Recent Progress on Microfluidics Integrated with Fiber-Optic Sensors for On-Site Detection
Abstract
:1. Introduction
2. Optical Detection with Microfluidics Using Fiber-Optic Sensors
2.1. Integration Modes
2.2. Optical Detection
2.2.1. Raman Detection
2.2.2. UV-Vis Detection
2.2.3. Fluorescence Detection
2.2.4. SPR/LSPR Detection
3. Applications
3.1. Biological Analysis
3.2. Food Safety
3.3. Environmental Monitoring
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalaf, E.M.; Sanaan Jabbar, H.; Mireya Romero-Parra, R.; Raheem Lateef Al-Awsi, G.; Setia Budi, H.; Altamimi, A.S.; Abdulfadhil Gatea, M.; Falih, K.T.; Singh, K.; Alkhuzai, K.A. Smartphone-assisted microfluidic sensor as an intelligent device for on-site determination of food contaminants: Developments and applications. Microchem. J. 2023, 190, 108692. [Google Scholar] [CrossRef]
- Masson, J.-F. Portable and field-deployed surface plasmon resonance and plasmonic sensors. Analyst 2020, 145, 3776–3800. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ji, M.; Hu, Y.; Li, G.; Xiao, X. Progress of environmental sample preparation for elemental analysis. J. Chromatogr. A 2022, 1681, 463458. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Lu, Z.; Hu, Y.; Xie, Z.; Li, G. Research progress on sample pretreatment methods for migrating substances from food contact materials. J. Sep. Sci. 2021, 44, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Yang, J.; Su, R.; Zhou, W.; Zhang, Y.; Zhong, Y.; Huang, S.; Chen, Y.; Li, G. Recent progress in fast sample preparation techniques. Anal. Chem. 2020, 92, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Li, Y.; Liu, Y.; Li, G.; Xiao, X. Recent advances in sample preparation techniques in China. J. Sep. Sci. 2019, 43, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Avino, A.; Molins-Legua, C.; Campins-Falco, P. Combining high performance thin layer chromatography with minispectrometer-fiber optic probe-coupled to smartphone for in place analysis: Lactose quantification in several matrices. J. Chromatogr. A 2022, 1661, 462694. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging droplet microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Han, J.; Choi, J.-W.; Ahn, C.H. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2015, 132, 46–57. [Google Scholar] [CrossRef]
- Duan, C.; Li, J.; Zhang, Y.; Ding, K.; Geng, X.; Guan, Y. Portable instruments for on-site analysis of environmental samples. TrAC Trends Anal. Chem. 2022, 154, 116653. [Google Scholar] [CrossRef]
- Battat, S.; Weitz, D.A.; Whitesides, G.M. An outlook on microfluidics: The promise and the challenge. Lab Chip 2022, 22, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xiang, X.; Ye, Q.; Wu, Q.; Zhang, J.; Lin, J.-M. Advances in nanomaterial-based microfluidic platforms for on-site detection of foodborne bacteria. TrAC Trends Anal. Chem. 2022, 147, 116509. [Google Scholar] [CrossRef]
- Dalili, A.; Samiei, E.; Hoorfar, M. A review of sorting, separation and isolation of cells and microbeads for biomedical applications: Microfluidic approaches. Analyst 2018, 144, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Dhwaj, A.; Verma, D.; Prabhakar, A. Cost-effective microabsorbance detection based nanoparticle immobilized microfluidic system for potential investigation of diverse chemical contaminants present in drinking water. Anal. Chim. Acta 2022, 1205, 339734. [Google Scholar] [CrossRef]
