A Survey on Blood Pressure Measurement Technologies: Addressing Potential Sources of Bias
Abstract
:1. Introduction
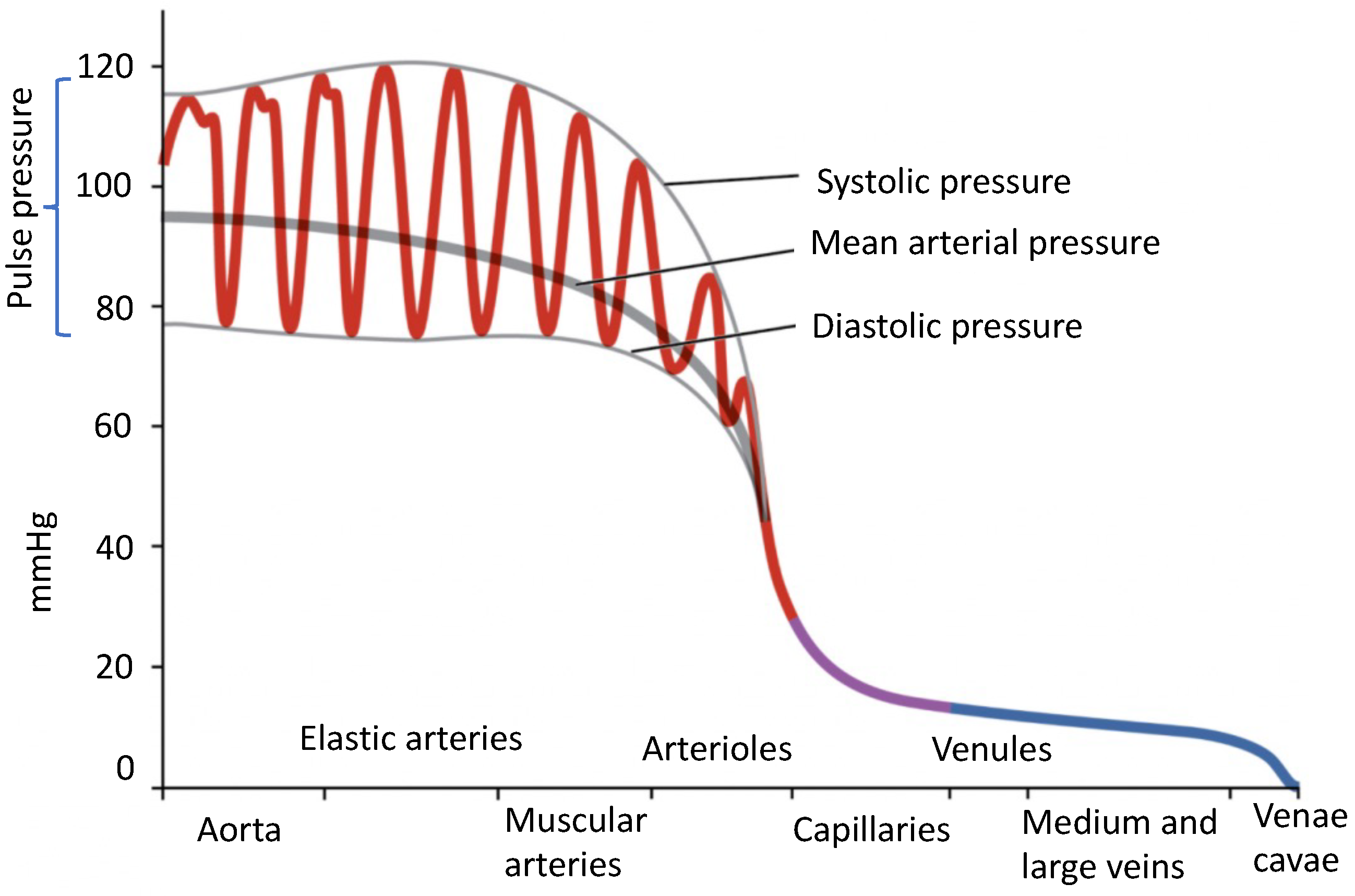
2. A Review of Blood Pressure Physiology
2.1. Blood Pressure Definition
- Systolic blood pressure (SBP), which represents the maximum pressure inside the arteries during cardiac contraction;
- Diastolic blood pressure (DBP), which represents the minimum arterial pressure during cardiac rest;
2.2. Factors Impacting Blood Pressure

2.2.1. Cardiac Output
2.2.2. Compliance
2.2.3. Blood Volume
2.2.4. Blood Viscosity
2.2.5. Blood Vessel Length and Diameter
2.3. Blood Pressure Norms
3. Blood Pressure Measurement Methods
3.1. Invasive Blood Pressure Measurement
3.2. Non-Invasive Blood Pressure Measurement
3.2.1. Auscultatory
3.2.2. Oscillometric
3.2.3. Ultrasound
4. Cuff-Based Blood Pressure Measurement Technologies
4.1. BP Measurement Device Standards
4.2. Commercial Cuff-Based BP Measurement Devices
4.3. Standard Blood Pressure Measurement Conditions
- To maintain a stable BP measurement environment, it is recommended to refrain from opening and closing windows and doors. [42].
- The temperature and relative humidity of the BP measurement environment should be in the range of 15–25 °C and 20–85%, respectively [42].
- The patient should not smoke, eat, or drink for at least 30 min before measuring [55].
- The patient should have adequate rest time before the measurement to stabilize BP.
- The patient should not speak and should remain quiet during the measurement [55].
- The patient should sit on a chair with back and arm supports and without crossing their legs [56].
- The patient’s arm should be placed and remain at the same level as the heart throughout the BP measurement [42].
- The antecubital fossa (the area between the anatomical arm and the forearm) should be 2-3 cm above the lower end of the cuff [57].
- During the measurement, the patient’s feet should remain flat on the floor [55].
- Measuring BP should be performed using direct contact of the cuff with the upper part of the arm (not over sleeves) [56].
5. Potential Sources of Bias in Blood Pressure Technologies
5.1. Biases Related to Blood Pressure Measurement Devices
5.1.1. Main Blood Pressure Measurement Unit
5.1.2. Consumables of Blood Pressure Measurement Devices
5.2. Subject-Specific Biases
5.2.1. Demographic Features
5.2.2. Subject-Specific Factors
5.2.3. Subject-Specific Background Medical Conditions
5.2.4. Eating, Drinking, and Smoking
5.2.5. Circadian Rhythm
5.3. Biases Related to the Acquisition Session
5.3.1. Seasonal Variations and Ambient Temperature
5.3.2. Cuff Position
5.3.3. Body Position
5.3.4. Arm Position
5.3.5. Leg Position
5.3.6. Left vs. Right Arm
5.3.7. Cuff Size and Tightness
5.3.8. Rest Period before Measuring BP
5.3.9. Number of Measurements
5.3.10. Clothing
6. Cuff-Less Blood Pressure Technologies
7. Future Perspectives: Using Machine Learning for Bias Removal and Individualized BP-Level Risk Assessment

8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). 2021. Available online: https://www.who.int/news-room/factsheets/detail/cardiovascular-diseases-(cvds) (accessed on 21 June 2021).
- Ahmad, F.B.; Anderson, R.N. The leading causes of death in the US for 2020. JAMA 2021, 325, 1829–1830. [Google Scholar] [CrossRef]
- Leonardi-Bee, J.; Bath, P.M.; Phillips, S.J.; Sandercock, P.A. Blood Pressure and Clinical Outcomes in the International Stroke Trial. Stroke 2002, 33, 1315–1320. [Google Scholar] [CrossRef]
- Lawes, C.M.; Bennett, D.A.; Feigin, V.L.; Rodgers, A. Blood Pressure and Stroke. Stroke 2004, 35, 776–785. [Google Scholar] [CrossRef]
- Vasan, R.S.; Larson, M.G.; Leip, E.P.; Evans, J.C.; O’Donnell, C.J.; Kannel, W.B.; Levy, D. Impact of High-Normal Blood Pressure on the Risk of Cardiovascular Disease. N. Engl. J. Med. 2001, 345, 1291–1297. [Google Scholar] [CrossRef]
- Zhou, B.; Bentham, J.; Cesare, M.D.; Bixby, H.; Danaei, G.; Cowan, M.J.; Paciorek, C.J.; Singh, G.; Hajifathalian, K.; Bennett, J.E.; et al. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- Brenner, B.M.; Garcia, D.L.; Anderson, S. Glomeruli and Blood Pressure: Less of One, More the Other? Am. J. Hypertens. 1988, 1, 335–347. [Google Scholar] [CrossRef]
- Mukkamala, R.; Hahn, J.O.; Inan, O.T.; Mestha, L.K.; Kim, C.S.; Töreyin, H.; Kyal, S. Toward ubiquitous blood pressure monitoring via pulse transit time: Theory and practice. IEEE Trans. Biomed. Eng. 2015, 62, 1879–1901. [Google Scholar] [CrossRef] [PubMed]
- Kalehoff, J.P.; Oparil, S. The Story of the Silent Killer. Curr. Hypertens. Rep. 2020, 22, 1–14. Available online: https://link.springer.com/article/10.1007/s11906-020-01077-7 (accessed on 27 August 2020). [CrossRef] [PubMed]
- Rastegar, S.; GholamHosseini, H.; Lowe, A. Non-invasive continuous blood pressure monitoring systems: Current and proposed technology issues and challenges. Phys. Eng. Sci. Med. 2020, 43, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Tholl, U.; Forstner, K.; Anlauf, M. Measuring blood pressure: Pitfalls and recommendations. Nephrol. Dial. Transplant. 2004, 19, 766–770. [Google Scholar] [CrossRef]
- World Health Organization. WHO Technical Specifications for Automated Non-Invasive Blood Pressure Measuring Devices with Cuff. 2020; World Health Organization: Geneva, Switzerland, 2020; pp. 1–68. [Google Scholar]
- Van Den Heuvel, J.F.; Lely, A.T.; Franx, A.; Bekker, M.N. Validation of the iHealth Track and Omron HEM-9210T automated blood pressure devices for use in pregnancy. Pregnancy Hypertens. 2019, 15, 37–41. [Google Scholar] [CrossRef]
- Pickering, T.G.; Shimbo, D.; Haas, D. Ambulatory Blood-Pressure Monitoring. N. Engl. J. Med. 2006, 354, 2368–2374. [Google Scholar] [CrossRef]
- Ding, X.R.; Zhao, N.; Yang, G.Z.; Pettigrew, R.I.; Lo, B.; Miao, F.; Li, Y.; Liu, J.; Zhang, Y.T. Continuous blood pressure measurement from invasive to unobtrusive: Celebration of 200th birth anniversary of Carl Ludwig. IEEE J. Biomed. Health Inform. 2016, 20, 1455–1465. [Google Scholar] [CrossRef]
- Sewell, K.; Halanych, J.H.; Russell, L.B.; Andreae, S.J.; Cherrington, A.L.; Martin, M.Y.; Pisu, M.; Safford, M.M. Blood pressure measurement biases in clinical settings, Alabama, 2010–2011. Prev. Chronic Dis. 2016, 13, E01. [Google Scholar] [CrossRef]
- Hoffman, K.M.; Trawalter, S.; Axt, J.R.; Oliver, M.N. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc. Natl. Acad. Sci. USA 2016, 113, 4296–4301. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Le Saux, M.; Siegel, R.; Goyal, M.; Chen, C.; Ma, Y.; Meltzer, A.C. Racial and ethnic disparities in the management of acute pain in US emergency departments: Meta-analysis and systematic review. Am. J. Emerg. Med. 2019, 37, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Valbuena, V.S.M.; Merchant, R.M.; Hough, C.L. Racial and ethnic bias in pulse oximetry and clinical outcomes. JAMA Intern. Med. 2022, 182, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Powers, B.; Vogeli, C.; Mullainathan, S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 2019, 366, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Reyna, M.A.; Nsoesie, E.O.; Clifford, G.D. Rethinking algorithm performance metrics for artificial intelligence in diagnostic medicine. JAMA 2022, 328, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Vokinger, K.N.; Feuerriegel, S.; Kesselheim, A.S. Mitigating bias in machine learning for medicine. Commun. Med. 2021, 1, 25. [Google Scholar] [CrossRef]
- Magder, S. The meaning of blood pressure. Crit. Care 2018, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Iaizzo, P.A. Handbook of Cardiac ANATOMY, Physiology, and Devices; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Mousavi, S.S.; Charmi, M.; Firouzmand, M.; Hemmati, M.; Moghadam, M.; Ghorbani, Y. Designing and Manufacturing a Mobile-Based Ambulatory Monitoring of Blood Pressure Using Electrocardiogram and Photoplethysmography Signals. Master’s Thesis, Zanjan University, Zanjan, Iran, 2018. [Google Scholar]
- Lowry, M.; Ashelford, S. Assessing the Pulse Rate in Adult Patients. Nurs. Times 2015, 111, 18–20. Available online: https://www.nursingtimes.net/clinical-archive/assessment-skills/assessing-the-pulse-rate-in-adult-patients-31-08-2015/ (accessed on 11 September 2015).
