SPR and Double Resonance LPG Biosensors for Helicobacter pylori BabA Antigen Detection
Abstract
1. Introduction
1.1. Current Diagnostic Methods
1.2. Immunosensors
1.3. Biosensors
2. Reagents and Materials
2.1. Bacterial Strain
2.2. Cultivation Procedure
2.2.1. Cultivation of Bacteria
2.2.2. Preparation of Bacterial Suspensions
- preparation of a stock suspension with deionized water (density of 109 CFU/mL);
- preparation of ten-fold dilutions of this suspension at concentrations ranging from 108 to 102 CFU/mL.
2.3. Blood Antigen
3. Optical Platforms and Their Functionalization for Bacterial Sensing
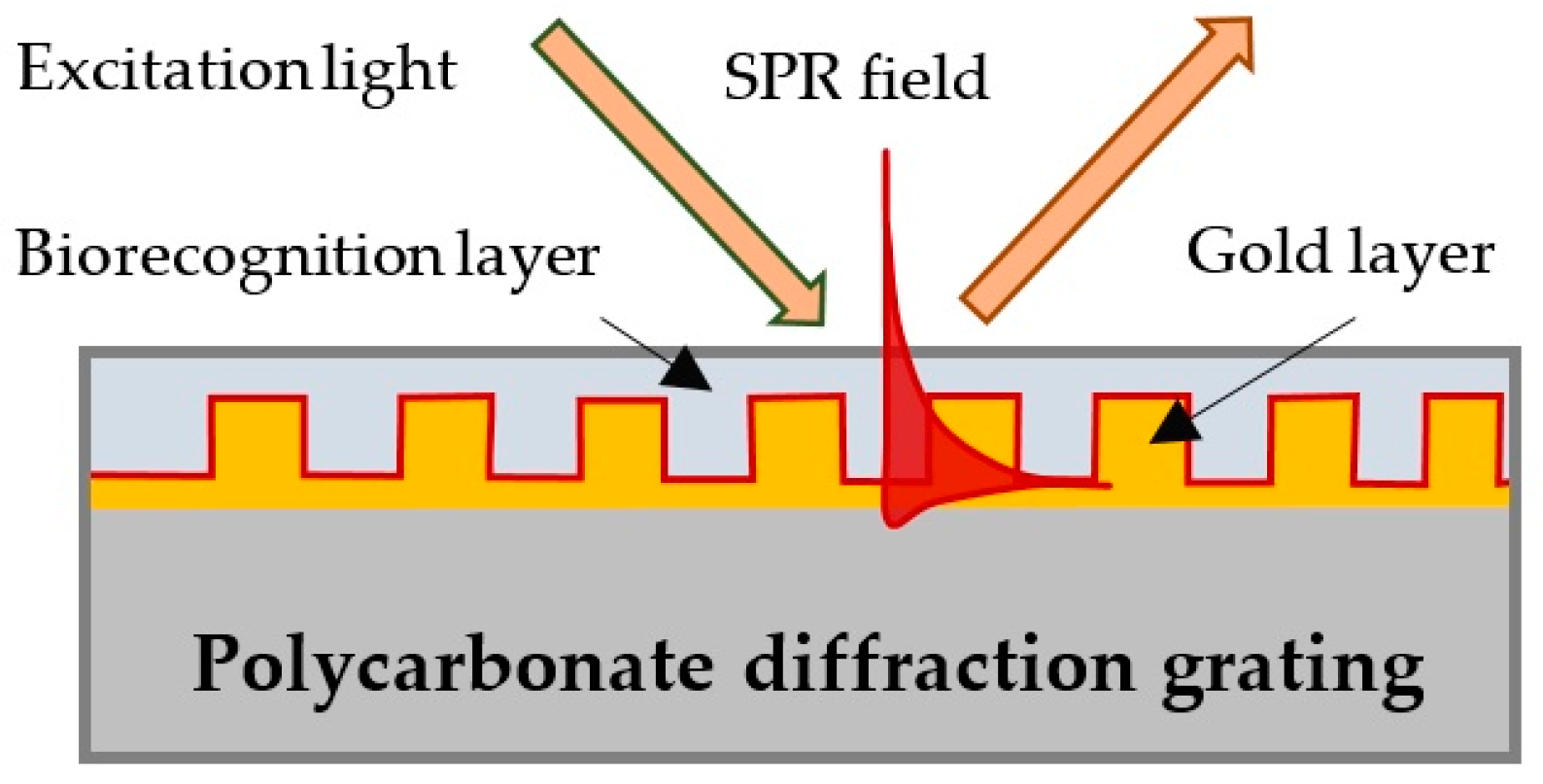
3.1. SPR Platforms
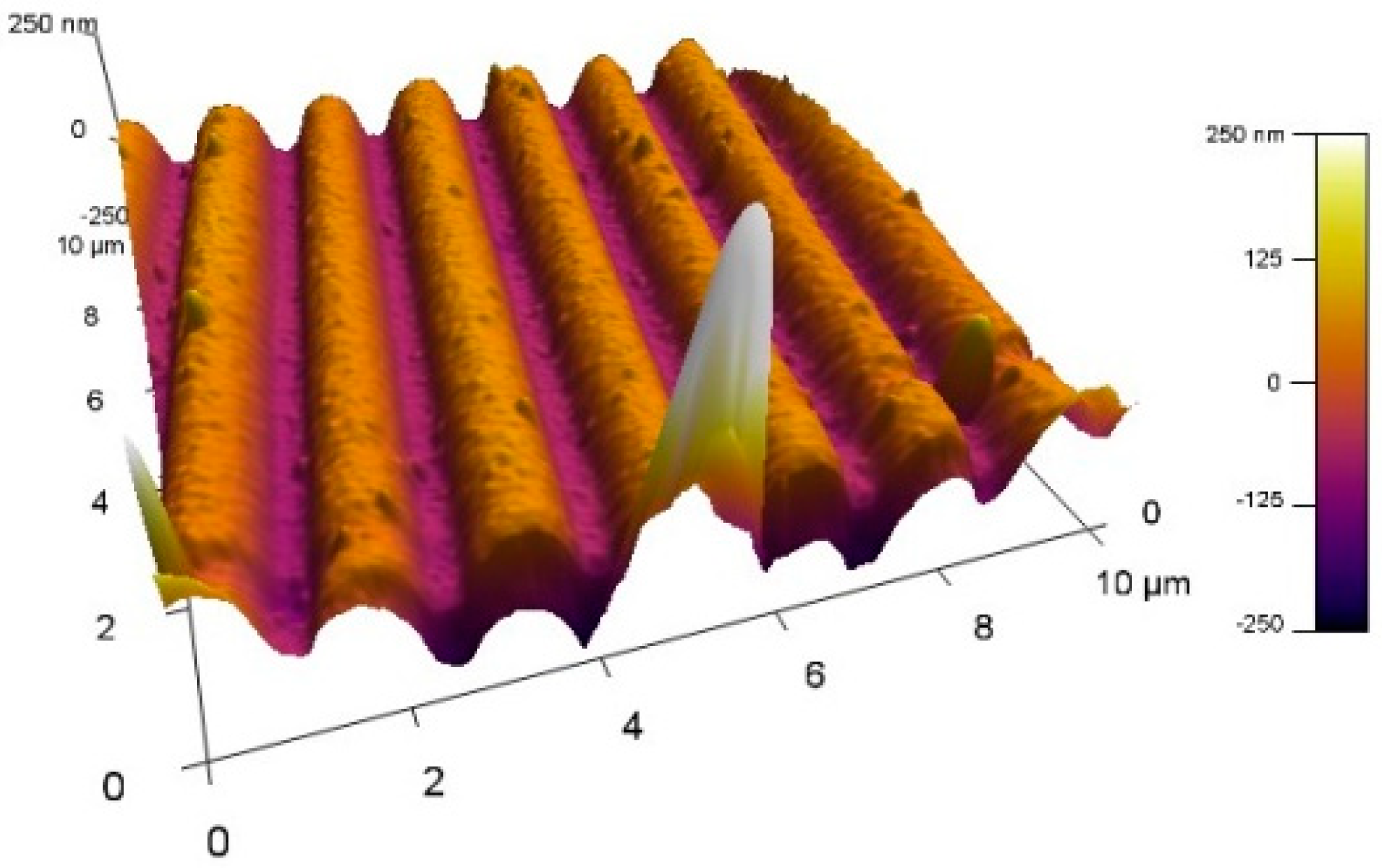
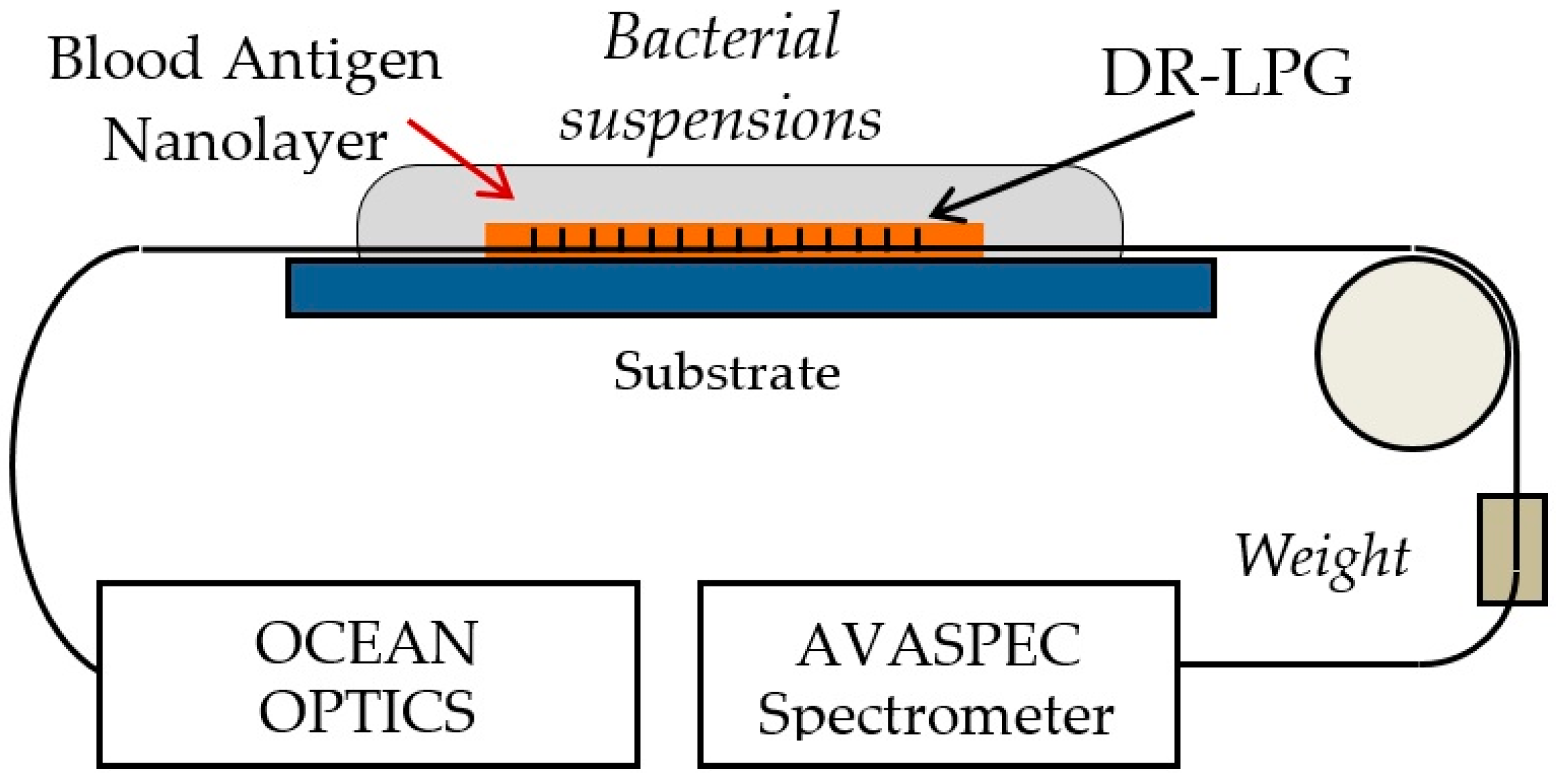
3.2. Double Resonance Long Period Gratings (DR LPG)
3.3. Functionalization of the Platforms
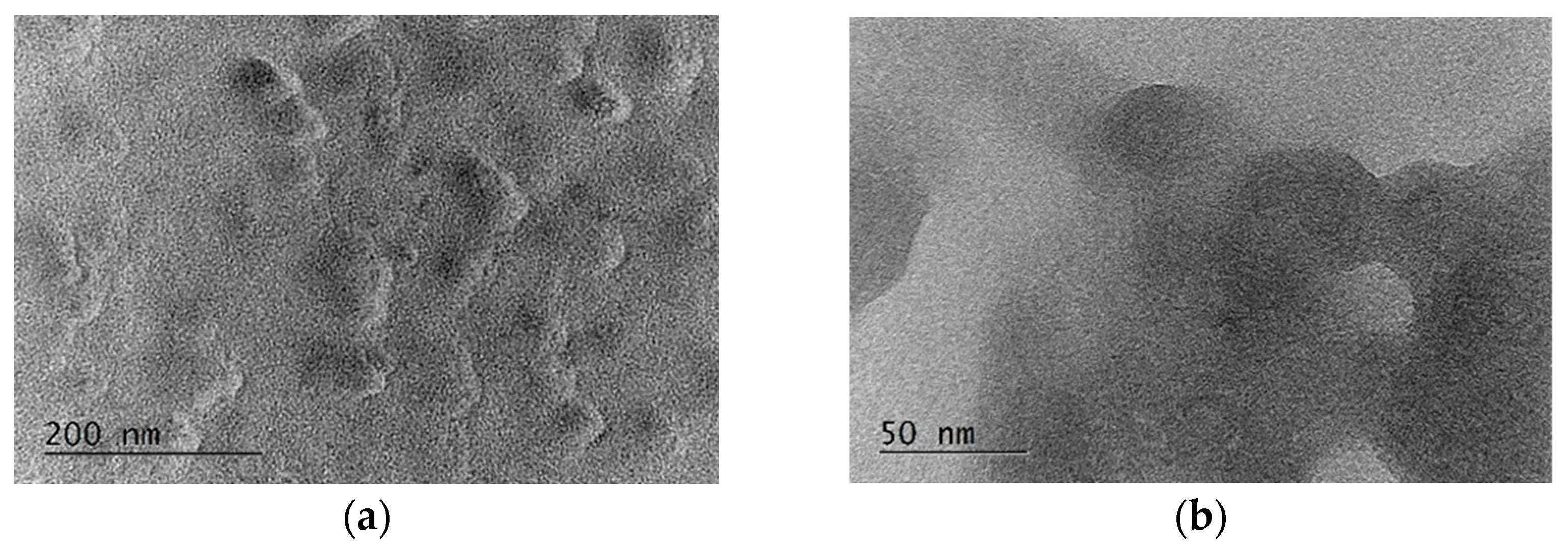
3.4. Characterization of the Deposited Film of Blood Antigen Molecules
3.5. Incubation of SPR Chips and Measurement Procedure
3.6. Incubation of the LPG Structures and Measurement Procedure
4. Results and Discussion
4.1. Results of SPR Measurements
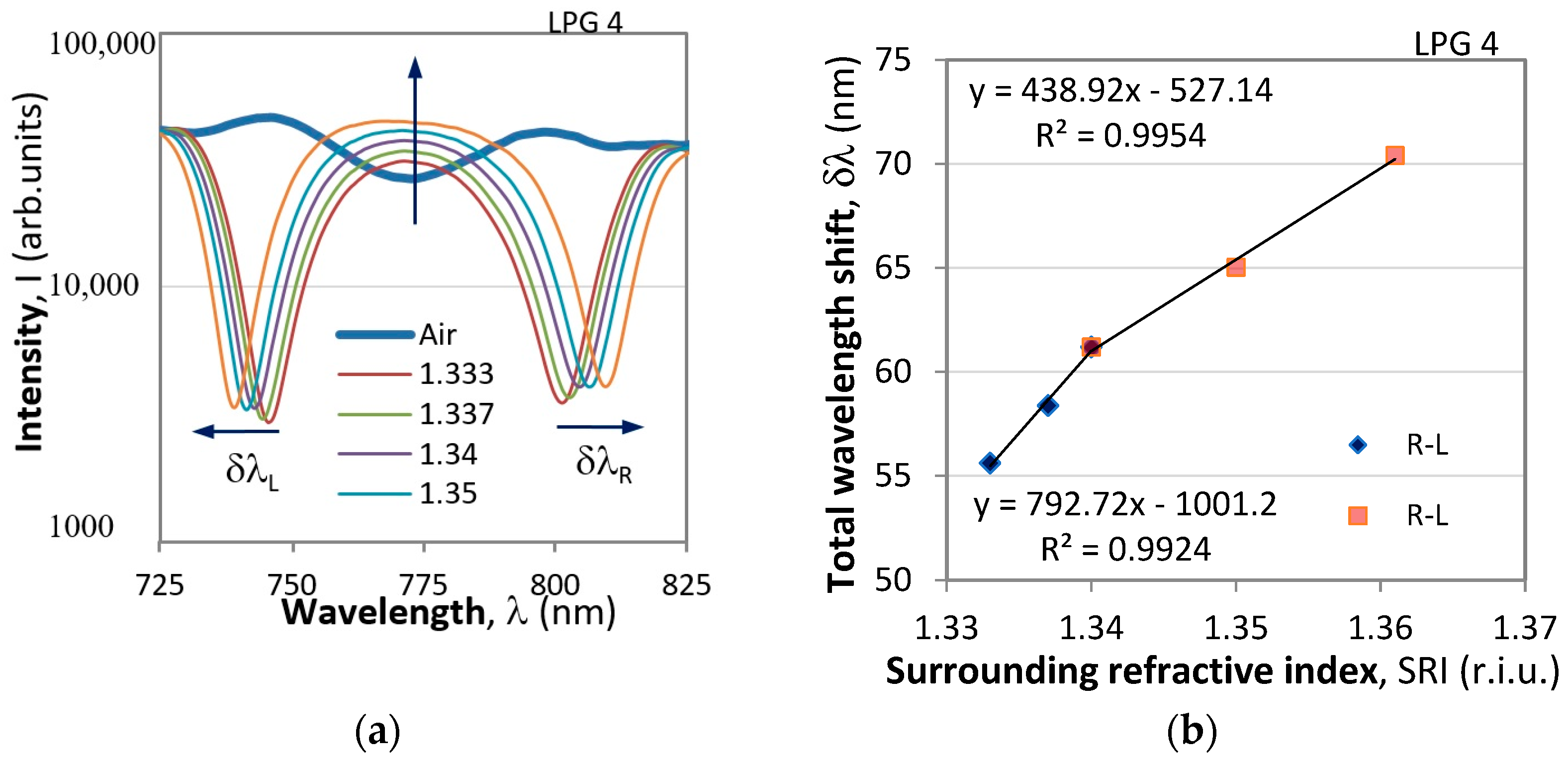