- Dong, R.; Li, Y.; Liu, S.; Li, W.; Tao, L.; Chen, C.; Qian, Z.; Yang, Y. On-chip spectroscopic monitoring of erythrocyte oxygenation under hematocrit and oxygen gradients. J. Sci. Adv. Mater. Devices 2022, 7, 100515. [Google Scholar] [CrossRef]
- Dong, J.; Li, G.; Xia, L. Microfluidic magnetic spatial confinement strategy for the enrichment and ultrasensitive detection of MCF-7 and Escherichia coli O157:H7. Anal. Chem. 2022, 94, 16901–16909. [Google Scholar] [CrossRef]
- Blue, R.; Uttamchandani, D. Recent advances in optical fiber devices for microfluidics integration. J. Biophotonics 2016, 9, 13–25. [Google Scholar] [CrossRef]
- Zhu, Y.; Fang, Q. Analytical detection techniques for droplet microfluidics—A review. Anal. Chim. Acta 2013, 787, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, Y.; Ju, H.; Lu, G. From lab to field: Surface-enhanced Raman scattering-based sensing strategies for on-site analysis. TrAC Trends Anal. Chem. 2022, 146, 116488. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, X.; Xia, L.; Li, G.; Shui, L. Microfluidic magnetic analyte delivery technique for separation, enrichment, and fluorescence detection of ultratrace biomarkers. Anal. Chem. 2021, 93, 8273–8280. [Google Scholar] [CrossRef]
- Moradi, V.; Akbari, M.; Wild, P. A fluorescence-based pH sensor with microfluidic mixing and fiber optic detection for wide range pH measurements. Sens. Actuators A 2019, 297, 111507. [Google Scholar] [CrossRef]
- Xia, L.; Li, G. Recent progress of microfluidics in surface-enhanced Raman spectroscopic analysis. J. Sep. Sci. 2021, 44, 1752–1768. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, L.; Xue, Q.; Fan, J.; Hu, Q.; Tang, C.; Shi, H.; Hu, B.; Tian, J. Dynamic liquid surface enhanced raman scattering platform based on soft tubular microfluidics for label-free cell detection. Anal. Chem. 2019, 91, 7973–7979. [Google Scholar] [CrossRef] [PubMed]
- Leitao, C.; Pereira, S.O.; Marques, C.; Cennamo, N.; Zeni, L.; Shaimerdenova, M.; Ayupova, T.; Tosi, D. Cost-effective fiber optic solutions for biosensing. Biosensors 2022, 12, 575. [Google Scholar] [CrossRef]
- Elsherif, M.; Salih, A.E.; Muñoz, M.G.; Alam, F.; AlQattan, B.; Antonysamy, D.S.; Zaki, M.F.; Yetisen, A.K.; Park, S.; Wilkinson, T.D.; et al. Optical fiber sensors: Working principle, applications, and limitations. Adv. Photonics Res. 2022, 3, 2100371. [Google Scholar] [CrossRef]
- Chau, Y.F.; Jheng, C. Structurally and materially sensitive hybrid surface plasmon modes in periodic silver-shell nanopearl and its dimer arrays. J. Nanopart. Res. 2013, 15, 1424. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kim, B. Lab-on-a-chip pathogen sensors for food safety. Sensors 2012, 12, 10713–10741. [Google Scholar] [CrossRef]
- Gao, D.; Yang, X.; Teng, P.; Kong, D.; Liu, Z.; Yang, J.; Luo, M.; Li, Z.; Wen, X.; Yuan, L.; et al. On-line SERS detection of adenine in DNA based on the optofluidic in-fiber integrated GO/PDDA/AgNPs. Sens. Actuators B 2021, 332, 129517. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Fang, W.; Tong, L.; Zhang, L. Ultra-sensitive nanofiber fluorescence detection in a microfluidic chip. Sensors 2015, 15, 4890–4898. [Google Scholar] [CrossRef]