- Betts, J.; Desaix, P.; Johnson, E.; Johnson, J.; Korol, O.; Kruse, D.; Poe, B.; Wise, J.; Womble, M.; Young, K. Anatomy and Physiology; OpenStax: Houston, TX, USA, 2013. [Google Scholar]
- Vlachopoulos, C.; O’Rourke, M.; Nichols, W.W. McDonald’s Blood Flow in Arteries, 6th ed.; Hodder Arnold: London, UK, 2011. [Google Scholar] [CrossRef]
- Lapum, J.L.; Verkuyl, M.; Garcia, W.; St-Amant, O.; Tan, A. Vital Sign Measurement across the Lifespan—2nd Canadian Edition; Ryerson University: Toronto, ON, Canada, 2021; Available online: https://pressbooks.library.torontomu.ca/vitalsign/ (accessed on 21 January 2021).
- Sapra, A.; Malik, A.; Bhandari, P. Vital sign assessment. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- DeSaix, P.; Betts, G.J.; Johnson, E.; Johnson, J.E.; Oksana, K.; Kruse, D.H.; Poe, B.; Wise, J.A.; Young, K.A. Anatomy & Physiology (OpenStax), 2013. Available online: https://assets.openstax.org/oscms-prodcms/media/documents/AnatomyandPhysiology-OP.pdf (accessed on 20 April 2022).
- Glasser, S. Vascular compliance and cardiovascular disease: A risk factor or a marker? Am. J. Hypertens. 1997, 10, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Bazett, H. The time relations of the blood-pressure changes after excision of the adrenal glands, with some observations on blood volume changes. J. Physiol. 1920, 53, 320. [Google Scholar] [CrossRef] [PubMed]
- Letcher, R.L.; Chien, S.; Pickering, T.G.; Sealey, J.E.; Laragh, J.H. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects. Role of fibrinogen and concentration. Am. J. Med. 1981, 70, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.; Sanye-Hajari, J.; Bek, T. Increased blood pressure induces a diameter response of retinal arterioles that increases with decreasing arteriolar diameter. Invest. Ophthalmol. Vis. Sci. 2007, 48, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Azegami, T.; Uchida, K.; Tokumura, M.; Mori, M. Blood Pressure Tracking From Childhood to Adulthood. Front. Pediatr. 2021, 9, 785356. [Google Scholar] [CrossRef]
- Falkner, B.; Gidding, S.S.; Baker-Smith, C.M.; Brady, T.M.; Flynn, J.T.; Malle, L.M.; South, A.M.; Tran, A.H.; Urbina, E.M. Pediatric Primary Hypertension: An Underrecognized Condition: A Scientific Statement From the American Heart Association. Hypertension 2023, 80, e101–e111. [Google Scholar] [CrossRef]
- Cole, E. Measuring central venous pressure. Nurs. Stand. 2007, 22, 40–42. [Google Scholar] [CrossRef]
- Webster, J. Medical instrumentation: Application and Design; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Kumar, R.; Dubey, P.K.; Zafer, A.; Kumar, A.; Yadav, S. Past, present and future of blood pressure measuring instruments and their calibration. Measurement 2021, 172, 108845. [Google Scholar] [CrossRef]
- Peter, L.; Noury, N.; Cerny, M. A review of methods for non-invasive and continuous blood pressure monitoring: Pulse transit time method is promising? Irbm 2014, 35, 271–282. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, S.H. Blood pressure measurements and hypertension in infants, children, and adolescents: From the postmercury to mobile devices. Clin. Exp. Pediatr. 2022, 65, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Yarows, S.A.; Qian, K. Accuracy of aneroid sphygmomanometers in clinical usage: University of Michigan experience. Blood Press. Monit. 2001, 6, 101–106. [Google Scholar] [CrossRef]
- Canzanello, V.J.; Jensen, P.L.; Schwartz, G.L. Are Aneroid Sphygmomanometers Accurate in Hospital and Clinic Settings? Arch. Intern. Med. 2001, 161, 729. [Google Scholar] [CrossRef] [PubMed]
- Mion, D.; Pierin, A. How accurate are sphygmomanometers? J. Hum. Hypertens. 1998, 12, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Ogedegbe, G.; Pickering, T. Principles and techniques of blood pressure measurement. Cardiol. Clin. 2010, 28, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Association for the Advancement of Medical Instrumentation and others. ANSI/AAMI SP 10-1987; Association for the Advancement of Medical Instrumentation: Arlington, VA, USA, 1987. [Google Scholar]
- Geršak, G.; Žemva, A.; Drnovšek, J. A procedure for evaluation of non-invasive blood pressure simulators. Med. Biol. Eng. Comput. 2009, 47, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.S.; Alpert, B.; Mieke, S.; Asmar, R.; Atkins, N.; Eckert, S.; Frick, G.; Friedman, B.; Graßl, T.; Ichikawa, T.; et al. A universal standard for the validation of Blood Pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) collaboration statement. Hypertension 2018, 71, 368–374. [Google Scholar] [CrossRef]
- ISO 81060-2:2018/Amd.1:2020; Non-Invasive Sphygmomanometers—Part 2: Clinical Investigation of Intermittent Automated Measurement—Amendment 1. International Organization for Standardization: Geneva, Switzerland, 2020.
