C.; Li, J.; Sun, J.; Fan, Y.; Zhang, J.; Lu, L.; Zhang, R.; Wang, B.; Huang, L.; Chen, Q. Transport of intensity equation: A tutorial. Opt. Lasers Eng. 2020, 135, 106187. [Google Scholar] [CrossRef]
- Shen, C.; Liang, M.; Pan, A.; Yang, C. Non-iterative complex wave-field reconstruction based on Kramers–Kronig relations. Photonics Res. 2021, 9, 1003–1012. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Ma, S.; Wang, Y.; Liu, S.; Liu, Z. Single-frame two-color illumination computational imaging based on Kramers–Kronig relations. Appl. Phys. Lett. 2023, 123, 141107. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Tian, L. High-fidelity intensity diffraction tomography with a non-paraxial multiple-scattering model. Opt. Express 2022, 30, 32808–32821. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Kim, J.; Moon, T.; Seong, B.; Kim, W.; Yoo, C.H.; Choi, J.K.; Joo, C. Polarization-sensitive intensity diffraction tomography. Light Sci. Appl. 2023, 12, 124. [Google Scholar] [CrossRef]
- Wang, F.; Bian, Y.; Wang, H.; Lyu, M.; Pedrini, G.; Osten, W.; Barbastathis, G.; Situ, G. Phase imaging with an untrained neural network. Light Sci. Appl. 2020, 9, 77. [Google Scholar] [CrossRef]
- Wang, K.; Song, L.; Wang, C.; Ren, Z.; Zhao, G.; Dou, J.; Di, J.; Barbastathis, G.; Zhou, R.; Zhao, J.; et al. On the use of deep learning for phase recovery. Light Sci. Appl. 2024, 13, 4. [Google Scholar] [CrossRef]
- Matlock, A.; Zhu, J.; Tian, L. Multiple-scattering simulator-trained neural network for intensity diffraction tomography. Opt. Express 2023, 31, 4094–4107. [Google Scholar] [CrossRef]
- Pierré, W.; Hervé, L.; Paviolo, C.; Mandula, O.; Remondiere, V.; Morales, S.; Grudinin, S.; Ray, P.F.; Dhellemmes, M.; Arnoult, C.; et al. 3D time-lapse imaging of a mouse embryo using intensity diffraction tomography embedded inside a deep learning framework. Appl. Opt. 2022, 61, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.M.; Rodrigo, J.A.; Alieva, T. Optical diffraction tomography with fully and partially coherent illumination in high numerical aperture label-free microscopy [Invited]. Appl. Opt. 2018, 57, A205–A214. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.M.; Rodrigo, J.A.; Alieva, T. Partially coherent illumination engineering for enhanced refractive index tomography. Opt. Lett. 2018, 43, 4699–4702. [Google Scholar] [CrossRef]
- Hugonnet, H.; Lee, M.; Park, Y. Optimizing illumination in three-dimensional deconvolution microscopy for accurate refractive index tomography. Opt. Express 2021, 29, 6293–6301. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, N.; Bai, Z.; Zhou, S.; Chen, Q.; Zuo, C. Optimization analysis of partially coherent illumination for refractive index tomographic microscopy. Opt. Lasers Eng. 2021, 143, 106624. [Google Scholar] [CrossRef]
- Cao, R.; Kellman, M.; Ren, D.; Eckert, R.; Waller, L. Self-calibrated 3D differential phase contrast microscopy with optimized illumination. Biomed. Opt. Express 2022, 13, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Isikman, S.O.; Greenbaum, A.; Luo, W.; Coskun, A.F.; Ozcan, A. Giga-pixel lensfree holographic microscopy and tomography using color image sensors. PLoS ONE 2012, 7, e45044. [Google Scholar] [CrossRef] [PubMed]
- Merola, F.; Memmolo, P.; Miccio, L.; Savoia, R.; Mugnano, M.; Fontana, A.; d’Ippolito, G.; Sardo, A.; Iolascon, A.; Gambale, A.; et al. Tomographic flow cytometry by digital holography. Light Sci. Appl. 2017, 6, e16241. [Google Scholar] [CrossRef] [PubMed]
- Villone, M.M.; Memmolo, P.; Merola, F.; Mugnano, M.; Miccio, L.; Maffettone, P.L.; Ferraro, P. Full-angle tomographic phase microscopy of flowing quasi-spherical cells. Lab A Chip 2018, 18, 126–131. [Google Scholar] [CrossRef]
- Pirone, D.; Lim, J.; Merola, F.; Miccio, L.; Mugnano, M.; Bianco, V.; Cimmino, F.; Visconte, F.; Montella, A.; Capasso, M.; et al. Stain-free identification of cell nuclei using tomographic phase microscopy in flow cytometry. Nat. Photonics 2022, 16, 851–859. [Google Scholar] [CrossRef]
- Pirone, D.; Sirico, D.; Miccio, L.; Bianco, V.; Mugnano, M.; Giudice, D.; Pasquinelli, G.; Valente, S.; Lemma, S.; Iommarini, L.; et al. 3D imaging lipidometry in single cell by in-flow holographic tomography. Opto-Electron. Adv. 2023, 6, 220048. [Google Scholar] [CrossRef]