- Chen, Y.S.; Huang, C.H.; Pai, P.C.; Seo, J.; Lei, K.F. A review on microfluidics-based impedance biosensors. Biosensors 2023, 13, 83. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2015–2019). Anal. Chem. 2020, 92, 397–430. [Google Scholar] [CrossRef]
- Ding, Y.; Howes, P.D.; de Mello, A.J. Recent advances in droplet microfluidics. Anal. Chem. 2019, 92, 132–149. [Google Scholar] [CrossRef]
- Gao, D.; Yang, X.; Teng, P.; Luo, M.; Zhang, H.; Liu, Z.; Yang, J.; Li, Z.; Wen, X.; Yuan, L.; et al. In-fiber optofluidic online SERS detection of trace uremia toxin. Opt. Lett. 2021, 46, 1101–1104. [Google Scholar] [CrossRef]
- Zheng, Z.; Shi, M.; Xu, Y.; Liu, S.; Zhong, H.; Shou, Q.; Huang, J.; Luan, T.; Li, Z.; Xing, X. Light-induced dynamic assembly of gold nanoparticles in a lab-on-fiber platform for surface-enhanced raman scattering detection. ACS Appl. Nano Mater. 2022, 5, 8005–8011. [Google Scholar] [CrossRef]
- Li, H.; Chu, R.; Cao, J.; Zhou, F.; Guo, K.; Zhang, Q.; Wang, H.; Liu, Y. Sensitive and reproducible on-chip SERS detection by side-polished fiber probes integrated with microfluidic chips. Measurement 2023, 218, 113203. [Google Scholar] [CrossRef]
- Yazdi, S.H.; White, I.M. Optofluidic surface enhanced Raman spectroscopy microsystem for sensitive and repeatable on-site detection of chemical contaminants. Anal. Chem. 2012, 84, 7992–7998. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Tian, M.; Li, R.; Quan, X.; Jia, Q. A novel organic-inorganic hybrid monolithic column prepared in-situ in a microchip and its application for the determination of 2-amino-4-chlorophenol in chlorzoxazone tablets. Talanta 2013, 115, 801–805. [Google Scholar] [CrossRef]
- Zhong, N.; Chen, M.; Wang, Z.; Xin, X.; Li, B. Photochemical device for selective detection of phenol in aqueous solutions. Lab Chip 2018, 18, 1621–1632. [Google Scholar] [CrossRef]
- Waechter, H.; Bescherer, K.; Durr, J.C.; Oleschuk, D.R.; Loock, H. 405 nm Absorption detection in nanoliter volumes. Anal. Chem. 2009, 81, 9048–9054. [Google Scholar] [CrossRef]
- Mei, H.; Pan, J.; Zhang, Z.; Zhang, L.; Tong, L. Coiled optical nanofiber for optofluidic absorbance detection. ACS Sens. 2019, 4, 2267–2271. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Song, D.; Li, Z.; Han, S.; Long, F.; Zhu, A. Simple, rapid, and sensitive on-site detection of Hg2+ in water samples through combining portable evanescent wave optofluidic biosensor and fluorescence resonance energy transfer principle. Anal. Chim. Acta 2021, 1155, 338351. [Google Scholar] [CrossRef]
- Parker, H.E.; Sengupta, S.; Harish, A.V.; Soares, R.R.G.; Joensson, H.N.; Margulis, W.; Russom, A.; Laurell, F. A Lab-in-a-Fiber optofluidic device using droplet microfluidics and laser-induced fluorescence for virus detection. Sci. Rep. 2022, 12, 3539. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, H.; Zhuo, Y.; Song, D.; Li, C.; Zhu, A.; Long, F. Reusable smartphone-facilitated mobile fluorescence biosensor for rapid and sensitive on-site quantitative detection of trace pollutants. Biosens. Bioelectron. 2022, 199, 113863. [Google Scholar] [CrossRef]
