Ultra-Long-Term-EEG Monitoring (ULTEEM) Systems: Towards User-Friendly Out-of-Hospital Recordings of Electrical Brain Signals in Epilepsy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
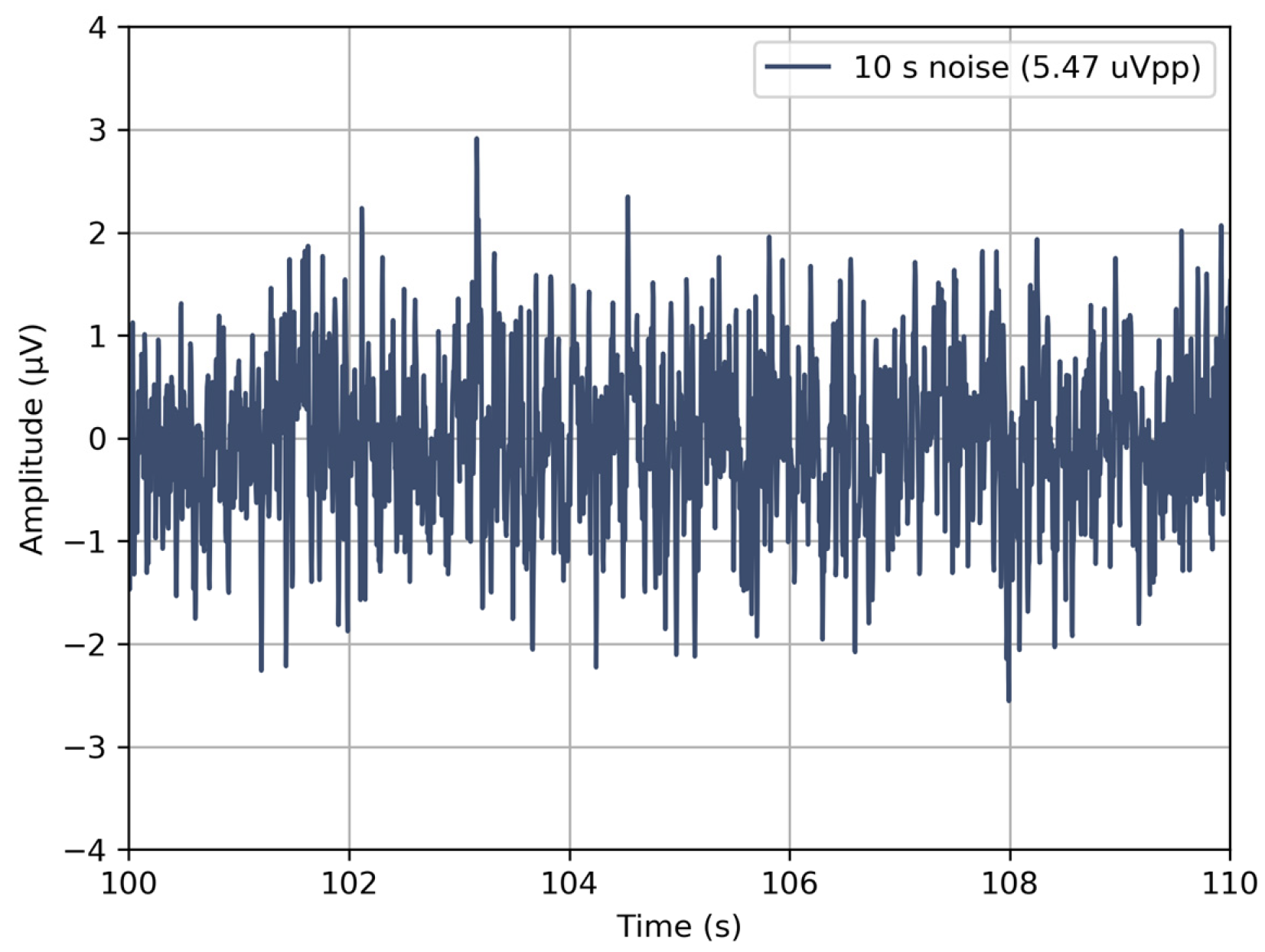
3.1. Verification of the Devices