- Bianco, V.; Massimo, D.; Pirone, D.; Giugliano, G.; Mosca, N.; Summa, M.; Scerra, G.; Memmolo, P.; Miccio, L.; Russo, T.; et al. Label-Free Intracellular Multi-Specificity in Yeast Cells by Phase-Contrast Tomographic Flow Cytometry. Small Methods 2023, 7, e2300447. [Google Scholar] [CrossRef]
- Wang, Z.; Bianco, V.; Pirone, D.; Memmolo, P.; Villone, M.; Maffettone, P.L.; Ferraro, P. Dehydration of plant cells shoves nuclei rotation allowing for 3D phase-contrast tomography. Light Sci. Appl. 2021, 10, 187. [Google Scholar] [CrossRef]
- Pirone, D.; Memmolo, P.; Merola, F.; Miccio, L.; Mugnano, M.; Capozzoli, A.; Curcio, C.; Liseno, A.; Ferraro, P. Rolling angle recovery of flowing cells in holographic tomography exploiting the phase similarity. Appl. Opt. 2021, 60, A277. [Google Scholar] [CrossRef]
- Sung, Y. Hyperspectral Three-Dimensional Refractive-Index Imaging Using Snapshot Optical Tomography. Phys. Rev. Appl. 2023, 19, 014064. [Google Scholar] [CrossRef]
- Luo, Z.; Yurt, A.; Stahl, R.; Carlon, M.S.; Ramalho, A.S.; Vermeulen, F.; Lambrechts, A.; Braeken, D.; Lagae, L. Fast compressive lens-free tomography for 3D biological cell culture imaging. Opt. Express 2020, 28, 26935–26952. [Google Scholar] [CrossRef]
- Jung, J.; Kim, K.; Yoon, J.; Park, Y. Hyperspectral optical diffraction tomography. Opt. Express 2016, 24, 2006–2012. [Google Scholar] [CrossRef]
- Sung, Y. Spectroscopic Microtomography in the Visible Wavelength Range. Phys. Rev. Appl. 2018, 10, 054041. [Google Scholar] [CrossRef]
- Juntunen, C.; Abramczyk, A.R.; Woller, I.M.; Sung, Y. Hyperspectral Three-Dimensional Absorption Imaging Using Snapshot Optical Tomography. Phys. Rev. Appl. 2022, 18, 034055. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, P.; Kuś, A.; Krauze, W.; Tamborski, S.; Ziemczonok, M.; Kuźbicki, Ł.; Szkulmowski, M.; Kujawińska, M. Near-infrared, wavelength, and illumination scanning holographic tomography. Biomed. Opt. Express 2022, 13, 5971–5988. [Google Scholar] [CrossRef] [PubMed]
- Juntunen, C.; Abramczyk, A.R.; Shea, P.; Sung, Y. Spectroscopic Microtomography in the Short-Wave Infrared Wavelength Range. Sensors 2023, 23, 5164. [Google Scholar] [CrossRef] [PubMed]
- Tamamitsu, M.; Toda, K.; Shimada, H.; Honda, T.; Takarada, M.; Okabe, K.; Nagashima, Y.; Horisaki, R.; Ideguchi, T. Label-free biochemical quantitative phase imaging with mid-infrared photothermal effect. Optica 2020, 7, 359–366. [Google Scholar] [CrossRef]
- Yurdakul, C.; Zong, H.; Bai, Y.; Cheng, J.X.; Ünlü, M.S. Bond-selective interferometric scattering microscopy. J. Phys. D Appl. Phys. 2021, 54, 364002. [Google Scholar] [CrossRef]
- Zhang, D.; Lan, L.; Bai, Y.; Majeed, H.; Kandel, M.E.; Popescu, G.; Cheng, J.X. Bond-selective transient phase imaging via sensing of the infrared photothermal effect. Light Sci. Appl. 2019, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Matlock, A.; Zhu, H.; Song, Z.; Zhu, J.; Wang, B.; Chen, F.; Zhan, Y.; Chen, Z.; Xu, Y.; et al. Bond-selective intensity diffraction tomography. Nat. Commun. 2022, 13, 7767. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yin, J.; Cheng, J.X. Bond-selective imaging by optically sensing the mid-infrared photothermal effect. Sci. Adv. 2021, 7, eabg1559. [Google Scholar] [CrossRef]
- Pu, Y.; Centurion, M.; Psaltis, D. Harmonic holography: A new holographic principle. Appl. Opt. 2008, 47, A103–A110. [Google Scholar] [CrossRef]
- Shaffer, E.; Pavillon, N.; Kühn, J.; Depeursinge, C. Digital holographic microscopy investigation of second harmonic generated at a glass/air interface. Opt. Lett. 2009, 34, 2450–2452. [Google Scholar] [CrossRef]
- Shaffer, E.; Marquet, P.; Depeursinge, C. Real time, nanometric 3D-tracking of nanoparticles made possible by second harmonic generation digital holographic microscopy. Opt. Express 2010, 18, 17392–17403. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Grange, R.; Pu, Y.; Psaltis, D. Three-dimensional harmonic holographic microcopy using nanoparticles as probes for cell imaging. Opt. Express 2009, 17, 2880–2891. [Google Scholar] [CrossRef] [PubMed]
- Masihzadeh, O.; Schlup, P.; Bartels, R.A. Label-free second harmonic generation holographic microscopy of biological specimens. Opt. Express 2010, 18, 9840–9851. [Google Scholar] [CrossRef]
- Smith, D.R.; Winters, D.G.; Bartels, R.A. Submillisecond second harmonic holographic imaging of biological specimens in three dimensions. Proc. Natl. Acad. Sci. USA 2013, 110, 18391–18396. [Google Scholar] [CrossRef]