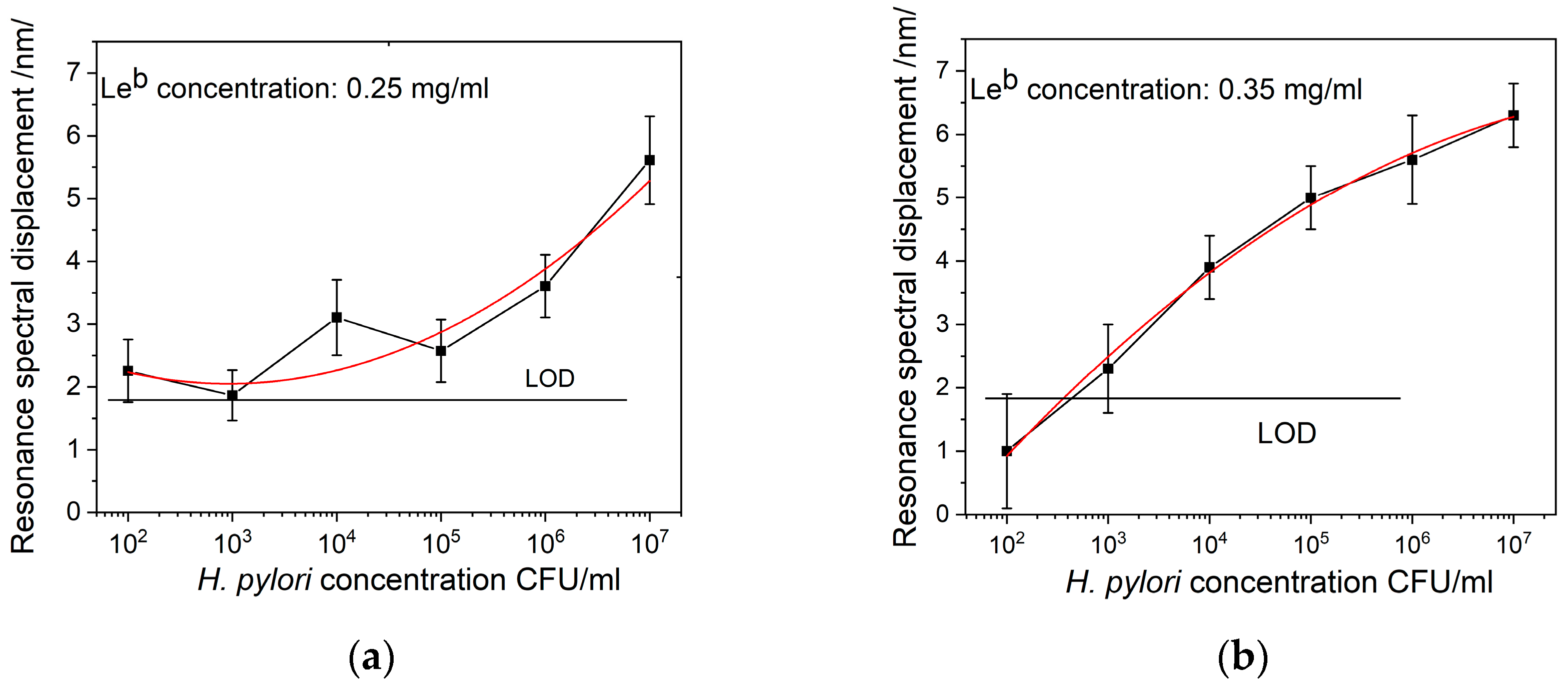
4.2. Results of DR LPG Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stefano, K.; Rosalia, A.; Chiara, B.; Federica, G.; Marco, M.; Gioacchino, L.; Fabiola, F.; Francesco, D.M.; Gian, L.D.A. Non-invasive tests for the diagnosis of Helicobacter pylori: State of the art. Acta Biomed. 2018, 89 (Suppl. S8), 58–64. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Hungin, P.; Jones, R.; Axon, A.; Graham, D.Y.; Tytgat, G.; The European Helicobacter pylori Study Group (EHPSG). Current concepts in the management of Helicobacter pylori infection—The Maastricht 2-2000 Consensus Report. Aliment. Pharmacol. Ther. 2002, 16, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Kuipers, E.; Bazzoli, F.; EL-Omar, E.; Graham, D.; Hunt, R.; Rokkas, T.; Vakil, N.; et al. Current concepts in the management of Helicobacter pylori infection. The Maastricht-3 2005 Consensus Report. Gut 2007, 56, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Atherton, J.; Anthony, T.; Axon, R.; Bazzoli, F.; Gensini, G.F.; Gisbert, J.P.; Graham, D.Y.; et al. Management of Helicobacter pylori infection. The