- Pan, L.; Zou, M.; Ma, F.; Kong, L.; Zhang, C.; Yang, L.; Zhu, A.; Long, F.; Liu, X.Y.; Lin, N. Fast dopamine detection based on evanescent wave detection platform. Anal. Chim. Acta 2022, 1191, 339312. [Google Scholar] [CrossRef]
- Aray, A.; Chiavaioli, F.; Arjmand, M.; Trono, C.; Tombelli, S.; Giannetti, A.; Cennamo, N.; Soltanolkotabi, M.; Zeni, L.; Baldini, F. SPR-based plastic optical fibre biosensor for the detection of C-reactive protein in serum. J. Biophotonics 2016, 9, 1077–1084. [Google Scholar] [CrossRef]
- Guo, T.; Liu, F.; Liang, X.; Qiu, X.; Huang, Y.; Xie, C.; Xu, P.; Mao, W.; Guan, B.O.; Albert, J. Highly sensitive detection of urinary protein variations using tilted fiber grating sensors with plasmonic nanocoatings. Biosens. Bioelectron. 2016, 78, 221–228. [Google Scholar] [CrossRef]
- Qu, J.H.; Ordutowski, H.; Van Tricht, C.; Verbruggen, R.; Barcenas Gallardo, A.; Bulcaen, M.; Ciwinska, M.; Gutierrez Cisneros, C.; Devriese, C.; Guluzade, S.; et al. Point-of-care therapeutic drug monitoring of adalimumab by integrating a FO-SPR biosensor in a self-powered microfluidic cartridge. Biosens. Bioelectron. 2022, 206, 114125. [Google Scholar] [CrossRef]
- Gahlaut, S.K.; Pathak, A.; Gupta, B.D.; Singh, J.P. Portable fiber-optic SPR platform for the detection of NS1-antigen for dengue diagnosis. Biosens. Bioelectron. 2022, 196, 113720. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, D.; Qin, Y. Novel strategy for fabrication of sensing layer on thiol-functionalized fiber-optic tapers and their application as SERS probes. Talanta 2019, 194, 895–902. [Google Scholar] [CrossRef]
- Strobbia, P.; Ran, Y.; Crawford, B.M.; Cupil-Garcia, V.; Zentella, R.; Wang, H.N.; Sun, T.P.; Vo-Dinh, T. Inverse molecular sentinel-integrated fiberoptic sensor for direct and in situ detection of miRNA targets. Anal. Chem. 2019, 91, 6345–6352. [Google Scholar] [CrossRef]
- Li, S.; Xia, L.; Zhang, H.; Li, W.; Li, K.; Chen, X. Inline integration of offset MMF-capillary-MMF structure as a portable and compact fiber-optic surface-enhanced Raman scattering microfluidic chip. Appl. Opt. 2018, 57, 10548–10552. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Li, S.; Chen, L.; Zhou, H.; Zhu, J.; Zheng, Z. Fiber-optic SERS microfluidic chip based on light-induced gold nano-particle aggregation. Opt. Commun. 2015, 352, 148–154. [Google Scholar] [CrossRef]
- Pu, H.; Xiao, W.; Sun, D.-W. SERS-microfluidic systems: A potential platform for rapid analysis of food contaminants. Trends Food Sci. Technol. 2017, 70, 114–126. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.; Jung, J.H. Fully integrated optofluidic SERS platform for real-time and continuous characterization of airborne microorganisms. Biosens. Bioelectron. 2020, 169, 112611. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, Z.; Wu, J.; Li, S.; Li, W.; Zhang, H.; Xia, L. A Raman immunosensor based on SERS and microfluidic chip for all-fiber detection of brain natriuretic peptide. Infrared Phys. Technol. 2022, 125, 104252. [Google Scholar] [CrossRef]
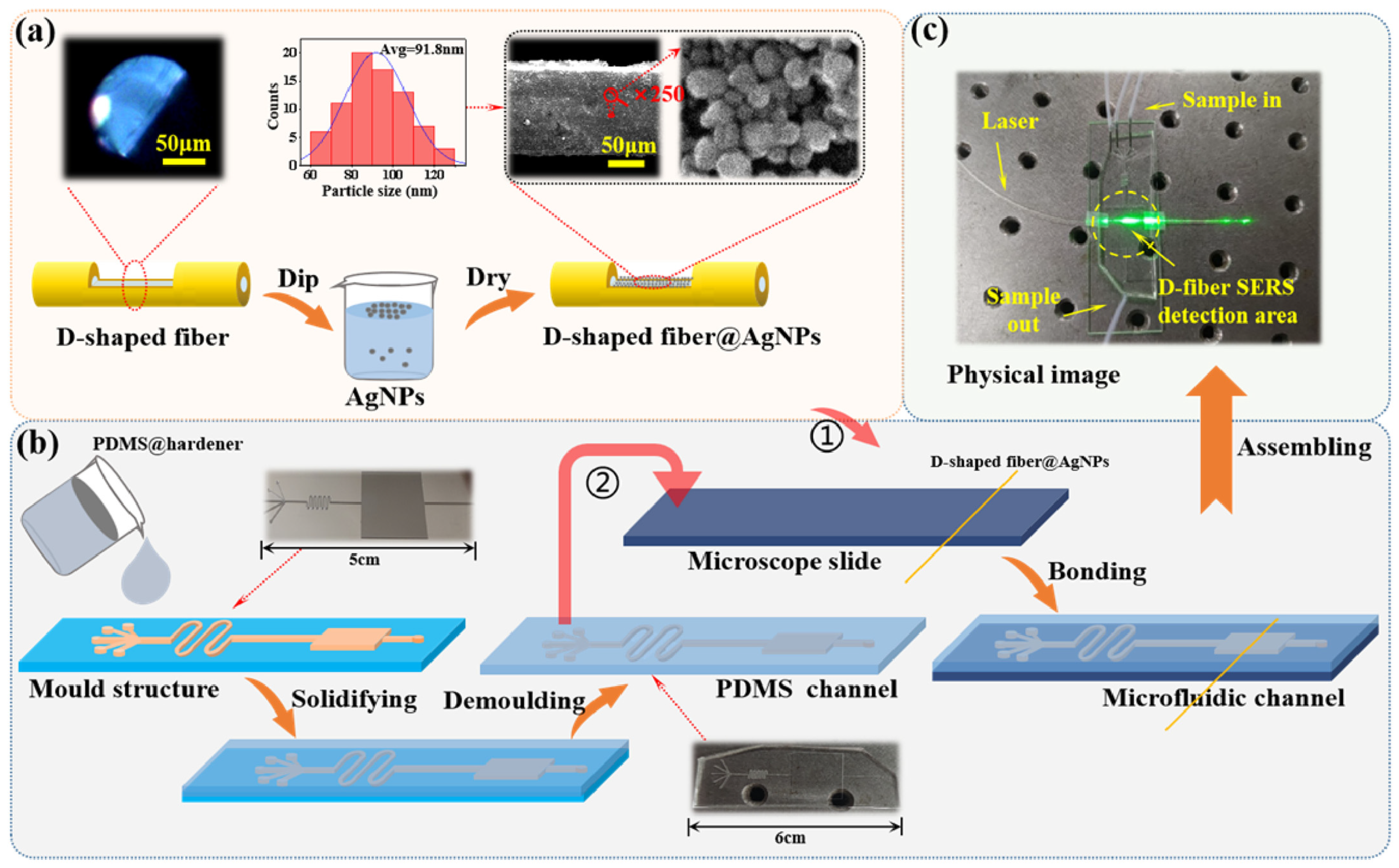