- Yu, W.; Li, X.; Hu, R.; Qu, J.; Liu, L. Full-field measurement of complex objects illuminated by an ultrashort pulse laser using delay-line sweeping off-axis interferometry. Opt. Lett. 2021, 46, 2803–2806. [Google Scholar] [CrossRef]
- Li, X.; Yu, W.; Hu, R.; Qu, J.; Liu, L. Fast polarization-sensitive second-harmonic generation microscopy based on off-axis interferometry. Opt. Express 2023, 31, 3143–3152. [Google Scholar] [CrossRef]
- Hu, C.; Field, J.J.; Kelkar, V.; Chiang, B.; Wernsing, K.; Toussaint, K.C.; Bartels, R.A.; Popescu, G. Harmonic optical tomography of nonlinear structures. Nat. Photonics 2020, 14, 564–569. [Google Scholar] [CrossRef]
- Yu, W.; Li, X.; Wang, B.; Qu, J.; Liu, L. Optical diffraction tomography of second-order nonlinear structures in weak scattering media: Theoretical analysis and experimental consideration. Opt. Express 2022, 30, 45724–45737. [Google Scholar] [CrossRef]
- Moon, J.; Kang, S.; Hong, J.H.; Yoon, S.; Choi, W. Synthetic aperture phase imaging of second harmonic generation field with computational adaptive optics. arXiv 2023, arXiv:2304.14018. [Google Scholar]
- Débarre, D.; Supatto, W.; Pena, A.M.; Fabre, A.; Tordjmann, T.; Combettes, L.; Schanne-Klein, M.C.; Beaurepaire, E. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat. Methods 2006, 3, 47–53. [Google Scholar] [CrossRef]
- Olivier, N.; Aptel, F.; Plamann, K.; Schanne-Klein, M.C.; Beaurepaire, E. Harmonic microscopy of isotropic and anisotropic microstructure of the human cornea. Opt. Express 2010, 18, 5028–5040. [Google Scholar] [CrossRef]
- Hugonnet, H.; Han, H.; Park, W.; Park, Y. Improving Specificity and Axial Spatial Resolution of Refractive Index Imaging by Exploiting Uncorrelated Subcellular Dynamics. ACS Photonics 2023, 18, 257–266. [Google Scholar] [CrossRef]
- Verrier, N.; Atlan, M. Absolute measurement of small-amplitude vibrations by time-averaged heterodyne holography with a dual local oscillator. Opt. Lett. 2013, 38, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Verrier, N.; Alloul, L.; Gross, M. Vibration of low amplitude imaged in amplitude and phase by sideband versus carrier correlation digital holography. Opt. Lett. 2015, 40, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Verrier, N.; Alexandre, D.; Gross, M. Laser Doppler holographic microscopy in transmission: Application to fish embryo imaging. Opt. Express 2014, 22, 9368–9379. [Google Scholar] [CrossRef] [PubMed]
- Puyo, L.; Paques, M.; Fink, M.; Sahel, J.A.; Atlan, M. In vivo laser Doppler holography of the human retina. Biomed. Opt. Express 2018, 9, 4113–4129. [Google Scholar] [CrossRef]
- Puyo, L.; Spahr, H.; Pfäffle, C.; Hüttmann, G.; Hillmann, D. Retinal blood flow imaging with combined full-field swept-source optical coherence tomography and laser Doppler holography. Opt. Lett. 2022, 47, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Brodoline, A.; Rawat, N.; Alexandre, D.; Cubedo, N.; Gross, M. 4D compressive sensing holographic microscopy imaging of small moving objects. Opt. Lett. 2019, 44, 2827–2830. [Google Scholar] [CrossRef]
- Brodoline, A.; Rawat, N.; Alexandre, D.; Cubedo, N.; Gross, M. 4D compressive sensing holographic imaging of small moving objects with multiple illuminations. Appl. Opt. 2019, 58, G127–G134. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Bedrossian, M.; Lee, J.; Ho, G.H.; Shapiro, M.G.; Nadeau, J.L. Genetically encoded phase contrast agents for digital holographic microscopy. Nano Lett. 2020, 20, 8127–8134. [Google Scholar] [CrossRef] [PubMed]
- Horstmeyer, R.; Heintzmann, R.; Popescu, G.; Waller, L.; Yang, C. Standardizing the resolution claims for coherent microscopy. Nat. Photonics 2016, 10, 68–71. [Google Scholar] [CrossRef]
- He, Y.; Zhou, N.; Ziemczonok, M.; Wang, Y.; Lei, L.; Duan, L.; Zhou, R. Standardizing image assessment in optical diffraction tomography. Opt. Lett. 2023, 48, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.; Yoon, J.; Heo, J.; Choi, C.; Park, Y. Three-dimensional label-free imaging and quantification of lipid droplets in live hepatocytes. Sci. Rep. 2016, 6, 36815. [Google Scholar] [CrossRef]
- Stasiewicz, K.; Krajewski, R.; Jaroszewicz, L.; Kujawińska, M.; Świłło, R. Influence of tapering process on changes of optical fiber refractive index distribution along a structure. Opto-Electron. Rev. 2010, 18, 102–109. [Google Scholar] [CrossRef]