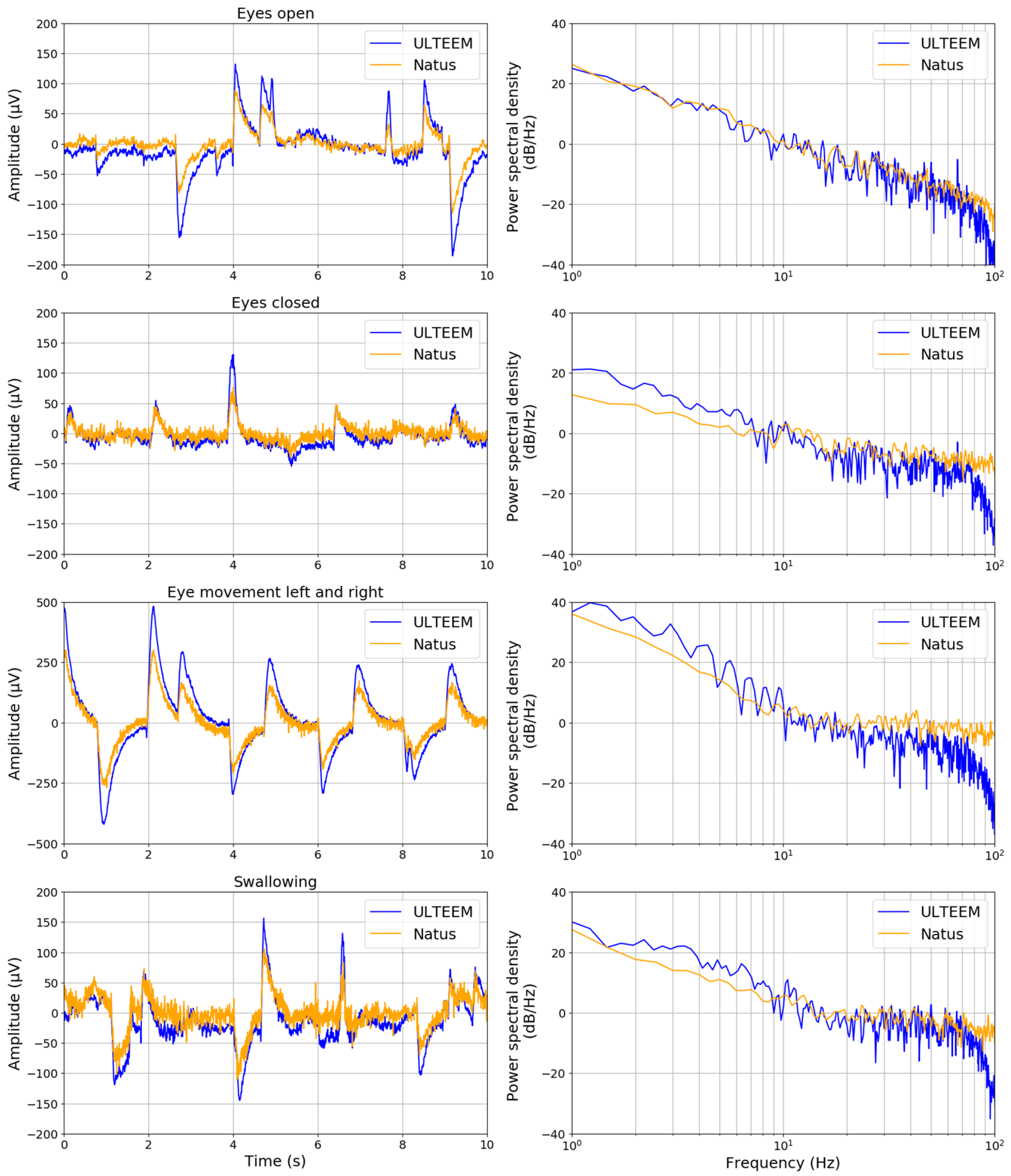
3.2. ULTEEM Pilot Clinical Study
- keeping their eyes open and fixing their gaze as much as possible;
- keeping the eyes closed and relaxing;
- performing eye movements (left and right) without turning their head;
- swallowing repetitively.