Maastricht-IV/Florence Consensus Report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Mabe, K.; Hanawa, T.; Kamiya, S. Urease-positive bacteria in the stomach induce a false-positive reaction in a urea breath test for diagnosis of Helicobacter pylori infection. J. Med. Microbiol. 2008, 57, 814–819. [Google Scholar] [CrossRef]
- Patel, S.K.; Pratap, C.B.; Jain, A.K.; Gulati, A.K.; Nath, G. Diagnosis ofHelicobacter pylori: What should be the gold standard? World J. Gastroenterol. 2014, 20, 12847–12859. [Google Scholar] [CrossRef] [PubMed]
- Godbole, G.; Mégraud, F.; Bessède, E. Review: Diagnosis of Helicobacter pylori Infection. Helicobacter 2020, 25 (Suppl. S1), e12735. [Google Scholar] [CrossRef]
- Xu, C.; Soyfoo, D.M.; Wu, Y.; Xu, S. Virulence of Helicobacter pylori outer membrane proteins: An updated review. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1821–1830. [Google Scholar] [CrossRef]
- Oleastro, M.; Ménard, A. The role of Helicobacter pylori outer membrane proteins in adherence and pathogenesis. Biology 2013, 2, 1110–1134. [Google Scholar] [CrossRef]
- Alipour, M. Molecular Mechanism of Helicobacter Pylori-Induced Gastric Cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Forma, A.; Sitarz, M.; Portincasa, P.; Garruti, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori BabA in Adaptation for Gastric Colonization. World J. Gastroenterol. 2017, 23, 4158–4169. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, J.; Sonden, B.; Hurtig, M.; Olfat, F.O.; Forsberg, L.; Roche, N.; Angstrom, J.; Larsson, T.; Teneberg, S.; Karlsson, K.; et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 2002, 297, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Senkovich, O.A.; Yin, J.; Ekshyyan, V.; Conant, C.; Traylor, J.; Adegboyega, P.; McGee, D.J.; Rhoads, R.E.; Slepenkov, S.; Testerman, T.L. Helicobacter pylori AlpA and AlpB bind host laminin and influence gastric inflammation in gerbils. Infect. Immun. 2011, 79, 3106–3116. [Google Scholar] [CrossRef]
- Boren, T.; Falk, P.; Roth, K.A.; Larson, G.; Normark, S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 1993, 262, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Ilver, D.; Arnqvist, A.; Ogren, J.; Frick, I.M.; Kersulyte, D.; Incecik, E.T.; Berg, D.E.; Covacci, A.; Engstrand, L.; Borén, T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 