- Fan, S.; Smith-Dryden, S.; Zhao, J.; Gausmann, S.; Schulzgen, A.; Li, G.; Saleh, B. Optical Fiber Refractive Index Profiling by Iterative Optical Diffraction Tomography. J. Light. Technol. 2018, 36, 5754–5763. [Google Scholar] [CrossRef]
- Smith-Dryden, S.; Fan, S.; Li, G.; Saleh, B. Iterative optical diffraction tomography with embedded regularization. Opt. Express 2023, 31, 116–124. [Google Scholar] [CrossRef]
- Sullivan, A.C.; McLeod, R.R. Tomographic reconstruction of weak, replicated index structures embedded in a volume. Opt. Express 2007, 15, 14202–14212. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yoon, J.; Park, Y. Large-scale optical diffraction tomography for inspection of optical plastic lenses. Opt. Lett. 2016, 41, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Sarmis, M.; Simon, B.; Debailleul, M.; Colicchio, B.; Georges, V.; Delaunay, J.J.; Haeberle, O. High resolution reflection tomographic diffractive microscopy. J. Mod. Opt. 2010, 57, 740–745. [Google Scholar] [CrossRef]
- Ziemczonok, M.; Kus, A.; Wasylczyk, P.; Kujawinska, M. 3D-printed biological cell phantom for testing 3D quantitative phase imaging systems. Sci. Rep. 2019, 9, 18872. [Google Scholar] [CrossRef]
- Krauze, W.; Kuś, A.; Ziemczonok, M.; Haimowitz, M.; Chowdhury, S.; Kujawińska, M. 3d scattering microphantom sample to assess quantitative accuracy in tomographic phase microscopy techniques. Sci. Rep. 2022, 12, 19586. [Google Scholar] [CrossRef]
- Ziemczonok, M.; Kus, A.; Kujawinska, M. Optical diffraction tomography meets metrology—Measurement accuracy on cellular and subcellular level. Measurement 2022, 195, 111106. [Google Scholar] [CrossRef]
- Lee, D.; Lee, M.; Kwak, H.; Kim, Y.S.; Shim, J.; Jung, J.H.; Park, W.s.; Park, J.H.; Lee, S.; Park, Y. High-fidelity optical diffraction tomography of live organisms using iodixanol refractive index matching. Biomed. Opt. Express 2022, 13, 6404–6415. [Google Scholar] [CrossRef]
- Bhaduri, B.; Edwards, C.; Pham, H.; Zhou, R.; Nguyen, T.H.; Goddard, L.L.; Popescu, G. Diffraction phase microscopy: Principles and applications in materials and life sciences. Adv. Opt. Photon. 2014, 6, 57–119. [Google Scholar] [CrossRef]
- Hugonnet, H.; Lee, M.; Shin, S.; Park, Y. Vectorial inverse scattering for dielectric tensor tomography: Overcoming challenges of reconstruction of highly scattering birefringent samples. Opt. Express 2023, 31, 29654–29663. [Google Scholar] [CrossRef]
- Lee, K.; Kim, K.; Jung, J.; Heo, J.; Cho, S.; Lee, S.; Chang, G.; Jo, Y.; Park, H.; Park, Y. Quantitative phase imaging techniques for the study of cell pathophysiology: From principles to applications. Sensors 2013, 13, 4170–4191. [Google Scholar] [CrossRef]
- Balasubramani, V.; Kujawińska, M.; Allier, C.; Anand, V.; Cheng, C.J.; Depeursinge, C.; Hai, N.; Juodkazis, S.; Kalkman, J.; Kuś, A.; et al. Roadmap on Digital Holography-Based Quantitative Phase Imaging. J. Imaging 2021, 7, 252. [Google Scholar] [CrossRef]
- Rappaz, B.; Marquet, P.; Cuche, E.; Emery, Y.; Depeursinge, C.; Magistretti, P.J. Measurement of the integral refractive index and dynamic cell morphometry of living cells with digital holographic microscopy. Opt. Express 2005, 13, 9361–9373. [Google Scholar] [CrossRef]
- Kleiber, A.; Kraus, D.; Henkel, T.; Fritzsche, W. Tomographic imaging flow cytometry. Lab A Chip 2021, 21, 3655–3666. [Google Scholar] [CrossRef]
- Pirone, D.; Mugnano, M.; Memmolo, P.; Merola, F.; Lama, G.C.; Castaldo, R.; Miccio, L.; Bianco, V.; Grilli, S.; Ferraro, P. Three-Dimensional Quantitative Intracellular Visualization of Graphene Oxide Nanoparticles by Tomographic Flow Cytometry. Nano Lett. 2021, 21, 5958–5966. [Google Scholar] [CrossRef] [PubMed]
- Isikman, S.O.; Bishara, W.; Zhu, H.; Ozcan, A. Optofluidic tomography on a chip. Appl. Phys. Lett. 2011, 98, 161109. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Lue, N.; Hamza, B.; Martel, J.; Irimia, D.; Dasari, R.R.; Choi, W.; Yaqoob, Z.; So, P. Three-dimensional holographic refractive-index measurement of continuously flowing cells in a microfluidic channel. Phys. Rev. Appl. 2014, 1, 014002. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.lynceetec.com/ (accessed on 20 February 2024).
- Available online: https://phiab.com/ (accessed on 20 February 2024).
- Available online: https://telight.eu/ (accessed on 20 February 2024).
- Available online: https://www.holmarc.com/index.php (accessed on 20 February 2024).
- Available online: https://www.phasics.com/en/ (accessed on 20 February 2024).