- Hassler, C.; Burnier, M. Circadian variations in blood pressure. Am. J. Cardiovasc. Drugs 2005, 5, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Toftegaard, T.S.; Bertelsen, O.W. Challenges in blood pressure self-measurement. Int. J. Telemed. Appl. 2012, 2012, 437350. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.S.; Palatini, P.; Parati, G.; O’Brien, E.; Januszewicz, A.; Lurbe, E.; Persu, A.; Mancia, G.; Kreutz, R.; European Society of Hypertension Council and the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J. Hypertens. 2021, 39, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Li, J.; Zheng, D.; Liu, C. Sources of automatic office blood pressure measurement error: A systematic review. Physiol. Meas. 2022, 43, 09TR02. [Google Scholar] [CrossRef]
- Muntner, P.; Shimbo, D.; Carey, R.M.; Charleston, J.B.; Gaillard, T.; Misra, S.; Myers, M.G.; Ogedegbe, G.; Schwartz, J.E.; Townsend, R.R.; et al. Measurement of blood pressure in humans: A scientific statement from the American Heart Association. Hypertension 2019, 73, e35–e66. [Google Scholar] [CrossRef]
- Kallioinen, N.; Hill, A.; Horswill, M.S.; Ward, H.E.; Watson, M.O. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: A systematic review. J. Hypertens. 2017, 35, 421. [Google Scholar] [CrossRef] [PubMed]
- ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2013, 31, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Yong, P.G.; Geddes, L.A. The effect of cuff pressure deflation rate on accuracy in indirect measurement of blood pressure with the auscultatory method. J. Clin. Monit. 1987, 3, 155–159. [Google Scholar] [CrossRef]
- Speechly, C.; Bignell, N.; Turner, M. Sphygmomanometer calibration: Why, how and how often? Aust. Fam. Physician 2007, 36, 834–837. [Google Scholar]
- Turner, M.J.; Baker, A.B.; Kam, P.C. Effects of systematic errors in blood pressure measurements on the diagnosis of hypertension. Blood Press. Monit. 2004, 9, 249–253. [Google Scholar] [CrossRef]
- Yayan, E.H.; Zengin, M. A Key Point in Medical Measurements: Device Calibration and Knowledge Level of Healthcare Professionals. Int. J. Caring Sci. 2020, 13, 1346–1354. [Google Scholar]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005, 45, 142–161. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Sex Differences in Regulation of Blood Pressure. In Advances in Experimental Medicine and Biology; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 139–151. [Google Scholar] [CrossRef]
- Sandberg, K.; Ji, H. Sex differences in primary hypertension. Biol. Sex Differ. 2012, 3, 7. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Merz, C.N.B.; Cheng, S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020, 5, 255. [Google Scholar] [CrossRef]
- Somani, Y.B.; Baross, A.W.; Brook, R.D.; Milne, K.J.; McGowan, C.L.; Swaine, I.L. Acute response to a 2-minute isometric exercise test predicts the blood pressure-lowering efficacy of isometric resistance training in young adults. Am. J. Hypertens. 2018, 31, 362–368. [Google Scholar] [CrossRef]
- Olatunji, L.; Aaron, A.; Michael, O.; Oyeyipo, I. Water ingestion affects orthostatic challenge-induced blood pressure and heart rate responses in young healthy subjects: Gender implications. Niger. J. Physiol. Sci. 2011, 26, 11–18. [Google Scholar]
- Kho, Y.L.; Yi, S.G.; Lee, E.H.; Chung, M.H. Acute Effects of Tobacco and Non-tobacco Cigarette Smoking on the Blood Pressure and Heart Rate. J. Environ. Health Sci. 2006, 32, 222–226. [Google Scholar]
- Papakonstantinou, E.; Kechribari, I.; Sotirakoglou, K.; Tarantilis, P.; Gourdomichali, T.; Michas, G.; Kravvariti, V.; Voumvourakis, K.; Zampelas, A. Acute effects of coffee consumption on self-reported gastrointestinal symptoms, blood pressure and stress indices in healthy individuals. Nutr. J. 2016, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Monnard, C.R.; Grasser, E.K. Water ingestion decreases cardiac workload time-dependent in healthy adults with no effect of gender. Sci. Rep. 2017, 7, 7939. [Google Scholar] [CrossRef] [PubMed]
- Helfer, S.G.; McCubbin, J.A. Does gender affect the relation between blood pressure and pain sensitivity? Int. J. Behav. Med. 2001, 8, 220–229. [Google Scholar] [CrossRef]
- Harshfield, G.A.; Alpert, B.S.; Willey, E.S.; Somes, G.W.; Murphy, J.K.; Dupaul, L.M. Race and gender influence ambulatory blood pressure patterns of adolescents. Hypertension 1989, 14, 598–603. [Google Scholar] [CrossRef]
- Costa-Hong, V.A.; Muela, H.C.S.; Macedo, T.A.; Sales, A.R.K.; Bortolotto, L.A. Gender differences of aortic wave reflection and influence of menopause on central blood pressure in patients with arterial hypertension. BMC Cardiovasc. Disord. 2018, 18, 123. [Google Scholar] [CrossRef]
- Ki, J.H.; Oh, M.K.; Lee, S.H. Differences in blood pressure measurements obtained using an automatic oscillometric sphygmomanometer depending on clothes-wearing status. Korean J. Fam. Med. 2013, 34, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Poole, J.C.; Treiber, F.A.; Harshfield, G.A.; Hanevold, C.D.; Snieder, H. Ethnic and gender differences in ambulatory blood pressure trajectories: Results from a 15-year longitudinal study in youth and young adults. Circulation 2006, 114, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Song, B.M.; Kim, H.C.; Shim, J.S.; Lee, M.H.; Choi, D.P. Inter-arm difference in brachial blood pressure in the general population of Koreans. Korean Circ. J. 2016, 46, 374–383. [Google Scholar] [CrossRef]
- Lan, Y.L.; Chen, T.L. Prevalence of high blood pressure and its relationship with body weight factors among inpatients with schizophrenia in Taiwan. Asian Nurs. Res. 2012, 6, 13–18. [Google Scholar] [CrossRef]
- Privšek, E.; Hellgren, M.; Råstam, L.; Lindblad, U.; Daka, B. Epidemiological and clinical implications of blood pressure measured in seated versus supine position. Medicine 2018, 97, e11603. [Google Scholar] [CrossRef]
- Cui, J.; Hopper, J.L.; Harrap, S.B. Genes and family environment explain correlations between blood pressure and body mass index. Hypertension 2002, 40, 7–12. [Google Scholar] [CrossRef]
- Vallée, A.; Perrine, A.L.; Deschamps, V.; Blacher, J.; Olié, V. Relationship between dynamic changes in body weight and blood pressure: The ESTEBAN survey. Am. J. Hypertens. 2019, 32, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Giggey, P.P.; Wendell, C.R.; Zonderman, A.B.; Waldstein, S.R. Greater coffee intake in men is associated with steeper age-related increases in blood pressure. Am. J. Hypertens. 2011, 24, 310–315. [Google Scholar] [CrossRef]
- Bourgeois, B.; Watts, K.; Thomas, D.M.; Carmichael, O.; Hu, F.B.; Heo, M.; Hall, J.E.; Heymsfield, S.B. Associations between height and blood pressure in the United States population. Medicine 2017, 96, e9233. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.H.; Nanas, S.; Dyer, A.; Liu, K.; Mcdonald, A.; Schoenberger, J.A.; Shekelle, R.B.; Stamler, R.; Stamler, J. The role of weight in the positive association between age and blood pressure. Am. J. Epidemiol. 1986, 124, 612–623. [Google Scholar] [CrossRef]
- Carrico, R.J.; Sun, S.S.; Sima, A.P.; Rosner, B. The predictive value of childhood blood pressure values for adult elevated blood pressure. Open J. Pediatr. 2013, 3, 116. [Google Scholar] [CrossRef]
- Jones, D.W.; Hall, J.E. Racial and Ethnic Differences in Blood Pressure. Circulation 2006, 114, 2757–2759. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.T.; Chen, L.; Cherrington, A.L.; Moise, N.; Jaeger, B.C.; Foti, K.; Sakhuja, S.; Wozniak, G.; Abdalla, M.; Muntner, P. Racial and ethnic differences in blood pressure among US adults, 1999–2018. Hypertension 2021, 78, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.S.; Kaufman, J.S. Race and Hypertension. Hypertension 1998, 32, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Fryar, C.D.; Ostchega, Y.; Hales, C.M.; Zhang, G.; Kruszon-Moran, D. Hypertension Prevalence and Control among Adults: United States, 2015–2016; National Center for Health Statistics: Hyattsville, MA, USA, 2017. Available online: https://www.cdc.gov/nchs/products/databriefs/db289.htm (accessed on October 2017).
- Staessen, J.A.; Bieniaszewski, L.; O’Brien, E.; Gosse, P.; Hayashi, H.; Imai, Y.; Kawasaki, T.; Otsuka, K.; Palatini, P.; Thijs, L.; et al. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. The “Ad Hoc’ Working Group. Hypertension 1997, 29, 30–39. [Google Scholar] [CrossRef]
- Mayet, J.; Chapman, N.; Li, C.K.C.; Shahi, M.; Poulter, N.R.; Sever, P.S.; Foale, R.A.; Thom, S.A.M. Ethnic differences in the hypertensive heart and 24-hour blood pressure profile. Hypertension 1998, 31, 1190–1194. [Google Scholar] [CrossRef]
- He, Q.; Ding, Z.Y.; Fong, D.Y.T.; Karlberg, J. Blood pressure is associated with body mass index in both normal and obese children. Hypertension 2000, 36, 165–170. [Google Scholar] [CrossRef]
- Jena, S.K.; Pattnaik, M. Relationship between body mass index and blood pressure in school students. CHRISMED J. Health Res. 2018, 5, 187. [Google Scholar]
- Neter, J.E.; Stam, B.E.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Influence of weight reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2003, 42, 878–884. [Google Scholar] [CrossRef]