1998, 279, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.A.; Appelmelk, B.J.; Rasko, D.A.; Moran, A.P.; Hynes, S.O.; MacLean, L.L.; Chan, K.H.; Michael, F.S.; Logan, S.M.; O’Rourke, J.; et al. Lipopolysaccharide structures of Helicobacter pylori genomic strains 26695 and J99, mouse model H-pylori Sydney strain, H-pylori P466 carrying sialyl Lewis X, and H-pylori UA915 expressing Lewis B—Classification of H-pylori lipopolysaccharides into glycotype families. Eur. J. Biochem. 2000, 267, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Dyankov, G.; Najdenski, H.; Kasovski, V.; Zaharieva, M.; Kisov, H.; Hikova, E.; Malinowski, N. Detection of Helicobacter Pylori in saliva by Surface Plasmon Resonance. In Proceedings of the 7th BIOMATSEN, Oludeniz, Turkey, 22–28 April 2022. [Google Scholar]
- Gupta, S.; Jain, U.; Murti, B.T.; Putri, A.D.; Tiwari, A.; Chauhan, N. Nanohybrid-based immunosensor prepared for Helicobacter pylori BabA antigen detection through immobilized antibody assembly with@ Pdnano/rGO/PEDOT sensing platform. Sci. Rep. 2020, 10, 21217. [Google Scholar] [CrossRef]