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; De Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.C.; Clevers, H. Imaging organoids: A bright future ahead. Nat. Methods 2018, 15, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Fei, K.; Zhang, J.; Yuan, J.; Xiao, P. Present application and perspectives of organoid imaging technology. Bioengineering 2022, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Keshara, R.; Kim, Y.H.; Grapin-Botton, A. Organoid imaging: Seeing development and function. Annu. Rev. Cell Dev. Biol. 2022, 38, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Quan, H.; Seok, S.H.; Sung, Y.; Joo, C. Quantitative refractive index tomography of millimeter-scale objects using single-pixel wavefront sampling. Optica 2022, 9, 1073–1083. [Google Scholar] [CrossRef]
- Mudry, E.; Chaumet, P.C.; Belkebir, K.; Maire, G.; Sentenac, A. Mirror-assisted tomographic diffractive microscopy with isotropic resolution. Opt. Lett. 2010, 35, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Sun, J.; Zhang, R.; Ye, R.; Li, J.; Bai, Z.; Zhou, S.; Chen, Q.; Zuo, C. Quasi-Isotropic High-Resolution Fourier Ptychographic Diffraction Tomography with Opposite Illuminations. ACS Photonics 2023, 10, 2461–2466. [Google Scholar] [CrossRef]
- Pohl, D.; Denk, W.; Lanz, M.O. Image recording with resolution λ/20. Appl. Phys. Lett 1984, 44, 651–653. [Google Scholar] [CrossRef]
- Pendry, J.B. Negative refraction makes a perfect lens. Phys. Rev. Lett. 2000, 85, 3966. [Google Scholar] [CrossRef]
- Hao, X.; Kuang, C.; Liu, X.; Zhang, H.; Li, Y. Microsphere based microscope with optical super-resolution capability. Appl. Phys. Lett. 2011, 99, 203102. [Google Scholar] [CrossRef]
- Micó, V.; Zheng, J.; Garcia, J.; Zalevsky, Z.; Gao, P. Resolution enhancement in quantitative phase microscopy. Adv. Opt. Photon. 2019, 11, 135–214. [Google Scholar] [CrossRef]
- Astratov, V. Label-Free Super-Resolution Microscopy; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Astratov, V.N.; Sahel, Y.B.; Eldar, Y.C.; Huang, L.; Ozcan, A.; Zheludev, N.; Zhao, J.; Burns, Z.; Liu, Z.; Narimanov, E.; et al. Roadmap on Label-Free Super-Resolution Imaging. Laser Photonics Rev. 2023, 17, 2200029. [Google Scholar] [CrossRef]
- Godavarthi, C.; Zhang, T.; Maire, G.; Chaumet, P.C.; Giovannini, H.; Talneau, A.; Belkebir, K.; Sentenac, A. Superresolution with full-polarized tomographic diffractive microscopy. J. Opt. Soc. Am. A 2015, 32, 287–292. [Google Scholar] [CrossRef]