- Tibana, R.A.; Pereira, G.B.; Navalta, J.W.; Bottaro, M.; Prestes, J. Acute effects of resistance exercise on 24-h blood pressure in middle aged overweight and obese women. Int. J. Sports Med. 2013, 34, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Rontoyanni, V.G.; Protogerou, A.D.; Georgoulia, A.; Xenos, K.; Chrysou, J.; Sfikakis, P.P.; Sidossis, L.S. Acute effects of beer on endothelial function and hemodynamics: A single-blind, crossover study in healthy volunteers. Nutrition 2013, 29, 1122–1126. [Google Scholar] [CrossRef]
- Fantin, F.; Bulpitt, C.J.; Zamboni, M.; Cheek, E.; Rajkumar, C. Arterial compliance may be reduced by ingestion of red wine. J. Hum. Hypertens. 2016, 30, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Kayrak, M.; Ulgen, M.S.; Yazici, M.; Yilmaz, R.; Demir, K.; Dogan, Y.; Ozhan, H.; Alihanoglu, Y.; Koc, F.; Bodur, S. A comparison of blood pressure and pulse pressure values obtained by oscillometric and central measurements in hypertensive patients. Blood Press. 2010, 19, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.M.; Vilaça-Alves, J.; Noleto, M.V.; Silva, J.S.; Costa, A.M.; Silva, C.N.F.; Póvoa, T.I.R.; Lehnen, A.M. Acute blood pressure response in hypertensive elderly women immediately after water aerobics exercise: A crossover study. Clin. Exp. Hypertens. 2017, 39, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Netea, R.T.; Lenders, J.W.M.; Smits, P.; Thien, T. Both body and arm position significantly influence blood pressure measurement. J. Hum. Hypertens. 2003, 17, 459–462. [Google Scholar] [CrossRef]
- Talukder, M.R.R.; Rutherford, S.; Phung, D.; Islam, M.Z.; Chu, C. The effect of drinking water salinity on blood pressure in young adults of coastal Bangladesh. Environ. Pollut. 2016, 214, 248–254. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Wang, B.; Yang, H.; Ban, J.; Liu, F.; Li, T. Acute effects of temperature exposure on blood pressure: An hourly level panel study. Environ. Int. 2019, 124, 493–500. [Google Scholar] [CrossRef]
- Azar, R.R.; Frangieh, A.H.; Mroué, J.; Bassila, L.; Kasty, M.; Hage, G.; Kadri, Z. Acute effects of waterpipe smoking on blood pressure and heart rate: A real-life trial. Inhal. Toxicol. 2016, 28, 339–342. [Google Scholar] [CrossRef]
- Krzesiński, P.; Stańczyk, A.; Gielerak, G.; Piotrowicz, K.; Banak, M.; Wójcik, A. The diagnostic value of supine blood pressure in hypertension. Arch. Med. Sci. 2016, 12, 310–318. [Google Scholar] [CrossRef]
- Li, Y.; Thijs, L.; Zhang, Z.Y.; Asayama, K.; Hansen, T.W.; Boggia, J.; Björklund-Bodegård, K.; Yang, W.Y.; Niiranen, T.J.; Ntineri, A.; et al. Opposing age-related trends in absolute and relative risk of adverse health outcomes associated with out-of-office blood pressure. Hypertension 2019, 74, 1333–1342. [Google Scholar] [CrossRef]
- Walker, K.A.; Sharrett, A.R.; Wu, A.; Schneider, A.L.C.; Albert, M.; Lutsey, P.L.; Bandeen-Roche, K.; Coresh, J.; Gross, A.L.; Windham, B.G.; et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA 2019, 322, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Vita, J.A.; Keyes, M.J.; Larson, M.G.; Hamburg, N.M.; Levy, D.; Mitchell, G.F.; Osypiuk, E.W.; Vasan, R.S.; Benjamin, E.J. Relation of season and temperature to endothelium-dependent flow-mediated vasodilation in subjects without clinical evidence of cardiovascular disease (from the Framingham Heart Study). Am. J. Cardiol. 2007, 100, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Barba, G.; Troiano, E.; Russo, P.; Strazzullo, P.; Siani, A. Body mass, fat distribution and blood pressure in Southern Italian children: Results of the ARCA project. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Hara, A.; Asayama, K.; Miyazaki, S.; Kikuya, M.; Imai, Y.; Ohkubo, T. Antihypertensive drug effects according to the pretreatment self-measured home blood pressure: The HOMED-BP study. BMJ Open 2020, 10, e040524. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, M.Z.; Cheng, Z.Y.; Kang, F.; Nie, Y.H.; Mi, X.Y.; Li, H.Y.; Jin, L.; Zhang, Y.W.; Bai, Y.N. Effects of outdoor temperature on blood pressure in a prospective cohort of northwest China. Biomed. Environ. Sci. 2021, 34, 89–100. [Google Scholar] [CrossRef]
- Kang, Y.; Han, Y.; Guan, T.; Wang, X.; Xue, T.; Chen, Z.; Jiang, L.; Zhang, L.; Zheng, C.; Wang, Z.; et al. Clinical blood pressure responses to daily ambient temperature exposure in China: An analysis based on a representative nationwide population. Sci. Total Environ. 2020, 705, 135762. [Google Scholar] [CrossRef]
- Lewington, S.; Li, L.; Sherliker, P.; Guo, Y.; Millwood, I.; Bian, Z.; Whitlock, G.; Yang, L.; Collins, R.; Chen, J.; et al. Seasonal variation in blood pressure and its relationship with outdoor temperature in 10 diverse regions of China. J. Hypertens. 2012, 30, 1383–1391. [Google Scholar] [CrossRef]
- Ohkubo, T.; Hozawa, A.; Yamaguchi, J.; Kikuya, M.; Ohmori, K.; Michimata, M.; Matsubara, M.; Hashimoto, J.; Hoshi, H.; Araki, T.; et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure. J. Hypertens. 2002, 20, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Routledge, F.; Mc Fetridge-Durdle, J. Nondipping blood pressure patterns among individuals with essential hypertension: A review of the literature. Eur. J. Cardiovasc. Nurs. 2007, 6, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Pickering, T.G.; Kario, K. Nocturnal non-dipping: What does it augur? Curr. Opin. Nephrol. Hypertens. 2001, 10, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Ragot, S.; Genes, N.; Vaur, L.; Herpin, D. Comparison of three blood pressure measurement methods for the evaluation of two antihypertensive drugs: Feasibility, agreement, and reproducibility of blood pressure response. Am. J. Hypertens. 2000, 13, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Asayama, K.; Ohkubo, T.; Rakugi, H.; Miyakawa, M.; Mori, H.; Katsuya, T.; Ikehara, Y.; Ueda, S.; Ohya, Y.; Tsuchihashi, T.; et al. Direct comparison of the reproducibility of in-office and self-measured home blood pressures. J. Hypertens. 2022, 40, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Katebi, N.; Clifford, G.D. Deep sequence learning for assessing hypertension in pregnancy from Doppler signals. medRxiv 2022. [CrossRef]
- Katebi, N.; Bremer, W.; Nguyen, T.; Phan, D.; Jeff, J.; Armstrong, K.; Phabian-Millbrook, P.; Platner, M.; Carroll, K.; Shoai, B.; et al. Automated image transcription for perinatal blood pressure monitoring using mobile health technology. medRxiv 2023. [CrossRef]
- Forman, J.P. Diet and Lifestyle Risk Factors Associated With Incident Hypertension in Women. JAMA 2009, 302, 401. [Google Scholar] [CrossRef]
- Buckman, J.F.; Eddie, D.; Vaschillo, E.G.; Vaschillo, B.; Garcia, A.; Bates, M.E. Immediate and complex cardiovascular adaptation to an acute alcohol dose. Alcohol. Clin. Exp. Res. 2015, 39, 2334–2344. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Kawano, Y. Diurnal blood pressure variation and related behavioral factors. Hypertens. Res. 2010, 34, 281–285. [Google Scholar] [CrossRef]
- Jansen, R.W.; Penterman, B.J.; Lier, H.J.V.; Hoefnagels, W.H. Blood pressure reduction after oral glucose loading and its relation to age, blood pressure and insulin. Am. J. Cardiol. 1987, 60, 1087–1091. [Google Scholar] [CrossRef]
- Sidery, M.B.; Cowley, A.J.; MacDonald, I.A. Cardiovascular responses to a high-fat and a high-carbohydrate meal in healthy elderly subjects. Clin. Sci. 1993, 84, 263–270. [Google Scholar] [CrossRef]
- Burnier, M. Should we eat more potassium to better control blood pressure in hypertension? Nephrol. Dial. Transplant. 2019, 34, 184–193. [Google Scholar] [CrossRef]
- Nishiwaki, M.; Kora, N.; Matsumoto, N. Ingesting a small amount of beer reduces arterial stiffness in healthy humans. Physiol. Rep. 2017, 5, e13381. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.K.; Whitehouse, J.M.; Shine, G.; Towell, A. Habitual coffee and tea drinkers experienced increases in blood pressure after consuming low to moderate doses of caffeine; these increases were larger upright than in the supine posture. Food Funct. 2011, 2, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.R.; Stream, S.F.; Durocher, J.J.; Larson, R.A. Influence of acute alcohol ingestion on sympathetic neural responses to orthostatic stress in humans. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E771–E778. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Gośliński, M.; Wesołowska, A.; Berenda, K.; Popławski, C. Effects of acute consumption of Noni and chokeberry juices vs. Energy drinks on blood pressure, heart rate, and blood glucose in young adults. Evid. Based Complement. Alternat. Med. 2019, 2019, 6076751. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; Khan, M. An Experimental Study of Short-term Physiological Effects of a Single Dose of Energy Drink in Healthy Male Medical Students. Pak. J. Med. Health Sci. 2019, 13, 685–689. [Google Scholar]
- Morris, C.J.; Yang, J.N.; Scheer, F.A. The impact of the circadian timing system on cardiovascular and metabolic function. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2012; pp. 337–358. [Google Scholar] [CrossRef]
- Richards, J.; Gumz, M.L. Advances in understanding the peripheral circadian clocks. FASEB J. 2012, 26, 3602–3613. [Google Scholar] [CrossRef]