3.3. Proof-of-Concept ULTEEMNite Night-Long Recording
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baud, M.O.; Kleen, J.K.; Mirro, E.A.; Andrechak, J.C.; King-Stephens, D.; Chang, E.F.; Rao, V.R. Multi-day rhythms modulate seizure risk in epilepsy. Nat. Commun. 2018, 9, 88. [Google Scholar] [CrossRef]
- Karoly, P.J.; Rao, V.R.; Gregg, N.M.; Worrell, G.A.; Bernard, C.; Cook, M.J.; Baud, M.O. Cycles in epilepsy. Nat. Rev. Neurol. 2021, 17, 267–284. [Google Scholar] [CrossRef]
- Baud, M.O.; Schindler, K.; Rao, V.R. Under-sampling in epilepsy: Limitations of conventional EEG. Clin. Neurophysiol. Pract. 2021, 6, 41–49. [Google Scholar] [CrossRef]
- Schindler, K.A.; Nef, T.; Baud, M.O.; Tzovara, A.; Yilmaz, G.; Tinkhauser, G.; Gerber, S.M.; Gnarra, O.; Warncke, J.D.; Schütz, N.; et al. NeuroTec Sitem-Insel Bern: Closing the Last Mile in Neurology. Clin. Transl. Neurosci. 2021, 5, 13. [Google Scholar] [CrossRef]
- Eichenwald, K. A Mind Unraveled: A Memoir; Ballantine Books: New York City, NY, USA, 2018; Available online: https://catalog.oslri.net/Record/784085 (accessed on 20 December 2023).
- Rao, V.R. Chronic electroencephalography in epilepsy with a responsive neurostimulation device: Current status and future prospects. Expert Rev. Med. Devices 2021, 18, 1093–1105. [Google Scholar] [CrossRef]
- Krucoff, M.O.; Wozny, T.A.; Lee, A.T.; Rao, V.R.; Chang, E.F. Operative Technique and Lessons Learned from Surgical Implantation of the NeuroPace Responsive Neurostimulation® System in 57 Consecutive Patients. Oper. Neurosurg. 2021, 20, E98–E109. [Google Scholar] [CrossRef]
- Casson, A.J.; Smith, S.; Duncan, J.S.; Rodriguez-Villegas, E. Wearable EEG: What is it, why is it needed and what does it entail? In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–24 August 2008; pp. 5867–5870. [Google Scholar] [CrossRef]
- Casson, A.J. Wearable EEG and beyond. Biomed. Eng. Lett. 2019, 9, 53–71. [Google Scholar] [CrossRef]
- Kaongoen, N.; Choi, J.; Choi, J.W.; Kwon, H.; Hwang, C.; Hwang, G.; Kim, B.H.; Jo, S. The future of wearable EEG: A review of ear-EEG technology and its applications. J. Neural Eng. 2023, 20, 051002. [Google Scholar] [CrossRef]
- Luedke, M.W.; Blalock, D.V.; Goldstein, K.M.; Kosinski, A.S.; Sinha, S.R.; Drake, C.; Lewis, J.D.; Husain, A.M.; Lewinski, A.A.; Shapiro, A.; et al. Self-management of Epilepsy: A Systematic Review. In VA Evidence-Based Synthesis Program Reports; Department of Veterans Affairs (US): Washington, DC, USA, 2019. Available online: http://www.ncbi.nlm.nih.gov/books/NBK544549/ (accessed on 20 December 2023).
- IEC 60601-1; Medical Electrical Equipment-Part 1: General Requirements for Basic Safety and Essential Performance, 3.0. International Electrotechnical Commission: Geneva, Switzerland, 2005.
- IEC 80601-2-26:2019|IEC Webstore. Available online: https://webstore.iec.ch/publication/26376 (accessed on 20 December 2023).