- Bo, H.; Ke, Y.; Yong, Z.; Jie, Z. Microfluidic integrated D-shaped optical fiber SERS probe with high sensitivity and ability of multi-molecule detection. Opt. Express 2023, 31, 27304–27311. [Google Scholar] [CrossRef]
- Stoddart, P.R.; White, D.J. Optical fibre SERS sensors. Anal. Bioanal. Chem. 2009, 394, 1761–1774. [Google Scholar] [CrossRef]
- Verissimo, M.I.S.; Gamelas, J.A.F.; Fernandes, A.J.S.; Evtuguin, D.V.; Gomes, M. A new formaldehyde optical sensor: Detecting milk adulteration. Food Chem. 2020, 318, 126461. [Google Scholar] [CrossRef]
- Kamuri, M.F.; Zainal Abidin, Z.; Yaacob, M.H.; Hamidon, M.N.; Md Yunus, N.A.; Kamarudin, S. Separation and detection of Escherichia coli and saccharomyces cerevisiae using a microfluidic device integrated with an optical fibre. Biosensors 2019, 9, 40. [Google Scholar] [CrossRef]
- Liu, A.L.; Li, Z.Q.; Wu, Z.Q.; Xia, X.H. Study on the photocatalytic reaction kinetics in a TiO2 nanoparticles coated microreactor integrated microfluidics device. Talanta 2018, 182, 544–548. [Google Scholar] [CrossRef]
- Kabiri, S.; Kurkuri, M.D.; Kumeria, T.; Losic, D. Frit-free PDMS microfluidic device for chromatographic separation and on-chip detection. RSC Adv. 2014, 4, 15276–15280. [Google Scholar] [CrossRef]
- Zhu, J.M.; Shi, Y.; Zhu, X.Q.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H.; Han, X.T. Optofluidic marine phosphate detection with enhanced absorption using a Fabry-Perot resonator. Lab Chip 2017, 17, 4025–4030. [Google Scholar] [CrossRef]
- Choi, K.; Mudrik, J.M.; Wheeler, A.R. A guiding light: Spectroscopy on digital microfluidic devices using in-plane optical fibre waveguides. Anal. Bioanal. Chem. 2015, 407, 7467–7475. [Google Scholar] [CrossRef]
- Yuan, Y.; Jia, H.; Xu, D.; Wang, J. Novel method in emerging environmental contaminants detection: Fiber optic sensors based on microfluidic chips. Sci. Total Environ. 2023, 857, 159563. [Google Scholar] [CrossRef]
- Hu, Y.; Muhammad, T.; Wu, B.; Wei, A.; Yang, X.; Chen, L. A simple on-line detection system based on fiber-optic sensing for the realtime monitoring of fixed bed adsorption processes of molecularly imprinted polymers. J. Chromatogr. A 2020, 1622, 461112. [Google Scholar] [CrossRef]
- Bhat, M.P.; Kurkuri, M.; Losic, D.; Kigga, M.; Altalhi, T. New optofluidic based lab-on-a-chip device for the real-time fluoride analysis. Anal. Chim. Acta 2021, 1159, 338439. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Chen, H.; Wang, D.N.; Wang, Y.; Zhao, C. A microfluid fiber device for trace detection of aggregation induced emission molecules. IEEE Sens. J. 2022, 22, 5688–5694. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.G.; Hu, S.; Peng, Y. Applications of fiber-optic biochemical sensor in microfluidic chips: A review. Biosens. Bioelectron. 2020, 166, 112447. [Google Scholar] [CrossRef]
- C Chung, Y.; Jin, W.; Lee, B.; Canning, J.; Nakamura, K.; Yuan, L.; Zhang, L.; Tong, L. Microfluidic chip based microfiber/nanofiber sensors. In Proceedings of the 25th International Conference on Optical Fiber Sensors, Jeju, Republic of Korea, 24–28 April 2017; Volume 10323, p. 1032335. [Google Scholar] [CrossRef]