- Zhang, T.; Godavarthi, C.; Chaumet, P.C.; Maire, G.; Giovannini, H.; Talneau, A.; Allain, M.; Belkebir, K.; Sentenac, A. Far-field diffraction microscopy at λ/10 resolution. Optica 2016, 3, 609–612. [Google Scholar] [CrossRef]
- Zhang, T.; Unger, K.; Maire, G.; Chaumet, P.C.; Talneau, A.; Godhavarti, C.; Giovannini, H.; Belkebir, K.; Sentenac, A. Multi-wavelength multi-angle reflection tomography. Opt. Express 2018, 26, 26093–26105. [Google Scholar] [CrossRef]
- Park, C.; Shin, S.; Park, Y. Generalized quantification of three-dimensional resolution in optical diffraction tomography using the projection of maximal spatial bandwidths. J. Opt. Soc. Am. A 2018, 35, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Cao, W.; Chen, T.; Huang, X.; Le, T.; Zhu, S.; Wang, D.W.; Lee, H.J.; Zhang, D. Super-resolution imaging of non-fluorescent molecules by photothermal relaxation localization microscopy. Nat. Photonics 2023, 17, 330–337. [Google Scholar] [CrossRef]
- Baffou, G.; Bon, P.; Savatier, J.; Polleux, J.; Zhu, M.; Merlin, M.; Rigneault, H.; Monneret, S. Thermal Imaging of Nanostructures by Quantitative Optical Phase Analysis. ACS Nano 2012, 6, 2452–2458. [Google Scholar] [CrossRef]
- Ledwig, P.; Robles, F.E. Epi-mode tomographic quantitative phase imaging in thick scattering samples. Biomed. Opt. Express 2019, 10, 3605–3621. [Google Scholar] [CrossRef]
- Ledwig, P.; Robles, F.E. Quantitative 3D refractive index tomography of opaque samples in epi-mode. Optica 2021, 8, 6–14. [Google Scholar] [CrossRef]
- Kang, S.; Zhou, R.; Brelen, M.; Mak, H.K.; Lin, Y.; So, P.T.; Yaqoob, Z. Mapping nanoscale topographic features in thick tissues with speckle diffraction tomography. Light Sci. Appl. 2023, 12, 200. [Google Scholar] [CrossRef]
- Berdeu, A.; Flasseur, O.; Méès, L.; Denis, L.; Momey, F.; Olivier, T.; Grosjean, N.; Fournier, C. Reconstruction of in-line holograms: Combining model-based and regularized inversion. Opt. Express 2019, 27, 14951–14968. [Google Scholar] [CrossRef]