- Rapin, M. A Wearable Sensor Architecture for High-Quality Measurement of Multilead ECG and Frequency-Multiplexed EIT. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2018. [Google Scholar] [CrossRef]
- Chi, Y.M.; Jung, T.-P.; Cauwenberghs, G. Dry-Contact and Noncontact Biopotential Electrodes: Methodological Review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef]
- Shad, E.H.T.; Molinas, M.; Ytterdal, T. Impedance and Noise of Passive and Active Dry EEG Electrodes: A Review. IEEE Sens. J. 2020, 20, 14565–14577. [Google Scholar] [CrossRef]
- IEC 60601-2-25; Medical Electrical Equipment-Part 2-25: Particular Requirements for the Basic Safety and Essential Performance of Electrocardiographs, 2.0. International Electrotechnical Commission: Geneva, Switzerland, 2011.
- Zipp, P.; Ahrens, H. A model of bioelectrode motion artefact and reduction of artefact by amplifier input stage design. J. Biomed. Eng. 1979, 1, 273–276. [Google Scholar] [CrossRef]
- Chetelat, O.; Gentsch, R.; Krauss, J.; Luprano, J. Getting rid of the wires and connectors in physiological monitoring. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 1278–1282. [Google Scholar] [CrossRef]
- Chetelat, O.; Ferrario, D.; Proenca, M.; Porchet, J.-A.; Falhi, A.; Grossenbacher, O.; Delgado-Gonzalo, R.; Della Ricca, N.; Sartori, C. Clinical validation of LTMS-S: A wearable system for vital signs monitoring. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3125–3128. [Google Scholar] [CrossRef]
- Simblett, S.K.; Biondi, A.; Bruno, E.; Ballard, D.; Stoneman, A.; Lees, S.; Richardson, M.P.; Wykes, T. Patients’ experience of wearing multimodal sensor devices intended to detect epileptic seizures: A qualitative analysis. Epilepsy Behav. 2019, 102, 106717. [Google Scholar] [CrossRef]
- Chételat, O.; Caros, J.S.I. Floating Front-End Amplifier and One-Wire Measuring Devices. EP2101408B1, 16 May 2012. Available online: https://patents.google.com/patent/EP2101408B1/en?oq=EP2101408 (accessed on 14 January 2020).
- Skarpsno, E.S.; Mork, P.J.; Nilsen, T.I.L.; Holtermann, A. Sleep positions and nocturnal body movements based on free-living accelerometer recordings: Association with demographics, lifestyle, and insomnia symptoms. Nat. Sci. Sleep 2017, 9, 267–275. [Google Scholar] [CrossRef]
- Bandt, C. Statistics and contrasts of order patterns in univariate time series. Chaos Interdiscip. J. Nonlinear Sci. 2023, 33, 033124. [Google Scholar] [CrossRef]