- Wang, C.-W.; Fan, Z.H. Multi-sample immunoassay inside optical fiber capillary enabled by evanescent wave detection. Sens. Bio Sens. Res. 2016, 7, 7–11. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Yang, R.; Song, D.; Long, F.; Zhu, A. Integrated multichannel all-fiber optofluidic biosensing platform for sensitive and simultaneous detection of trace analytes. Anal. Chim. Acta 2018, 1040, 112–119. [Google Scholar] [CrossRef]
- Song, D.; Yang, R.; Fang, S.; Liu, Y.; Liu, J.; Xu, W.; Long, F.; Zhu, A. A novel dual-color total internal reflection fluorescence detecting platform using compact optical structure and silicon-based photodetector. Talanta 2019, 196, 78–84. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Albert, J. Review of plasmonic fiber optic biochemical sensors: Improving the limit of detection. Anal. Bioanal. Chem. 2015, 407, 3883–3897. [Google Scholar] [CrossRef]
- Sun, Y.S.; Li, C.J.; Hsu, J.C. Integration of curved D-Type optical fiber sensor with microfluidic chip. Sensors 2016, 17, 63. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, L.; Li, S.; Qi, R.; Chen, X.; Li, W. Highly sensitive refractive index detection based on compact HSC-SPR structure in a microfluidic chip. Sens. Actuators A 2019, 297, 111558. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, Q.; Wang, C.; Qian, Y.; Liu, G.; Wang, Y.; Fu, L. Surface plasmon resonance (SPR) biosensors for food allergen detection in food matrices. Biosens. Bioelectron. 2019, 142, 111449. [Google Scholar] [CrossRef]
- Kim, H.-M.; Park, J.-H.; Jeong, D.H.; Lee, H.-Y.; Lee, S.-K. Real-time detection of prostate-specific antigens using a highly reliable fiber-optic localized surface plasmon resonance sensor combined with micro fluidic channel. Sens. Actuators B 2018, 273, 891–898. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, H.J.; Park, J.H.; Lee, S.K. High-performance biosensor using a sandwich assay via antibody-conjugated gold nanoparticles and fiber-optic localized surface plasmon resonance. Anal. Chim. Acta 2022, 1213, 339960. [Google Scholar] [CrossRef]
- Jyoti; Kavita; Verma, R.K. Selective detection of urea as milk adulterant using LMR based fiber optic probe. J. Food Compos. Anal. 2022, 114, 104825. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Trinh, K.T.L.; Yoon, W.J.; Lee, N.Y.; Ju, H. Integration of a microfluidic polymerase chain reaction device and surface plasmon resonance fiber sensor into an inline all-in-one platform for pathogenic bacteria detection. Sens. Actuators B 2017, 242, 1–8. [Google Scholar] [CrossRef]
- Chang, T.C.; Sun, A.Y.; Huang, Y.C.; Wang, C.H.; Wang, S.C.; Chau, L.K. Integration of power-free and self-contained microfluidic chip with fiber optic particle plasmon resonance aptasensor for rapid detection of SARS-CoV-2 nucleocapsid protein. Biosensors 2022, 12, 785. [Google Scholar] [CrossRef]
- Rifat, A.A.; Mahdiraji, G.A.; Chow, D.M.; Shee, Y.G.; Ahmed, R.; Adikan, F.R. Photonic crystal fiber-based surface plasmon resonance sensor with selective analyte channels and ggraphene-silver deposited core. Sensors 2015, 15, 11499–11510. [Google Scholar] [CrossRef]
- Bing, P.; Wu, G.; Sui, J.; Zhang, H.; Tan, L.; Li, Z.; Yao, J. Double samples synchronous detection sensor based on up-core photonic crystal fiber. Optik 2020, 224, 165522. [Google Scholar] [CrossRef]