- Hower, I.M.; Harper, S.A.; Buford, T.W. Circadian rhythms, exercise, and cardiovascular health. J. Circadian Rhythm. 2018, 16, 7. [Google Scholar] [CrossRef]
- Boivin, J.M.; Boutte, E.; Fay, R.; Rossignol, P.; Zannad, F. Home blood pressure monitoring: A few minutes of rest before measurement may not be appropriate. Am. J. Hypertens. 2014, 27, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kario, K.; Chia, Y.C.; Turana, Y.; Chen, C.H.; Buranakitjaroen, P.; Nailes, J.; Hoshide, S.; Siddique, S.; Sison, J.; et al. The influence of the ambient temperature on blood pressure and how it will affect the epidemiology of hypertension in Asia. J. Clin. Hypertens. 2019, 22, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Sega, R.; Cesana, G.; Bombelli, M.; Grassi, G.; Stella, M.L.; Zanchetti, A.; Mancia, G. Seasonal variations in home and ambulatory blood pressure in the PAMELA population. J. Hypertens. 1998, 16, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.M.; Leineweber, M.J.; Thien, T. The effect of a change in ambient temperature on blood pressure in normotensives. J. Hum. Hypertens. 2001, 15, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, L.; Lewington, S.; Guo, Y.; Sherliker, P.; Bian, Z.; Collins, R.; Peto, R.; Liu, Y.; Yang, R.; et al. Outdoor temperature, blood pressure, and cardiovascular disease mortality among 23000 individuals with diagnosed cardiovascular diseases from China. Eur. Heart J. 2015, 36, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Deng, F.; Huang, J.; Wang, X.; Qin, Y.; Zheng, C.; Wei, H.; Shima, M.; Guo, X. Does ambient temperature interact with air pollution to alter blood pressure? A repeated-measure study in healthy adults. J. Hypertens. 2015, 33, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, S.; Cheong, H.K.; Ahn, B.; Choi, K. Effects of heat wave on body temperature and blood pressure in the poor and elderly. Environ. Health Toxicol. 2012, 27, e2012013. [Google Scholar] [CrossRef] [PubMed]
- Bilo, G.; Sala, O.; Perego, C.; Faini, A.; Gao, L.; Głuszewska, A.; Ochoa, J.E.; Pellegrini, D.; Lonati, L.M.; Parati, G. Impact of cuff positioning on blood pressure measurement accuracy: May a specially designed cuff make a difference? Hypertens. Res. 2017, 40, 573–580. [Google Scholar] [CrossRef]
- Hinghofer-Szalkay, H. Gravity, the hydrostatic indifference concept and the cardiovascular system. Eur. J. Appl. Physiol. 2011, 111, 163–174. [Google Scholar] [CrossRef]
- Al-Qatatsheh, A.; Morsi, Y.; Zavabeti, A.; Zolfagharian, A.; Salim, N.; Z Kouzani, A.; Mosadegh, B.; Gharaie, S. Blood pressure sensors: Materials, fabrication methods, performance evaluations and future perspectives. Sensors 2020, 20, 4484. [Google Scholar] [CrossRef] [PubMed]
- Sareen, P.; Saxena, K.; Sareen, B.; Taneja, B. Comparison of arm and calf blood pressure. Indian J. Anaesth. 2012, 56, 83. [Google Scholar] [CrossRef] [PubMed]
- Eşer, İ.; Khorshid, L.; Yapucu Güneş, Ü.; Demir, Y. The effect of different body positions on blood pressure. J. Clin. Nurs. 2007, 16, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Chachula, K.; Lieb, F.; Hess, F.; Welter, J.; Graf, N.; Dullenkopf, A. Non-invasive continuous blood pressure monitoring (ClearSight™ system) during shoulder surgery in the beach chair position: A prospective self-controlled study. BMC Anesthesiol. 2020, 20, 271. [Google Scholar] [CrossRef]
- Netea, R.T.; Smits, P.; Lenders, J.W.; Thien, T. Does it matter whether blood pressure measurements are taken with subjects sitting or supine? J. Hypertens. 1998, 16, 263–268. [Google Scholar] [CrossRef]
- Cicolini, G.; Pizzi, C.; Palma, E.; Bucci, M.; Schioppa, F.; Mezzetti, A.; Manzoli, L. Differences in blood pressure by body position (supine, Fowler’s, and sitting) in hypertensive subjects. Am. J. Hypertens. 2011, 24, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Adiyaman, A.; Verhoeff, R.; Lenders, J.W.; Deinum, J.; Thien, T. The position of the arm during blood pressure measurement in sitting position. Blood Press. Monit. 2006, 11, 309–313. [Google Scholar] [CrossRef]
- Mariotti, G.; Alli, C.; Avanzini, F.; Canciani, C.; Tullio, M.D.; Manzini, M.; Salmoirago, E.; Taioli, E.; Zussino, A.; Radice, M. Arm position as a source of error in blood pressure measurement. Clin. Cardiol. 1987, 10, 591–593. [Google Scholar] [CrossRef]
- Netea, R.; Lenders, J.; Smits, P.; Thien, T. Arm position is important for blood pressure measurement. J. Hum. Hypertens. 1999, 13, 105–109. [Google Scholar] [CrossRef]
- Foster-Fitzpatrick, L.; Ortiz, A.; Sibilano, H.; Marcantonio, R.; Braun, L.T. The Effects of Crossed Leg on Blood Pressure Measurement. Nurs. Res. 1999, 48, 105–108. [Google Scholar] [CrossRef]
- Adiyaman, A.; Tosun, N.; Elving, L.D.; Deinum, J.; Lenders, J.W.; Thien, T. The effect of crossing legs on blood pressure. Blood Press. Monit. 2007, 12, 189–193. [Google Scholar] [CrossRef]
- Pinar, R.; Sabuncu, N.; Oksay, A. Effects of crossed leg on blood pressure. Blood Press. 2004, 13, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Fred, H.L. Accurate blood pressure measurements and the other arm: The doctor is ultimately responsible. Tex. Heart Inst. J. 2013, 40, 217. [Google Scholar] [PubMed]
- Lane, D.; Beevers, M.; Barnes, N.; Bourne, J.; John, A.; Malins, S.; Beevers, D.G. Inter-Arm Differences in Blood Pressure: When Are They Clinically Significant. J. Hypertens. 2002, 20, 1089–1095. Available online: https://journals.lww.com/jhypertension/Abstract/2002/06000/Inter_arm_differences_in_blood_pressure__when_are.19.aspx (accessed on 1 March 2024). [CrossRef] [PubMed]
- Bur, A.; Hirschl, M.M.; Herkner, H.; Oschatz, E.; Kofler, J.; Woisetschläger, C.; Laggner, A.N. Accuracy of oscillometric blood pressure measurement according to the relation between cuff size and upper-arm circumference in critically ill patients. Crit. Care Med. 2000, 28, 371–376. [Google Scholar] [CrossRef]
- Palatini, P.; Asmar, R. Cuff challenges in blood pressure measurement. J. Clin. Hypertens. 2018, 20, 1100–1103. [Google Scholar] [CrossRef]
- Bakx, C.; Oerlemans, G.; van den Hoogen, H.; van Weel, C.; Thien, T. The influence of cuff size on blood pressure measurement. J. Hum. Hypertens. 1997, 11, 439–445. [Google Scholar] [CrossRef]
- Nikolic, S.B.; Abhayaratna, W.P.; Leano, R.; Stowasser, M.; Sharman, J.E. Waiting a few extra minutes before measuring blood pressure has potentially important clinical and research ramifications. J. Hum. Hypertens. 2013, 28, 56–61. [Google Scholar] [CrossRef]
- Sala, C.; Santin, E.; Rescaldani, M.; Magrini, F. How Long Shall the Patient Rest Before Clinic Blood Pressure Measurement? Am. J. Hypertens. 2006, 19, 713–717. [Google Scholar] [CrossRef]
- Mancia, G. Effects of blood-pressure measurement by the doctor on patient’s blood pressure and heart rate. Lancet 1983, 322, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Pickering, T.G.; White, W.B. When and how to use self (home) and ambulatory blood pressure monitoring. J. Am. Soc. Hypertens. 2008, 2, 119–124. [Google Scholar] [CrossRef]
- Sharma, M.; Barbosa, K.; Ho, V.; Griggs, D.; Ghirmai, T.; Krishnan, S.; Hsiai, T.; Chiao, J.C.; Cao, H. Cuff-less and continuous blood pressure monitoring: A methodological review. Technologies 2017, 5, 21. [Google Scholar] [CrossRef]
- Geddes, L.A. Handbook of Blood Pressure Measurement; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Mukkamala, R.; Hahn, J.O. Toward ubiquitous blood pressure monitoring via pulse transit time: Predictions on maximum calibration period and acceptable error limits. IEEE Trans. Biomed. Eng. 2018, 65, 1410–1420. [Google Scholar] [CrossRef]
- Nam, D.H.; Lee, W.B.; Hong, Y.S.; Lee, S.S. Measurement of spatial pulse wave velocity by using a clip-type pulsimeter equipped with a Hall sensor and photoplethysmography. Sensors 2013, 13, 4714–4723. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Chen, X.; Deng, N. Mechanism of magnetic pulse wave signal for blood pressure measurement. J. Biomed. Sci. Eng. 2016, 9, 29–36. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Teo, J.T.; Ng, S.H. Noninvasive monitoring of blood pressure using optical Ballistocardiography and Photoplethysmograph approaches. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 2425–2428. [Google Scholar] [CrossRef]
- Liu, S.H.; Cheng, D.C.; Su, C.H. A cuffless blood pressure measurement based on the impedance plethysmography technique. Sensors 2017, 17, 1176. [Google Scholar] [CrossRef]
- Chen, S.; Ji, Z.; Wu, H.; Xu, Y. A non-invasive continuous blood pressure estimation approach based on machine learning. Sensors 2019, 19, 2585. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Firouzmand, M.; Charmi, M.; Hemmati, M.; Moghadam, M.; Ghorbani, Y. Blood pressure estimation from appropriate and inappropriate PPG signals using A whole-based method. Biomed. Signal Process. Control 2019, 47, 196–206. [Google Scholar] [CrossRef]
- Division of Small Manufacturers Assistance, Office of Training and Assistance. Premarket Notification, 510 (k): Regulatory Requirements for Medical Devices; U.S. Dept. of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Devices and Radiological Health: Rockville, MD, USA; Washington, DC, USA, 1990.