- Bandt, C. Small Order Patterns in Big Time Series: A Practical Guide. Entropy 2019, 21, 613. [Google Scholar] [CrossRef]
- Prerau, M.J.; Brown, R.E.; Bianchi, M.T.; Ellenbogen, J.M.; Purdon, P.L. Sleep Neurophysiological Dynamics Through the Lens of Multitaper Spectral Analysis. Physiology 2017, 32, 60–92. [Google Scholar] [CrossRef]
- Pessa, A.A.B.; Ribeiro, H.V. ordpy: A Python package for data analysis with permutation entropy and ordinal network methods. Chaos Interdiscip. J. Nonlinear Sci. 2021, 31, 063110. [Google Scholar] [CrossRef]
- Elger, C.E.; Mormann, F. Seizure prediction and documentation—Two important problems. Lancet Neurol. 2013, 12, 531–532. [Google Scholar] [CrossRef]
- Lam, A.D.; Noebels, J. Night Watch on the Titanic: Detecting Early Signs of Epileptogenesis in Alzheimer Disease. Epilepsy Curr. 2020, 20, 369–374. [Google Scholar] [CrossRef]
- Vossel, K.A.; Beagle, A.J.; Rabinovici, G.D.; Shu, H.; Lee, S.E.; Naasan, G.; Hegde, M.; Cornes, S.B.; Henry, M.L.; Nelson, A.B.; et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013, 70, 1158–1166. [Google Scholar] [CrossRef]
- Csernus, E.A.; Werber, T.; Kamondi, A.; Horvath, A.A. The Significance of Subclinical Epileptiform Activity in Alzheimer’s Disease: A Review. Front. Neurol. 2022, 13, 856500. [Google Scholar] [CrossRef]
- Noebels, J. A perfect storm: Converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia 2011, 52, 39–46. [Google Scholar] [CrossRef]
- A Vossel, K.; Tartaglia, M.C.; Nygaard, H.B.; Zeman, A.Z.; Miller, B.L. Epileptic activity in Alzheimer’s disease: Causes and clinical relevance. Lancet Neurol. 2017, 16, 311–322. [Google Scholar] [CrossRef]
- Keret, O.; Hoang, T.D.; Xia, F.; Rosen, H.J.; Yaffe, K. Association of Late-Onset Unprovoked Seizures of Unknown Etiology with the Risk of Developing Dementia in Older Veterans. JAMA Neurol. 2020, 77, 710–715. [Google Scholar] [CrossRef]
- Huang, L.; Fu, C.; Li, J.; Peng, S. Late-onset epilepsy and the risk of dementia: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2022, 34, 1771–1779. [Google Scholar] [CrossRef]
- Shi, L.; Chen, S.-J.; Ma, M.-Y.; Bao, Y.-P.; Han, Y.; Wang, Y.-M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 40, 4–16. [Google Scholar] [CrossRef]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef]