- Gu, S.; Sun, W.; Li, M.; Li, Z.; Nan, X.; Feng, Z.; Deng, M. Simultaneous measurement of magnetic field and temperature based on photonic crystal fiber plasmonic sensor with dual-polarized modes. Optik 2022, 259, 169030. [Google Scholar] [CrossRef]
- Qing, Z.; Xu, J.; Hu, J.; Zheng, J.; He, L.; Zou, Z.; Yang, S.; Tan, W.; Yang, R. In situ amplification-based imaging of RNA in living cells. Angew. Chem. Int. Ed. 2019, 58, 11574–11585. [Google Scholar] [CrossRef]
- Ngo, L.T.; Wang, W.K.; Tseng, Y.T.; Chang, T.C.; Kuo, P.L.; Chau, L.K.; Huang, T.T. MutS protein-based fiber optic particle plasmon resonance biosensor for detecting single nucleotide polymorphisms. Anal. Bioanal. Chem. 2021, 413, 3329–3337. [Google Scholar] [CrossRef]
- Challa, P.K.; Peter, Q.; Wright, M.A.; Zhang, Y.; Saar, K.L.; Carozza, J.A.; Benesch, J.L.P.; Knowles, T.P.J. Real-time intrinsic fluorescence visualization and sizing of proteins and protein complexes in microfluidic devices. Anal. Chem. 2018, 90, 3849–3855. [Google Scholar] [CrossRef]
- Kim, H.M.; Jeong, D.H.; Lee, H.Y.; Park, J.H.; Lee, S.K. Design and validation of fiber optic localized surface plasmon resonance sensor for thyroglobulin immunoassay with high sensitivity and rapid detection. Sci. Rep. 2021, 11, 15985. [Google Scholar] [CrossRef]
- Hussain, M.; Liu, X.; Tang, S.; Zou, J.; Wang, Z.; Ali, Z.; He, N.; Tang, Y. Rapid detection of Pseudomonas aeruginosa based on lab-on-a-chip platform using immunomagnetic separation, light scattering, and machine learning. Anal. Chim. Acta 2022, 1189, 339223. [Google Scholar] [CrossRef]
- Qu, J.; Liu, Y.; Li, Y.; Li, J.; Meng, S. Microfluidic chip with fiber-tip sensors for synchronously monitoring concentration and temperature of glucose solutions. Sensors 2023, 23, 2478. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, J.; Wang, Q.; Xu, J.; Fang, S.; Chen, J.; Duan, M. Optical sensor for fluoride determination in tea sample based on evanescent-wave interaction and fiber-optic integration. Talanta 2017, 174, 372–379. [Google Scholar] [CrossRef]
- Lin, C.L.; Chang, W.H.; Wang, C.H.; Lee, C.H.; Chen, T.Y.; Jan, F.J.; Lee, G.B. A microfluidic system integrated with buried optical fibers for detection of Phalaenopsis orchid pathogens. Biosens. Bioelectron. 2015, 63, 572–579. [Google Scholar] [CrossRef]
- Vikas; Yadav, M.K.; Kumar, P.; Verma, R.K. Detection of adulteration in pure honey utilizing Ag-graphene oxide coated fiber optic SPR probes. Food Chem. 2020, 332, 127346. [Google Scholar] [CrossRef]
- Yazdi, S.H.; White, I.M. Multiplexed detection of aquaculture fungicides using a pump-free optofluidic SERS microsystem. Analyst 2013, 138, 100–103. [Google Scholar] [CrossRef]
- Oscar, S.V.; Fernando, O.L.; Del Pilar, C.M. Total polyphenols content in white wines on a microfluidic flow injection analyzer with embedded optical fibers. Food Chem. 2017, 221, 1062–1068. [Google Scholar] [CrossRef]
- Youngvises, N.; Nguyen, N.; Charoenrat, T.; Supaporn, K.; Jaroon, J.; Awadh, A. Miniaturized green analytical method for determination of silver ions using C-phycocyanin from cyanobacteria as an ecofriendly reagent. Chiang Mai J. Sci. 2021, 48, 221–230. Available online: https://epg.science.cmu.ac.th/ejournal (accessed on 5 June 2020).