- Alpert, B.S.; Dart, R.A.; Sica, D.A. Public-use blood pressure measurement: The kiosk quandary. J. Am. Soc. Hypertens. 2014, 8, 739–742. [Google Scholar] [CrossRef]
- Donawa, M.E. Continuing Evolution of the US FDA 510 (k) Process. Eur. Med. Device Technol. 2010, 1, 10–13. [Google Scholar]
- Alpert, B.S. Can ‘FDA-cleared’ blood pressure devices be trusted? A call to action. Blood Press. Monit. 2017, 22, 179–181. [Google Scholar] [CrossRef]
- Kans, J. Entrez Direct: E-utilities on the Unix Command Line; National Center for Biotechnology Information: Bethesda, MA, USA, 2013. [Google Scholar]
- Schwenck, J.; Punjabi, N.M.; Gaynanova, I. bp: Blood pressure analysis in R. PLoS ONE 2022, 17, e0268934. [Google Scholar] [CrossRef]
- Reynolds, D.A. Gaussian mixture models. Encycl. Biom. 2009, 741, 659–663. [Google Scholar]
- Mousavi, S.S.; Guo, Y.; Sarker, A.; Sameni, R. Learning from Two Decades of Blood Pressure Data: Demography-Specific Patterns Across 75 Million Patient Encounters. arXiv 2024, arXiv:2402.01598. [Google Scholar] [CrossRef]
- Praveen, D.; Peiris, D.; MacMahon, S.; Mogulluru, K.; Raghu, A.; Rodgers, A.; Chilappagari, S.; Prabhakaran, D.; Clifford, G.D.; Maulik, P.K.; et al. Cardiovascular disease risk and comparison of different strategies for blood pressure management in rural India. BMC Public Health 2018, 18, 1264. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Backus, B.; Six, A.; Kelder, J.; Bosschaert, M.; Mast, E.; Mosterd, A.; Veldkamp, R.; Wardeh, A.; Tio, R.; Braam, R.; et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int. J. Cardiol. 2013, 168, 2153–2158. [Google Scholar] [CrossRef]
- Guo, Y.; Mousavi, S.S.; Sameni, R.; Sarker, A. Leveraging Large Language Models for Analyzing Blood Pressure Variations Across Biological Sex from Scientific Literature. arXiv 2024, arXiv:2402.01826. [Google Scholar] [CrossRef]




| BP Category | SBP (mmHg) | DBP (mmHg) | |
|---|---|---|---|
| Normal BP | <120 | AND | <80 |
| Elevated | 120–129 | AND | <80 |
| High BP (Hypertension) Stage1 | 130–139 | OR | 80–89 |
| High BP (Hypertension) Stage2 | ≥140 | OR | ≥90 |
| Cuff | Arm Circumference (cm) | Bladder Width (cm) | Bladder Length (cm) |
|---|---|---|---|
| Newborn | <6 | 3 | 6 |
| Infant | 6–15 | 5 | 15 |
| Child | 16–21 | 8 | 21 |
| Small Adult | 22–26 | 10 | 24 |
| Adult | 27–34 | 13 | 30 |
| Large Adult | 35–52 | 20 | 42 |
| Adult Thigh | 45–52 | 20 | 42 |
| Ref. | Total N | Male | Female | ||||
|---|---|---|---|---|---|---|---|
| N | SBP | DBP | N | SBP | DBP | ||
| [69] | 20 | 10 | 126.0 ± 8.0 | 73.0 ± 5.0 | 10 | 122.0 ± 5.0 | 73.0 ± 5.0 |
| [69] | 26 | 13 | 117.0 ± 5.0 | 65.0 ± 7.0 | 13 | 103.0 ± 6.0 | 62.0 ± 8.0 |
| [70] | 37 | 22 | 121.2 ± 9.7 | 73.7 ± 8.5 | 15 | 117.4 ± 13.9 | 74.8 ± 12.2 |
| [71] | 39 | 24 | 122.9 ± 13.2 | 82.6 ± 10.1 | 15 | 110.5 ± 8.8 | 74.5 ± 7.3 |
| [72] | 40 | 20 | 128.2 ± 12.3 | 83.3 ± 5.8 | 20 | 117.0 ± 14.4 | 75.5 ± 12.3 |
| [73] | 45 | 23 | 119.0 ± 9.5 | 76.0 ± 4.7 | 22 | 111.0 ± 4.6 | 72.0 ± 4.6 |
| [74] | 55 | 26 | 129.1 ± 9.1 | 64.2 ± 8.3 | 29 | 108.0 ± 9.8 | 61.7 ± 6.7 |
| [75] | 92 | 55 | 105.6 ± 10.3 | 58.5 ± 9.3 | 37 | 103.4 ± 11.8 | 56.9 ± 8.9 |
| [75] | 107 | 42 | 114.3 ± 12.2 | 62.5 ± 13.3 | 65 | 100.3 ± 10.0 | 63.6 ± 10.9 |
| [76] | 122 | 52 | 137.0 ± 20.0 | 86.0 ± 12.0 | 70 | 145.0 ± 26.0 | 87.0 ± 15.0 |
| [77] | 141 | 117 | 128.8 ± 10.4 | 81.3 ± 5.3 | 24 | 126.0 ± 11.8 | 77.6 ± 7.4 |
| [78] | 312 | 142 | 116.3 ± 9.9 | 66.4 ± 7.1 | 170 | 112.3 ± 8.3 | 66.5 ± 6.8 |
| [78] | 351 | 184 | 113.7 ± 9.0 | 64.5 ± 6.6 | 167 | 109.8 ± 7.5 | 64.1 ± 5.9 |
| [79] | 806 | 237 | 120.6 ± 12.9 | 77.9 ± 8.7 | 569 | 112.7 ± 12.3 | 71.7 ± 8.4 |
| [80] | 1030 | 614 | 123.3 ± 12.3 | 77.3 ± 8.2 | 416 | 117.1 ± 10.6 | 73.9 ± 7.1 |
| [81] | 1298 | 638 | 127.4 ± 14.0 | 77.7 ± 10.5 | 660 | 124.4 ± 15.7 | 74.5 ± 9.7 |
| [82] | 1378 | 664 | 122.0 ± 10.5 | 72.0 ± 9.4 | 714 | 113.0 ± 9.9 | 68.2 ± 8.6 |
| [82] | 1534 | 767 | 132.0 ± 16.4 | 83.1 ± 9.3 | 767 | 126.0 ± 16.1 | 78.9 ± 9.2 |
| [83] | 2105 | 945 | 132.0 ± 18.0 | 79.0 ± 11.0 | 1160 | 122.0 ± 18.0 | 75.0 ± 10.0 |
| [84] | 2442 | 1577 | 129.7 ± 19.2 | 80.9 ± 10.6 | 865 | 123.2 ± 20.9 | 76.5 ± 10.3 |
| [85] | 2849 | 1505 | 124.6 ± 15.5 | 73.8 ± 15.5 | 1344 | 120.0 ± 18.3 | 71.2 ± 14.6 |
| [85] | 3654 | 1915 | 120.0 ± 21.8 | 70.5 ± 17.5 | 1739 | 115.0 ± 20.8 | 68.2 ± 16.6 |
| [85] | 6485 | 3379 | 121.7 ± 23.2 | 72.9 ± 17.4 | 3106 | 117.8 ± 22.2 | 70.4 ± 16.7 |
| [86] | 33,599 | 19,704 | 138.7 ± 18.4 | 81.3 ± 11.5 | 13895 | 132.1 ± 19.3 | 77.4 ± 11.6 |
| Ref. | N | Race | SBP | DBP |
|---|---|---|---|---|
| [75] | 199 | Black | 105.7 ± 10.9 | 63.2 ± 11.9 |
| White | 104.7 ± 10.9 | 57.9 ± 9.1 | ||
| [93] | 245 | White hypertensive | 145.0 ± 18.3 | 92.0 ± 10.7 |
| Black hypertensive | 142.0 ± 14.9 | 93.0 ± 10.8 | ||
| [78] | 663 | European American | 111.8 ± 8.3 | 64.3 ± 6.2 |
| African American | 114.1 ± 9.0 | 66.4 ± 6.9 | ||
| [85] | 6503 | Non-Hispanic Black | 122.4 ± 16.9 | 72.5 ± 21.3 |
| Mexican American | 117.6 ± 30.2 | 69.4 ± 24.1 | ||
| [85] | 9334 | Non-Hispanic White | 119.8 ± 32.2 | 71.7 ± 24.1 |
| Non-Hispanic Black | 122.4 ± 16.9 | 72.5 ± 21.3 | ||
| [85] | 10,139 | Non-Hispanic White | 119.8 ± 32.2 | 71.7 ± 24.1 |
| Mexican American | 117.6 ± 30.2 | 69.4 ± 24.1 |
| Ref. | N | BMI | SBP | DBP |
|---|---|---|---|---|
| [97] | 13 | 30.7 ± 4.2 | 124.7 ± 13.0 | 82.4 ± 10.1 |
| [98] | 17 | 24.3 ± 2.4 | 115.4 ± 6.2 | 68.5 ± 5.4 |
| [99] | 21 | 23.9 ± 3.3 | 115.4 ± 13.5 | 71.2 ± 9.4 |
| [72] | 40 | 23.6 ± 3.5 | 122.6 ± 14.4 | 79.4 ± 10.3 |
| [100] | 45 | 29.8 ± 4.7 | 174.0 ± 14.1 | 95.8 ± 11.5 |
| [73] | 45 | 22.6 ± 2.6 | 115.0 ± 6.7 | 74.0 ± 6.7 |
| [101] | 50 | 28.6 ± 3.9 | 133.9 ± 12.3 | 66.4 ± 9.7 |
| [102] | 57 | 25.7 ± 4.4 | 135.7 ± 24.8 | 79.5 ± 9.7 |
| [100] | 70 | 30.6 ± 5.6 | 168.3 ± 18.4 | 83.4 ± 9.4 |
| [103] | 88 | 22.0 ± 4.4 | 108.0 ± 10.0 | 65.0 ± 9.0 |
| [104] | 100 | 23.7 ± 2.9 | 132.9 ± 16.5 | 80.0 ± 10.4 |
| [105] | 165 | 21.3 ± 4.1 | 112.0 ± 10.0 | 67.0 ± 9.0 |