- Karageorgiou, E.; Vossel, K.A. Brain rhythm attractor breakdown in Alzheimer’s disease: Functional and pathologic implications. Alzheimers Dement. J. Alzheimers Assoc. 2017, 13, 1054–1067. [Google Scholar] [CrossRef]
- La, A.L.; Walsh, C.M.; Neylan, T.C.; Vossel, K.A.; Yaffe, K.; Krystal, A.D.; Miller, B.L.; Karageorgiou, E. Long-Term Trazodone Use and Cognition: A Potential Therapeutic Role for Slow-Wave Sleep Enhancers. J. Alzheimers Dis. 2019, 67, 911–921. [Google Scholar] [CrossRef]
- Halász, P.; Timofeev, I.; Szűcs, A. Derailment of Sleep Homeostatic Plasticity Affects the Most Plastic Brain Systems and Carries the Risk of Epilepsy. J. Integr. Neurosci. 2023, 22, 111. [Google Scholar] [CrossRef]




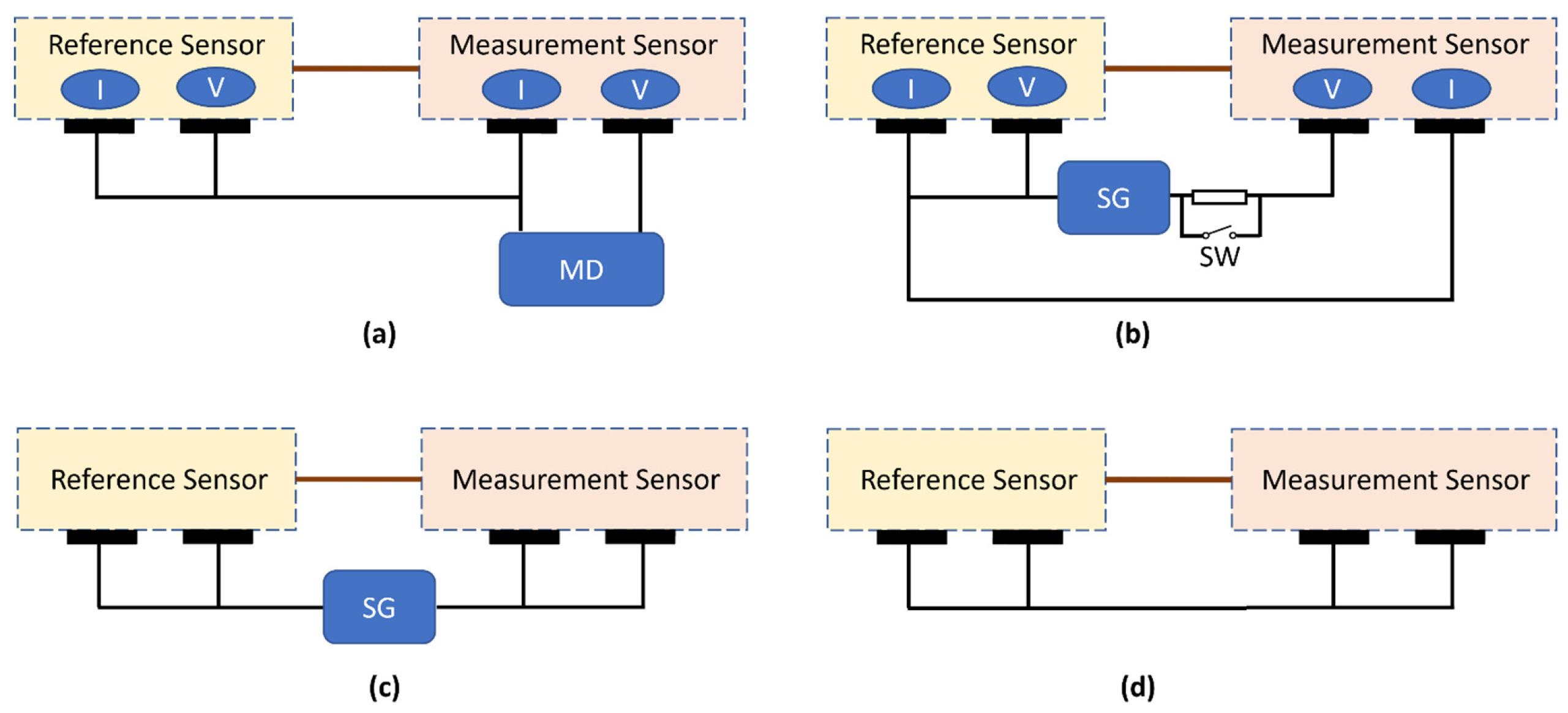



| Requirement | Unit | Value |
|---|---|---|
| Patient auxiliary current (DC) | µA | <10 NC 1 |
| Patient auxiliary current (AC) | µA | <100 NC 1 |
| Lower cut-off frequency of frequency response | Hz | <0.5 |
| Higher cut-off frequency of frequency response | Hz | >50 |
| Input-referred noise within the acquisition bandwidth | µVp-p 2 | <6 |
| Input impedance at 10 Hz | GΩ | >0.5 |
| Input leakage current | pA | <100 |
| Requirement | Unit | Measured Value |
|---|---|---|
| Patient auxiliary current (DC) | µA | <0.1 1 |
| Patient auxiliary current (AC) | µA | <0.1 1 |
| Lower cut-off frequency of frequency response | Hz | <0.5 |
| Higher cut-off frequency of frequency response | Hz | >50 |
| Input-referred noise within the acquisition bandwidth | µVp-p | <5.5 |
| Input impedance at 10 Hz | GΩ | >0.53 |
| Input leakage current | pA | <72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, G.; Seiler, A.; Chételat, O.; Schindler, K.A. Ultra-Long-Term-EEG Monitoring (ULTEEM) Systems: Towards User-Friendly Out-of-Hospital Recordings of Electrical Brain Signals in Epilepsy. Sensors 2024, 24, 1867. https://doi.org/10.3390/s24061867
Yilmaz G, Seiler A, Chételat O, Schindler KA. Ultra-Long-Term-EEG Monitoring (ULTEEM) Systems: Towards User-Friendly Out-of-Hospital Recordings of Electrical Brain Signals in Epilepsy. Sensors. 2024; 24(6):1867. https://doi.org/10.3390/s24061867
Chicago/Turabian StyleYilmaz, Gürkan, Andrea Seiler, Olivier Chételat, and Kaspar A. Schindler. 2024. "Ultra-Long-Term-EEG Monitoring (ULTEEM) Systems: Towards User-Friendly Out-of-Hospital Recordings of Electrical Brain Signals in Epilepsy" Sensors 24, no. 6: 1867. https://doi.org/10.3390/s24061867