- Alahmad, W.; Uraisin, K.; Nacapricha, D.; Kaneta, T. A miniaturized chemiluminescence detection system for a microfluidic paper-based analytical device and its application to the determination of chromium(iii). Anal. Methods 2016, 8, 5414–5420. [Google Scholar] [CrossRef]
- Prawpan, I.; Kamil, s.; Robert, K.; Wisaroot, S.; Wutthinan, J.; Nuanlaor, R.; Prapin, W.; Nathawut, C.; Rattikan, C.; Duangjai, N. Microfluidic analysis with front-face fluorometric detection for the determination of total inorganic iodine in drinking water. Anal. Sci. 2018, 34, 161–167. [Google Scholar] [CrossRef]
- Xiong, Y.; Tan, J.; Wang, C.; Wu, J.; Wang, Q.; Chen, J.; Fang, S.; Duan, M. A miniaturized evanescent-wave free chlorine sensor based on colorimetric determination by integrating on optical fiber surface. Sens. Actuators B 2017, 245, 674–682. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, B.; Zhu, H.; Jin, Y.; Shi, N.; Feng, J. Rapid on-chip quantification of ammonia nitrogen based on a ‘flow and react’ mechanism. Int. J. Environ. Anal. Chem. 2020, 102, 516–527. [Google Scholar] [CrossRef]








| Detection Method | Analyte | Sample | LOD (μg/L) | Time | Advantage | Ref. |
|---|---|---|---|---|---|---|
| Raman | Creatinine | Aqueous solution | 1.13 × 102 | 30 s | Short assay time/ Low sample consumption | [33] |
| Rhodamine 6G | Water pollutants | 4.79 × 10−2 | —— | High reusability/ Good controllability | [34] | |
| Levofloxacin | Tap water | 3.61 × 103 | 500 ms | Highly sensitive/ Reproducible | [35] | |
| Thiram | Aqueous solution | 50 | —— | Portable/Small footprint | [36] | |
| UV-vis | 2-amino-4-chlorophenol | Chlorzoxazone | 2.8 | 235 s | Fast pretreatment procedure | [37] |
| Phenol | Aqueous solution | 7.5 | 7 min | High sensitivity/ Rapid response time | [38] | |
| Tartrazine | Aqueous solution | 2.67 × 103 | —— | Low sample consumption/ Long absorption path length | [39] | |
| Chloramphenicol | Aqueous solution | 5.0 × 10−4 | 30 s | Small footprint/ Low power consumption | [40] | |
| Fluorescence | Hg2+ | Real water | 1.70 | 10 min | Portability/ Easy-to-operate | [41] |
| Fluorescein dye | Droplets | 3.32 | —— | High sensitivity/ Rapid response time | [42] | |
| Bisphenol A | Tap/Lake water | 1.8 × 10−2 | 15 min | Miniaturization/ Flexibility/Mobility | [43] | |
| Dopamine | Human serum | 4.59 | 60 s | Fast response/ High selectivity | [44] | |
| SPR/LSPR | C-reactive protein | Serum | 9.0 | —— | Easy to achieve/Real-time operation/ Label-free/Portable | [45] |
| Protein | Urine | 1.5 × 103 | —— | Low consumption/ Highly sensitive detection | [46] | |
| Adalimumab | Plasma | 350 | 12 min | Short assay time/ Low consumption | [47] | |
| Dengue virus NS1 protein | Blood serum | 60 | 25 min | Easy lab-made set-up/Fast | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Xia, L.; Xiao, X.; Li, G. Recent Progress on Microfluidics Integrated with Fiber-Optic Sensors for On-Site Detection. Sensors 2024, 24, 2067. https://doi.org/10.3390/s24072067
Wang W, Xia L, Xiao X, Li G. Recent Progress on Microfluidics Integrated with Fiber-Optic Sensors for On-Site Detection. Sensors. 2024; 24(7):2067. https://doi.org/10.3390/s24072067
Chicago/Turabian StyleWang, Weibin, Ling Xia, Xiaohua Xiao, and Gongke Li. 2024. "Recent Progress on Microfluidics Integrated with Fiber-Optic Sensors for On-Site Detection" Sensors 24, no. 7: 2067. https://doi.org/10.3390/s24072067
APA StyleWang, W., Xia, L., Xiao, X., & Li, G. (2024). Recent Progress on Microfluidics Integrated with Fiber-Optic Sensors for On-Site Detection. Sensors, 24(7), 2067. https://doi.org/10.3390/s24072067