- Dong, D.; Huang, X.; Li, L.; Mao, H.; Mo, Y.; Zhang, G.; Zhang, Z.; Shen, J.; Liu, W.; Wu, Z.; et al. Super-resolution fluorescence-assisted diffraction computational tomography reveals the three-dimensional landscape of the cellular organelle interactome. Light. Sci. Appl. 2020, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Pitrone, P.G.; Schindelin, J.; Stuyvenberg, L.; Preibisch, S.; Weber, M.; Eliceiri, K.W.; Huisken, J.; Tomancak, P. OpenSPIM: An open-access light-sheet microscopy platform. Nat. Methods 2013, 10, 598–599. [Google Scholar] [CrossRef]
- Choi, G.; Ryu, D.; Jo, Y.; Kim, Y.S.; Park, W.; Min, H.-s.; Park, Y. Cycle-consistent deep learning approach to coherent noise reduction in optical diffraction tomography. Opt. Express 2019, 27, 4927–4943. [Google Scholar] [CrossRef]
- Lim, J.; Ayoub, A.B.; Psaltis, D. Three-dimensional tomography of red blood cells using deep learning. Adv. Photonics 2020, 2, 026001. [Google Scholar] [CrossRef]
- Kamilov, U.S.; Papadopoulos, I.N.; Shoreh, M.H.; Goy, A.; Vonesch, C.; Unser, M.; Psaltis, D. Learning approach to optical tomography. Optica 2015, 2, 517–522. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Z.; Kamilov, U.S. Efficient and accurate inversion of multiple scattering with deep learning. Opt. Express 2018, 26, 14678–14688. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Pham, T.-a.; Gupta, H.; Unser, M.; Ma, J. Deep-learning projector for optical diffraction tomography. Opt. Express 2020, 28, 3905–3921. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.C.; Horstmeyer, R. Diffraction tomography with a deep image prior. Opt. Express 2020, 28, 12872–12896. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Ryu, D.; Baek, Y.; Cho, H.; Kim, G.; Kim, Y.S.; Lee, Y.; Kim, Y.; Ye, J.C.; Min, H.S.; et al. DeepRegularizer: Rapid resolution enhancement of tomographic imaging using deep learning. IEEE Trans. Med. Imaging 2021, 40, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Huh, J.; Kim, G.; Park, Y.K.; Ye, J.C. Missing Cone Artifact Removal in ODT Using Unsupervised Deep Learning in the Projection Domain. IEEE Trans. Comput. Imaging 2021, 7, 747–758. [Google Scholar] [CrossRef]
- Di, J.; Han, W.; Liu, S.; Wang, K.; Tang, J.; Zhao, J. Sparse-view imaging of a fiber internal structure in holographic diffraction tomography via a convolutional neural network. Appl. Opt. 2021, 60, A234–A242. [Google Scholar] [CrossRef]