- Cardos, A.I.; Maghiar, A.; Zaha, D.C.; Pop, O.; Fritea, L.; Miere, F.; Cavalu, S. Evolution of Diagnostic Methods for Helicobacter pylori Infections: From Traditional Tests to High Technology, Advanced Sensitivity and Discrimination Tools. Diagnostics 2022, 12, 508. [Google Scholar] [CrossRef]
- Saxena, K.; Chauhan, N.; Jain, U. Advances in diagnosis of Helicobacter pylori through biosensors: Point of care devices. Anal. Biochem. 2021, 630, 114325. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.; Alvandi, A.H.; Abdul-Tehrani, H.; Sadeghizadeh, M. Colorimetric detection of Helicobacter pylori DNA using isothermal helicase-dependent amplification and gold nanoparticle probes. Diagn. Microbiol. Infect. Dis. 2008, 62, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Duan, Y.; Li, Y.; Luo, Y. Fiber-Optic-Based Biosensor as an Innovative Technology for Point-of-Care Testing Detection of Foodborne Pathogenic Bacteria To Defend Food and Agricultural Product Safety. J. Agric. Food Chem. 2023, 71, 10982–10988. [Google Scholar] [CrossRef]
- Eftimov, T.; Genova-Kalou, P.; Dyankov, G.; Bock, W.; Mankov, V.; Ghaffari, S.; Veselinov, P.; Arapova, A.; Makouei, S. Capabilities of Double Resonance LPG and SPR Methods for Hypersensitive Detection of SARS Cov-2 Structural proteins: A Comparative Study. Biosensors 2023, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Dyankov, G.; Eftimov, T.; Malinovski, N.; Belina, E.; Kisov, H.; Mikulic, P.; Bock, W. Highly Efficient Biosensor based on MAPLE Deposited Hemoglobin on LPGs around Phase Matching Turning Point. Opt. Laser Technol. 2019, 123, 105907. [Google Scholar] [CrossRef]
- Dyankov, G.; Borisova, E.; Belina, E.; Kisov, H.; Angelov, I.; Gisbrecht, A.; Strijkova, V.; Malinowski, N. A Surface Plasmon Resonance Biosensor Based on Directly Immobilized Hemoglobin and Myoglobin. Sensors 2020, 20, 5572. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gu, L.; Ma, X.; Tian, X.; Fan, S.; Qin, M.; Lyu, M.; Wang, S. Rapid Detection of Helicobacter pylori by the Naked Eye Using DNA Aptamers. ACS Omega 2021, 6, 3771–3779. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyankov, G.; Eftimov, T.; Hikova, E.; Najdenski, H.; Kussovski, V.; Genova-Kalou, P.; Mankov, V.; Kisov, H.; Veselinov, P.; Ghaffari, S.S.; et al. SPR and Double Resonance LPG Biosensors for Helicobacter pylori BabA Antigen Detection. Sensors 2024, 24, 2118. https://doi.org/10.3390/s24072118