| [103] | 194 | 26.0 ± 5.0 | 120.3 ± 15.8 | 76.4 ± 11.3 |
| [106] | 280 | 28.7 ± 4.2 | 143.8 ± 14.3 | 92.4 ± 9.5 |
| [107] | 287 | 25.0 ± 3.9 | 139.2 ± 16.9 | 74.6 ± 12.0 |
| [78] | 312 | 24.0 ± 7.0 | 114.1 ± 9.0 | 66.4 ± 6.9 |
| [78] | 351 | 22.0 ± 5.0 | 111.8 ± 8.3 | 64.3 ± 6.2 |
| [108] | 389 | 29.4 ± 5.7 | 121.1 ± 16.3 | 53.8 ± 4.8 |
| [109] | 500 | 27.9 ± 5.3 | 123.0 ± 17.0 | 70.0 ± 11.0 |
| [109] | 599 | 28.1 ± 5.1 | 128.0 ± 18.0 | 72.0 ± 12.0 |
| [81] | 638 | 27.5 ± 3.5 | 127.4 ± 14.0 | 77.7 ± 10.5 |
| [81] | 660 | 27.3 ± 5.2 | 124.4 ± 15.7 | 74.5 ± 9.7 |
| [109] | 733 | 28.0 ± 5.2 | 122.0 ± 17.0 | 69.0 ± 11.0 |
| [109] | 735 | 28.2 ± 5.5 | 124.0 ± 18.0 | 71.0 ± 11.0 |
| [79] | 806 | 23.7 ± 3.0 | 115.0 ± 13.0 | 73.5 ± 8.9 |
| [108] | 833 | 27.5 ± 4.7 | 124.3 ± 9.5 | 68.5 ± 6.1 |
| [108] | 927 | 27.3 ± 5.6 | 117.1 ± 14.3 | 53.9 ± 4.6 |
| [83] | 945 | 26.1 ± 4.4 | 132.0 ± 18.0 | 79.0 ± 11.0 |
| [108] | 1030 | 30.8 ± 6.3 | 138.1 ± 18.6 | 71.9 ± 8.6 |
| [83] | 1160 | 25.7 ± 5.2 | 122.0 ± 18.0 | 75.0 ± 10.0 |
| [81] | 1298 | 27.4 ± 4.5 | 125.9 ± 14.9 | 76.1 ± 10.2 |
| [85] | 1344 | 30.0 ± 7.3 | 120.0 ± 18.3 | 71.2 ± 14.6 |
| [82] | 1378 | 23.4 ± 3.5 | 112.5 ± 10.1 | 70.0 ± 8.9 |
| [85] | 1505 | 27.1 ± 7.7 | 124.6 ± 15.5 | 73.8 ± 15.5 |
| [82] | 1534 | 26.5 ± 3.9 | 129.0 ± 16.2 | 81.0 ± 9.2 |
| [108] | 1559 | 28.8 ± 5.2 | 137.2 ± 16.4 | 71.8 ± 8.3 |
| [85] | 1739 | 28.6 ± 8.3 | 115.0 ± 20.8 | 68.2 ± 16.6 |
| [85] | 1915 | 27.7 ± 8.7 | 120.0 ± 21.8 | 70.5 ± 17.5 |
| [110] | 1937 | 19.2 ± 3.8 | 96.5 ± 13.3 | 60.6 ± 9.4 |
| [110] | 1968 | 19.5 ± 3.9 | 97.5 ± 13.2 | 61.3 ± 9.0 |
| [83] | 2105 | 25.9 ± 5.1 | 127.0 ± 19.0 | 77.0 ± 11.0 |
| [111] | 2423 | 24.3 ± 3.3 | 154.7 ± 16.2 | 90.1 ± 11.9 |
| [84] | 2442 | 24.9 ± 3.6 | 127.4 ± 20.1 | 79.4 ± 10.7 |
| [85] | 3106 | 26.9 ± 11.1 | 117.8 ± 22.2 | 70.4 ± 16.7 |
| [85] | 3379 | 27.5 ± 5.8 | 121.7 ± 23.2 | 72.9 ± 17.4 |
| [107] | 6887 | 25.7 ± 4.4 | 134.3 ± 20.2 | 79.6 ± 11.6 |
| [107] | 12,624 | 25.5 ± 4.4 | 131.9 ± 23.1 | 79.7 ± 11.9 |
| [107] | 17,921 | 25.6 ± 4.4 | 133.1 ± 22.4 | 79.9 ± 11.8 |
| [112] | 32,710 | 23.6 ± 3.3 | 123.6 ± 19.8 | 78.9 ± 12.4 |
| [113] | 417,907 | 23.8 ± 3.6 | 128.1 ± 19.0 | 76.1 ± 10.4 |
| [114] | 506,673 | 23.7 ± 3.4 | 131.0 ± 21.0 | 78.0 ± 11.0 |
| Ref. | N | Home | Clinic | ||||
|---|---|---|---|---|---|---|---|
| N | SBP | DBP | N | SBP | DBP | ||
| [118] | 454 | 199 | 144.0 ± 18.0 | 88.6 ± 10.0 | 255 | 160.0 ± 13.0 | 99.7 ± 4.0 |
| [119] | 574 | 287 | 125.7 ± 8.4 | 72.9 ± 8.6 | 287 | 139.2 ± 16.9 | 74.6 ± 12.0 |
| [111] | 4846 | 2423 | 152.4 ± 3.1 | 89.7 ± 9.3 | 2423 | 154.7 ± 16.2 | 90.1 ± 11.9 |
| [107] | 13,774 | 6887 | 127.3 ± 18.1 | 76.2 ± 9.9 | 6887 | 134.3 ± 20.2 | 79.6 ± 11.6 |
| [107] | 35,842 | 17,921 | 129.1 ± 18.6 | 76.9 ± 9.8 | 17921 | 133.1 ± 22.4 | 79.9 ± 11.8 |
| Ref. | BP | Sex | Obese Group | Non-Obese Group | Age (Years) | ||
|---|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | ||||
| [94] | SBP | Boys | 330 | 96.0 ± 13.3 | 331 | 90.0 ± 10.6 | 0.1–6.9 |
| SBP | Girls | 253 | 95.0 ± 13.2 | 251 | 90.0 ± 11.5 | ||
| [94] | DBP | Boys | 330 | 60.0 ± 10.7 | 331 | 60.0 ± 9.5 | 0.1–6.9 |
| DBP | Girls | 253 | 60.0 ± 11.0 | 251 | 60.0 ± 10.0 | ||
| [110] | SBP | Boys | 420 | 103.3 ± 14.8 | 1034 | 94.2 ± 11.8 | 6–11 |
| SBP | Girls | 401 | 100.7 ± 14.1 | 1050 | 93.5 ± 11.8 | ||
| [110] | DBP | Boys | 420 | 64.4 ± 9.8 | 1034 | 59.6 ± 8.7 | 6–11 |
| DBP | Girls | 401 | 63.0 ± 9.3 | 1050 | 58.8 ± 9.2 | ||
| [95] | SBP | Boys | 80 | 103.0 ± 13.0 | 143 | 98.0 ± 11.0 | 6–12 |
| SBP | Girls | 28 | 99.0 ± 14.0 | 144 | 94.0 ± 11.0 | ||
| [95] | DBP | Boys | 80 | 57.0 ± 9.0 | 143 | 55.0 ± 6.0 | 6–12 |
| DBP | Girls | 28 | 55.0 ± 11.0 | 144 | 50.0 ± 6.0 | ||
| Ref. | N | Conditions | SBP | DBP |
|---|---|---|---|---|
| [129] | 11 | B. drinking AF200 a | 120.0 ± 9.9 | 69.0 ± 3.3 |
| 90 min. A. drinking AF200 | 123 ± 6.6 | 74.0 ± 3.3 | ||
| [129] | 11 | B. drinking B350 b | 123.0 ± 6.6 | 71.0 ± 6.6 |
| 90 min. A. drinking B350 | 123.0 ± 9.9 | 76.0 ± 13.2 | ||
| [130] | 12 | B. drinking placebo | 133.5 ± 14.1 | 86.4 ± 8.7 |
| A. drinking placebo | 131.5 ± 11.8 | 82.9 ± 8.4 | ||
| [130] | 12 | B. drinking C67 d | 127.6 ± 9.1 | 81.9 ± 6.7 |
| A. drinking C67 | 135.6 ± 10.1 | 84.7 ± 6.0 | ||
| [130] | 12 | B. drinking C133 e | 126.9 ± 11.1 | 81.4 ± 7.7 |
| A. drinking C133 | 137.6 ± 14.1 | 86.5 ± 8.2 | ||
| [130] | 12 | B. drinking C200 f | 127.5 ± 10.2 | 81.1 ± 5.5 |
| A. drinking C200 | 132.7 ± 10.7 | 83.5 ± 8.2 | ||
| [131] | 15 | Pre-treatment of alcohol | 120.0 ± 11.6 | 64.0 ± 7.7 |
| Post-treatment of alcohol | 124.0 ± 15.5 | 69.0 ± 7.7 | ||
| [131] | 15 | Pre-treatment of placebo | 117.0 ± 7.7 | 64.0 ± 11.6 |
| Post-treatment of placebo | 123.0 ± 7.7 | 71.0 ± 7.7 | ||
| [99] | 18 | B. drinking alcohol c | 110.3 ± 12.0 | 80.0 ± 8.0 |
| A. drinking alcohol | 109.5 ± 11.4 | 76.2 ± 7.1 | ||
| [132] | 22 | B. drinking Noni juice | 119.6 ± 8.3 | 77.0 ± 6.6 |
| A. drinking Noni juice | 113.6 ± 8.5 | 72.0 ± 4.8 | ||
| [132] | 22 | B. drinking chokeberry juice | 125.6 ± 14 | 84.0 ± 9.8 |
| A. drinking chokeberry juice | 124.3 ± 16.1 | 81.0 ± 9.9 | ||
| [132] | 22 | B. consuming energy drink | 119.2 ± 14.8 | 73.9 ± 8.4 |
| A. consuming energy drink | 124.8 ± 14.1 | 84.8 ± 9.9 | ||
| [132] | 22 | B. drinking water | 124.3 ± 13.5 | 77.7 ± 9.2 |
| A. drinking water | 124.0 ± 11.4 | 75.8 ± 8.0 | ||
| [133] | 35 | B. drinking STING h | 123.0 ± 14.9 | 78.7 ± 10.5 |
| A. drinking STING | 123.7 ± 14.5 | 78.2 ± 9.8 | ||
| [70] | 37 | B. drinking 50 mL water | 119.6 ± 11.5 | 74.1 ± 10.1 |
| A. drinking 50 mL water | 122.5 ± 11.6 | 77.3 ± 7.7 | ||
| [70] | 37 | B. drinking 500 mL water | 116.9 ± 8.6 | 73.8 ± 10.0 |
| A. drinking 500 mL water | 125.8 ± 8.8 | 76.8 ± 10.7 | ||
| [71] | 39 | B. non-tobacco smoking | 120.0 ± 13.5 | 78.9 ± 10.1 |
| 65 min. A. non-tobacco smoking | 125.8 ± 8.8 | 76.6 ± 6.9 | ||
| [71] | 39 | B. tobacco smoking | 118.6 ± 12.8 | 79.7 ± 9.2 |
| 65 min. A. tobacco smoking | 116.9 ± 12.4 | 80.0 ± 8.9 | ||