- Bazow, B.; Phan, T.; Raub, C.B.; Nehmetallah, G. Three-dimensional refractive index estimation based on deep-inverse non-interferometric optical diffraction tomography (ODT-Deep). Opt. Express 2023, 31, 28382–28399. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Kim, G.; Moon, A.R.; Ryu, D.; Hugonnet, H.; Lee, M.J.; Shin, D.; Lee, S.J.; Lee, E.S.; Park, Y. Label-free histological analysis of retrieved thrombi in acute ischemic stroke using optical diffraction tomography and deep learning. J. Biophotonics 2023, 16, e202300067. [Google Scholar] [CrossRef] [PubMed]


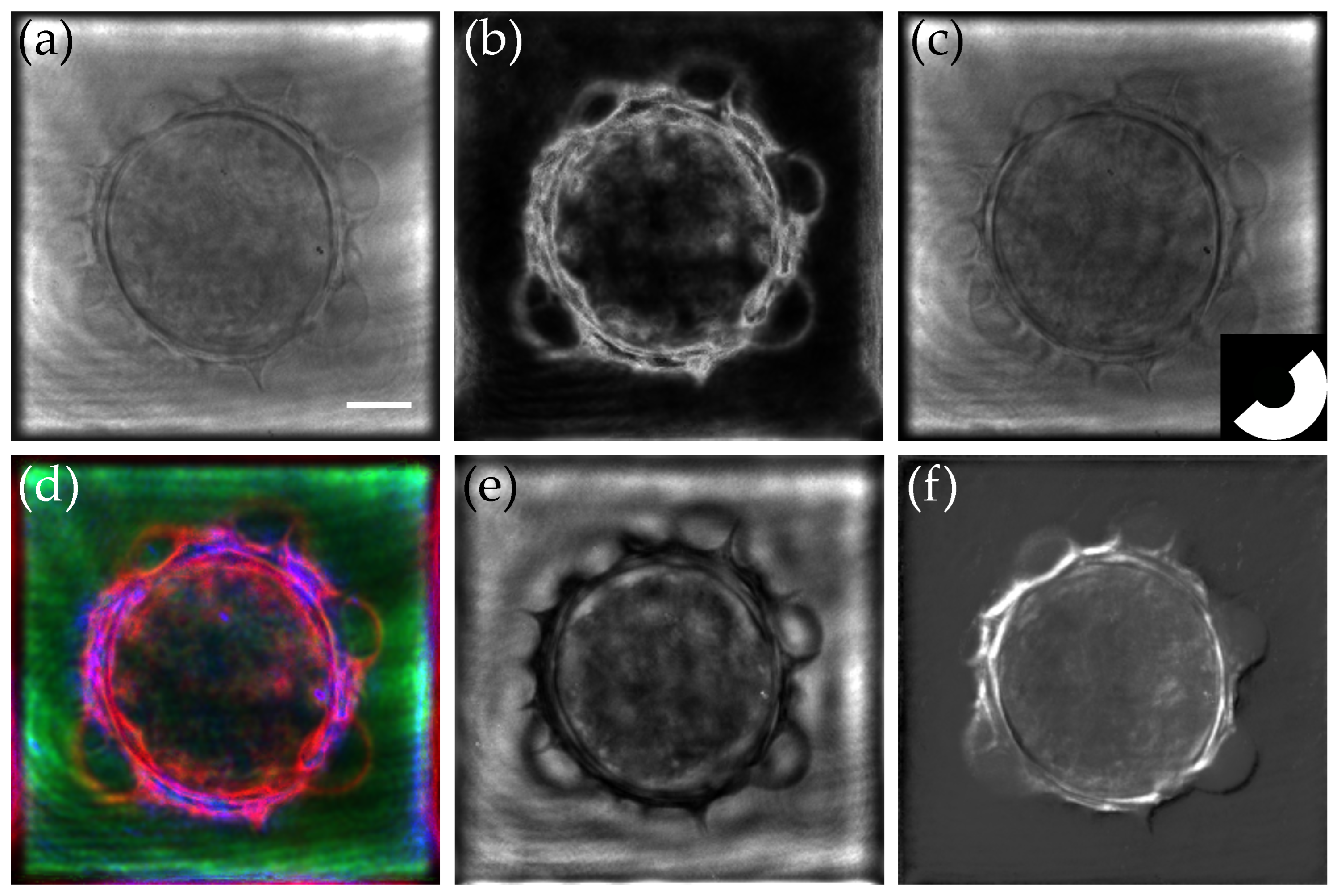
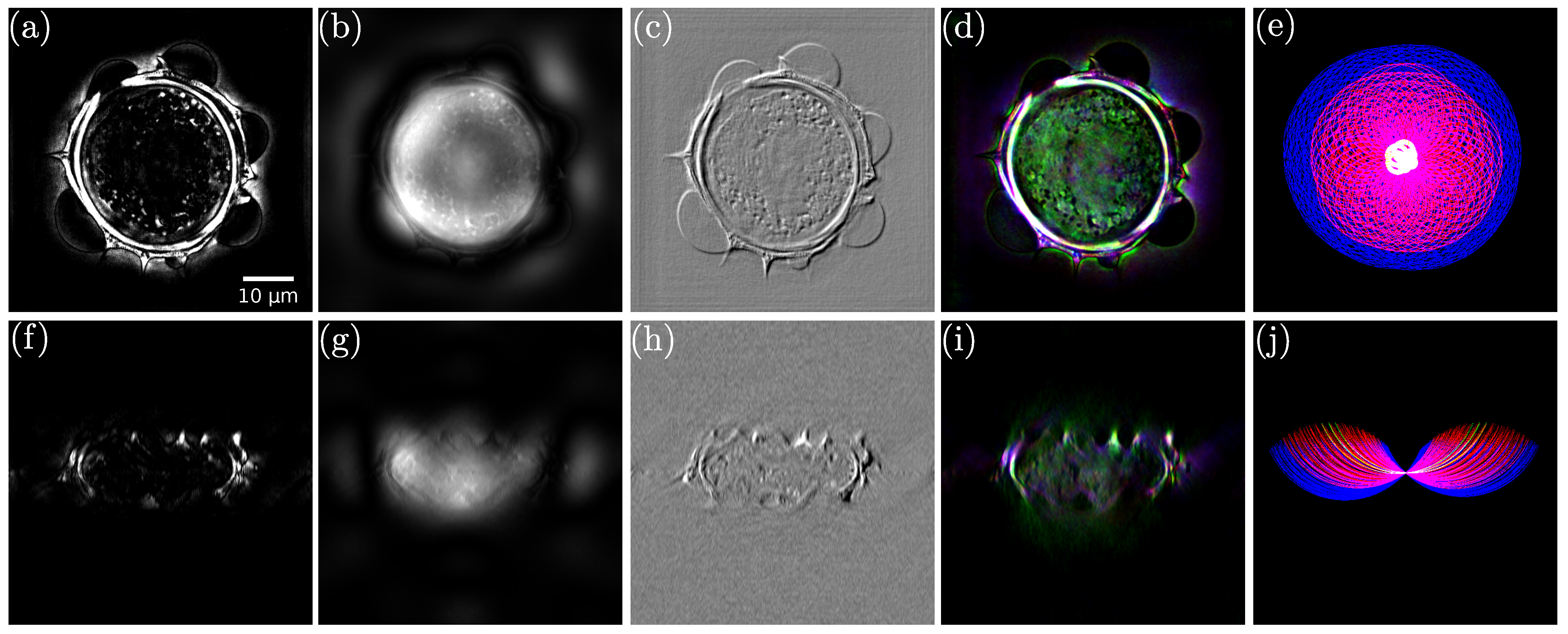



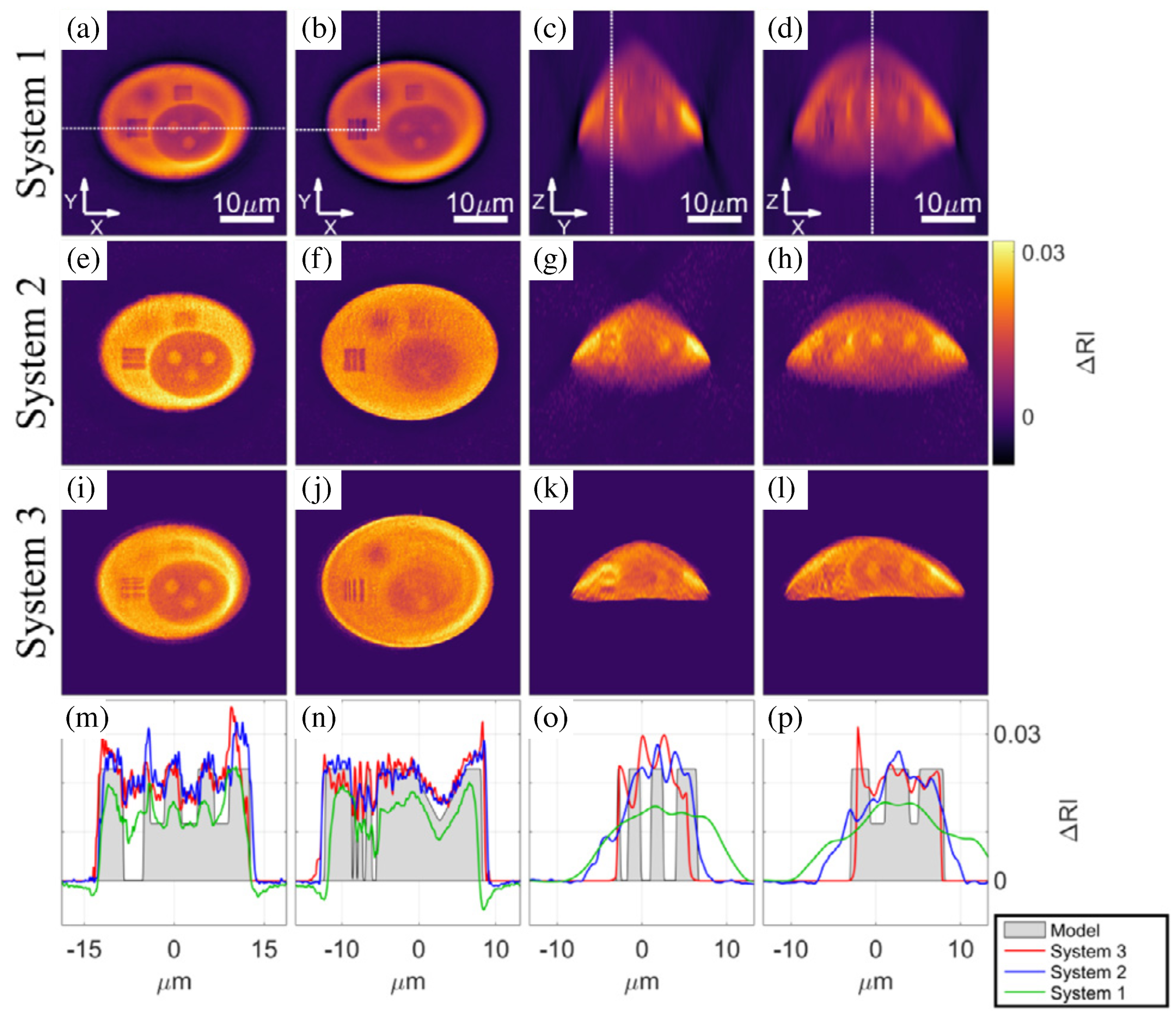
| Field of View | Lateral Resolution | Longitudinal Resolution | Acquisition Speed | Refractive Index Sensitivity | Cost | |
|---|---|---|---|---|---|---|
| Present situation | From about 100 × 100 at high-resolution to millimeter-size samples [240,280,322] | Sub 100 nm lateral resolution demonstrated [32,37] | Usually 2–3 times lower than lateral resolution. Isotropic resolution of about 180 nm demonstrated [37] | From a few seconds (e.g., rotating arm scanning [38,86]) to camera-speed limit only [193] | Δn = commonly obtained, even on biological samples, up to Δn = in large plastic samples [240] | From a few 100€ (lensless tomography) to about 50–70k€ (estimated) for a high-end 4Pi system (salaries and computers not included) |
| Challenges | Keeping high resolution in large volumes as in light-sheet microscopy | Development of nanometric super-resolution for unlabeled samples [311,312] | Isotropic resolution using standard configurations | High resolution with rapid acquisitions compatibility. Combination with other microscopic techniques [323] | Development and adoption of metrological approaches [273,282,283,284] | Development of open-source alternatives, such as the OpenSPIM initiative [324] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verrier, N.; Debailleul, M.; Haeberlé, O. Recent Advances and Current Trends in Transmission Tomographic Diffraction Microscopy. Sensors 2024, 24, 1594. https://doi.org/10.3390/s24051594
Verrier N, Debailleul M, Haeberlé O. Recent Advances and Current Trends in Transmission Tomographic Diffraction Microscopy. Sensors. 2024; 24(5):1594. https://doi.org/10.3390/s24051594
Chicago/Turabian StyleVerrier, Nicolas, Matthieu Debailleul, and Olivier Haeberlé. 2024. "Recent Advances and Current Trends in Transmission Tomographic Diffraction Microscopy" Sensors 24, no. 5: 1594. https://doi.org/10.3390/s24051594