Dyankov G, Eftimov T, Hikova E, Najdenski H, Kussovski V, Genova-Kalou P, Mankov V, Kisov H, Veselinov P, Ghaffari SS, et al. SPR and Double Resonance LPG Biosensors for Helicobacter pylori BabA Antigen Detection. Sensors. 2024; 24(7):2118. https://doi.org/10.3390/s24072118
Chicago/Turabian StyleDyankov, Georgi, Tinko Eftimov, Evdokiya Hikova, Hristo Najdenski, Vesselin Kussovski, Petia Genova-Kalou, Vihar Mankov, Hristo Kisov, Petar Veselinov, Sanaz Shoar Ghaffari, and et al. 2024. "SPR and Double Resonance LPG Biosensors for Helicobacter pylori BabA Antigen Detection" Sensors 24, no. 7: 2118. https://doi.org/10.3390/s24072118
APA StyleDyankov, G., Eftimov, T., Hikova, E., Najdenski, H., Kussovski, V., Genova-Kalou, P., Mankov, V., Kisov, H., Veselinov, P., Ghaffari, S. S., Kovacheva-Slavova, M., Vladimirov, B., & Malinowski, N. (2024). SPR and Double Resonance LPG Biosensors for Helicobacter pylori BabA Antigen Detection. Sensors, 24(7), 2118. https://doi.org/10.3390/s24072118