| [72] | 40 | B. drinking 200 mL cold espresso | 116.7 ± 9.7 | 75.3 ± 7.1 |
| A. drinking 200 mL cold espresso | 120.0 ± 11.1 | 79.5 ± 9.1 | ||
| [72] | 40 | B. drinking 200 mL filter coffee | 118.2 ± 12.3 | 77.1 ± 8.5 |
| A. drinking 200 mL filter coffee | 121.2 ± 10.6 | 79.1 ± 6.7 | ||
| [72] | 40 | B. drinking 200 mL cold inst. coffee | 116.7 ± 12.3 | 77.3 ± 8.5 |
| A. drinking 200 mL cold inst. coffee | 121.3 ± 11.4 | 79.6 ± 7.3 | ||
| [72] | 40 | B. drinking 200 mL hot inst. coffee | 118.5 ± 10.5 | 78.2 ± 9.3 |
| A. drinking 200 mL hot inst. coffee | 122.6 ± 11.8 | 80.2 ± 8.7 | ||
| [133] | 60 | B. drinking STING g | 121.2 ± 14.3 | 77.4 ± 9.6 |
| A. drinking STING | 126.5 ± 14.1 | 81.0 ± 9.0 | ||
| [123] | 72 | Pre-treatment beverage of juice | 117.0 ± 13.1 | 79.8 ± 10.1 |
| Post-treatment beverage of juice | 125.9 ± 13.2 | 85.4 ± 9.6 | ||
| [123] | 72 | Pre-treatment beverage of placebo | 126.2 ± 19.2 | 83.5 ± 13.9 |
| Post-treatment beverage of placebo | 130.7 ± 18.2 | 85.7 ± 12.9 | ||
| [123] | 72 | Pre-treatment beverage of alcohol | 116.9 ± 13.5 | 80.1 ± 8.7 |
| Post-treatment beverage of alcohol | 113.2 ± 12.6 | 79.9 ± 9.7 | ||
| [105] | 194 | B. water-pipe smoking | 120.3 ± 15.8 | 76.4 ± 11.3 |
| 15 min. A. water-pipe smoking | 121.1 ± 16.1 | 77.1 ± 10.8 |
| Ref. | N | Mean Daytime BP | Mean Nighttime BP |
|---|---|---|---|
| SBP/DBP | SBP/DBP | ||
| [93] | 46 | (149.0 ± 18.3)/(95.0 ± 10.7) | (132.0 ± 21.7)/(81.0 ± 13.5) |
| [93] | 46 | (145.0 ± 14.9)/(95.0 ± 11.5) | (136.0 ± 17.6)/(86.0 ± 11.5) |
| [106] | 280 | (144.7 ± 11.9)/(91.0 ± 8.6) | (128.2 ± 12.9)/(77.8 ± 9.0) |
| [78] | 312 | (119.5 ± 8.8)/(72.5 ± 6.6) | (108.7 ± 9.3)/(60.4 ± 7.2) |
| [78] | 351 | (117.7 ± 8.1)/(70.9 ± 6.4) | (105.9 ± 8.4)/(57.7 ± 6.1) |
| [107] | 17,921 | (129.3 ± 15.1)/(78.8 ± 9.3) | (112.9 ± 15.6)/(65.1 ± 9.6) |
| Ref. | N | Temp. (°C) | SBP | DBP |
|---|---|---|---|---|
| [140] | 19 | 7.5 ± 0.7 | 118.0 ± 7.8 | 65.0 ± 6.1 |
| [140] | 20 | 14.8 ± 1.3 | 116.0 ± 6.3 | 64.0 ± 5.4 |
| [140] | 20 | 2.0 ± 0.4 | 121.0 ± 7.6 | 65.0 ± 6.3 |
| [140] | 20 | −3.4 ± 3.0 | 125.0 ± 18.0 | 67.0 ± 5.8 |
| [142] | 39 | 25.0 | 117.0 | 65.0 |
| [142] | 39 | 17.6 | 117.0 | 66.0 |
| [142] | 39 | 22.7 | 122.0 | 65.0 |
| [142] | 39 | 21.6 | 119.0 | 65.0 |
| [104] | 100 | 15.7 ± 8.7 | 132.9 ± 16.5 | 80.0 ± 10.4 |
| [143] | 327 | 31.5 ± 1.0 | 133.7 ± 24.5 | 81.7 ± 15.4 |
| [109] | 500 | 25 ± 1 | 123.0 ± 17.0 | 70.0 ± 11.0 |
| [109] | 599 | 24 ± 1 | 128.0 ± 18.0 | 72.0 ± 12.0 |
| [109] | 733 | 26 ± 1 | 122.0 ± 17.0 | 69.0 ± 11.0 |
| [109] | 755 | 25 ± 1 | 124.0 ± 18.0 | 71.0 ± 11.0 |
| Ref. | N | Measuring Place | SBP | DBP |
|---|---|---|---|---|
| [100] | 45 | Upper Arm | 174.0 ± 14.1 | 95.8 ± 11.5 |
| Wrist | 163.8 ± 25.4 | 94.4 ± 11.5 | ||
| [100] | 70 | Upper Arm | 168.3 ± 18.4 | 83.4 ± 9.4 |
| Wrist | 159.2 ± 18.5 | 83.2 ± 10.5 | ||
| [147] | 250 | Arm | 127.7 ± 15.7 | 80.7 ± 11.2 |
| Leg | 143.0 ± 22.2 | 75.7 ± 11.9 |
| Ref. | N | Body Position | SBP | DBP |
|---|---|---|---|---|
| [130] | 12 | Supine | 116.2 ± 11.7 | 68.1 ± 6.6 |
| Upright | 133.5 ± 14.1 | 86.4 ± 8.7 | ||
| [102] | 57 | Sitting | 135.7 ± 24.8 | 79.5 ± 9.7 |
| Supine | 141.3 ± 25.5 | 84.6 ± 10.5 | ||
| [148] | 157 | Sitting | 102.8 ± 11.4 | 65.7 ± 8.2 |
| Standing | 99.9 ± 10.2 | 66.0 ± 8.7 | ||
| Supine | 107.9 ± 10.7 | 66.9 ± 9.6 | ||
| Supine; legs crossed | 107.0 ± 8.6 | 66.7 ± 7.3 | ||
| [149] | 229 | Supine | 129.8 ± 27.5 | 72.5 ± 14.5 |
| Beach chair | 114.6 ± 24.8 | 64.6 ± 11.2 | ||
| [150] | 245 | Sitting | 136.7 ± 21.9 | 86.0 ± 14 |
| Supine | 135.5 ± 20.3 | 83.5 ± 12.5 | ||
| [151] | 250 | Supine | 139.3 ± 14.0 | 80.1 ± 9.1 |
| Fowler’s | 138.1 ± 13.8 | 81.9 ± 9.4 | ||
| Sitting | 137.2 ± 13.7 | 83.0 ± 9.6 | ||
| [81] | 1298 | Sitting | 125.9 ± 14.9 | 76.1 ± 10.2 |
| Supine | 124.7 ± 14.1 | 71.7 ± 9.0 |
| Ref. | N | Arm Position | SBP | DBP |
|---|---|---|---|---|
| [102] | 57 | Arm high (at heart level) | 137.4 ± 29.0 | 78.2 ± 14.4 |
| Arm low (on the bed) | 142.1 ± 28.0 | 82.1 ± 13.4 | ||
| [154] | 69 | Arm high (at heart level) | 133.3 ± 20.7 | 77.7 ± 9.9 |
| Arm low (on chair arm-rest) | 143.0 ± 19.9 | 88.6 ± 9.1 |
| Ref. | N | Leg Position | SBP | DBP |
|---|---|---|---|---|
| [155] | 100 | Uncrossed | 146.5 ± 18.6 | 80.9 ± 11.2 |
| Crossed | 155.6 ± 19.3 | 84.9 ± 11.6 | ||
| [157] | 238 | Uncrossed | 145.3 ± 20.3 | 86.4 ± 10.8 |
| Crossed | 153.6 ± 20.2 | 92.1 ± 11.2 |
| Ref. | N | Right Arm | Left Arm |
|---|---|---|---|
| [102] | 57 | SBP: 138.3 ± 29.2 | SBP: 137.4 ± 29.0 |
| DBP: 77.8 ± 13.7 | DBP:78.2 ± 14.4 | ||
| [154] | 69 | SBP: 133.3 ± 20.7 | SBP: 131.8 ± 19.1 |
| DBP: 77.7 ± 9.9 | DBP: 78.0 ± 9.9 | ||
| [159] | 400 | SBP: 131.2 ± 21.0 | SBP: 129.4 ± 21.2 |
| DBP: 76.8 ± 11.9 | DBP: 77.1 ± 12.6 |
| Ref. | N | Cuff Size (cm) | SBP | DBP |
|---|---|---|---|---|
| [162] | 130 | 13 × 36 | 125.1 ± 19.2 | 75.4 ± 12.4 |
| 16 × 23 | 123.7 ± 19.7 | 74.4 ± 13.2 | ||
| 13 × 23 | 127.2 ± 19.2 | 77.0 ± 12.8 |
| Ref. | N | Before Resting | After Resting | Resting Time |
|---|---|---|---|---|
| [137] | 52 | SBP: 127.9 ± 12.0 | SBP: 121.5 ± 10.9 | 5 min |
| DBP: 78.0 ± 8.7 | DBP: 76.0 ± 9.0 |
| Ref. | N | Measuring Place | SBP | DBP |
|---|---|---|---|---|
| [77] | 141 | Sleeved | 128.5 ± 10.6 | 80.7 ± 6.3 |
| Rolled sleeves | 128.3 ± 11.1 | 80.9 ± 6.3 | ||
| Bare arm | 128.4 ± 10.8 | 80.8 ± 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, S.S.; Reyna, M.A.; Clifford, G.D.; Sameni, R. A Survey on Blood Pressure Measurement Technologies: Addressing Potential Sources of Bias. Sensors 2024, 24, 1730. https://doi.org/10.3390/s24061730
Mousavi SS, Reyna MA, Clifford GD, Sameni R. A Survey on Blood Pressure Measurement Technologies: Addressing Potential Sources of Bias. Sensors. 2024; 24(6):1730. https://doi.org/10.3390/s24061730
Chicago/Turabian StyleMousavi, Seyedeh Somayyeh, Matthew A. Reyna, Gari D. Clifford, and Reza Sameni. 2024. "A Survey on Blood Pressure Measurement Technologies: Addressing Potential Sources of Bias" Sensors 24, no. 6: 1730. https://doi.org/10.3390/s24061730






