Smartphone-Derived Seismocardiography: Robust Approach for Accurate Cardiac Energy Assessment in Patients with Various Cardiovascular Conditions
Abstract
:1. Introduction
2. Materials and Methods
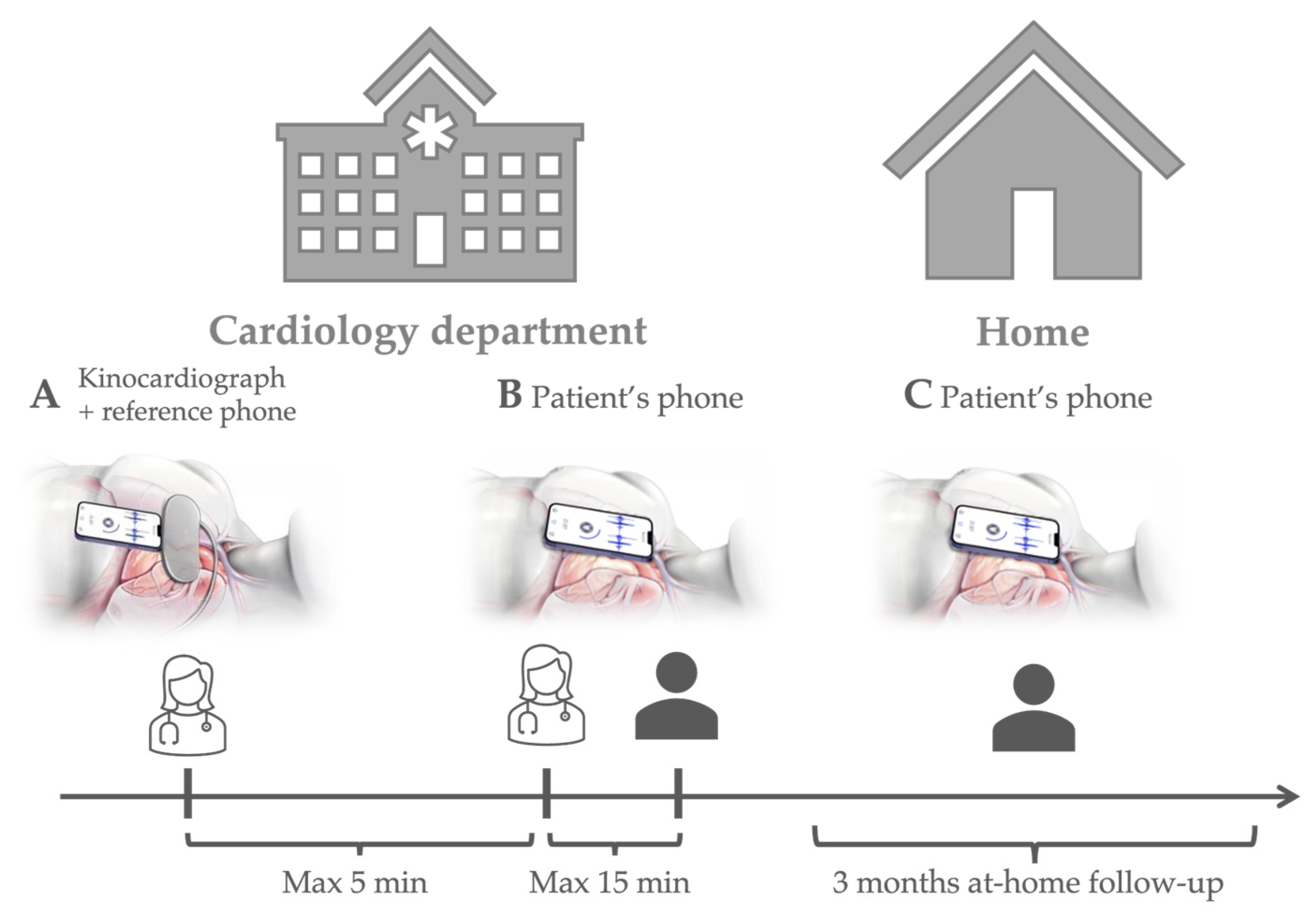
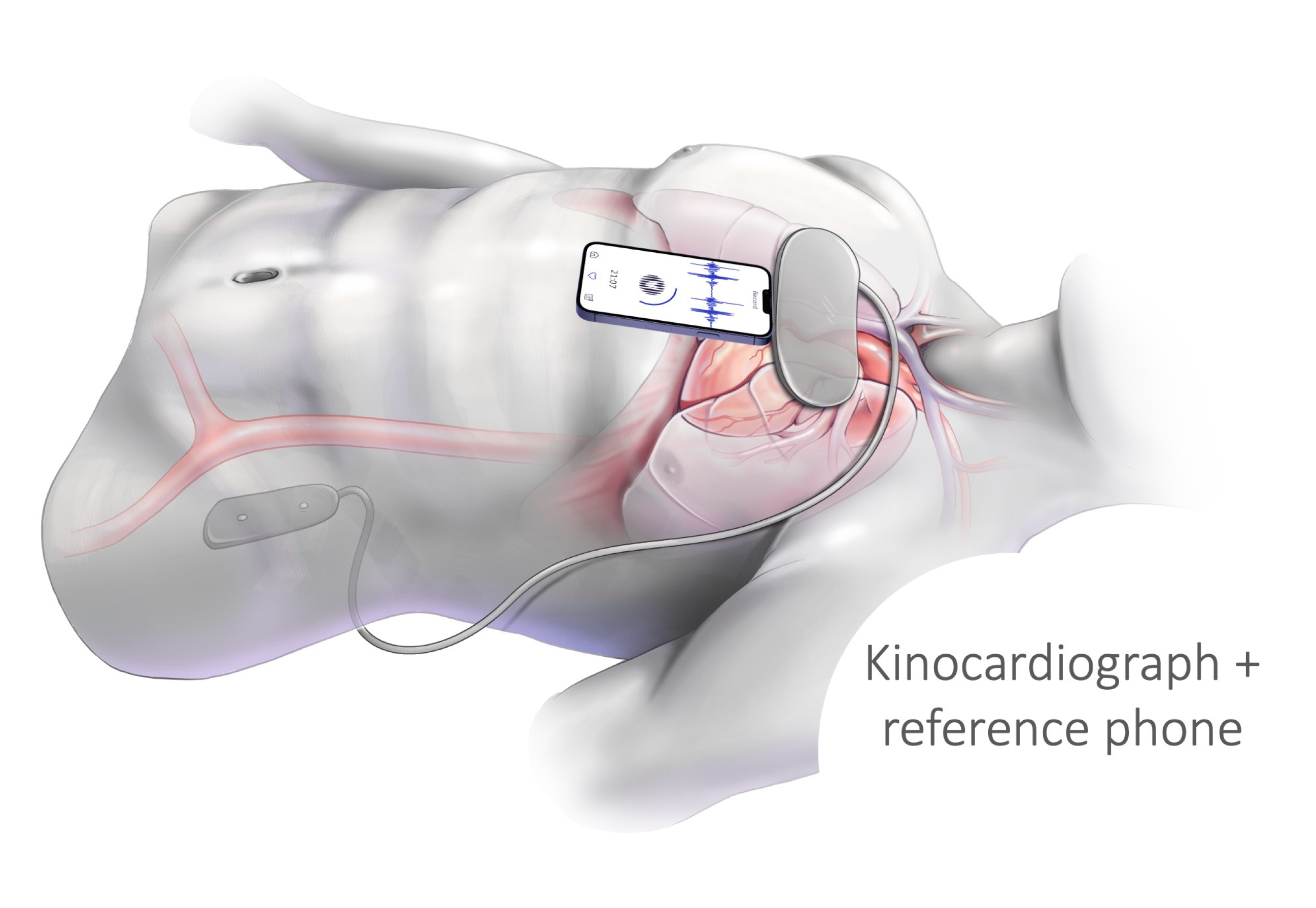
2.1. Protocol and Participants
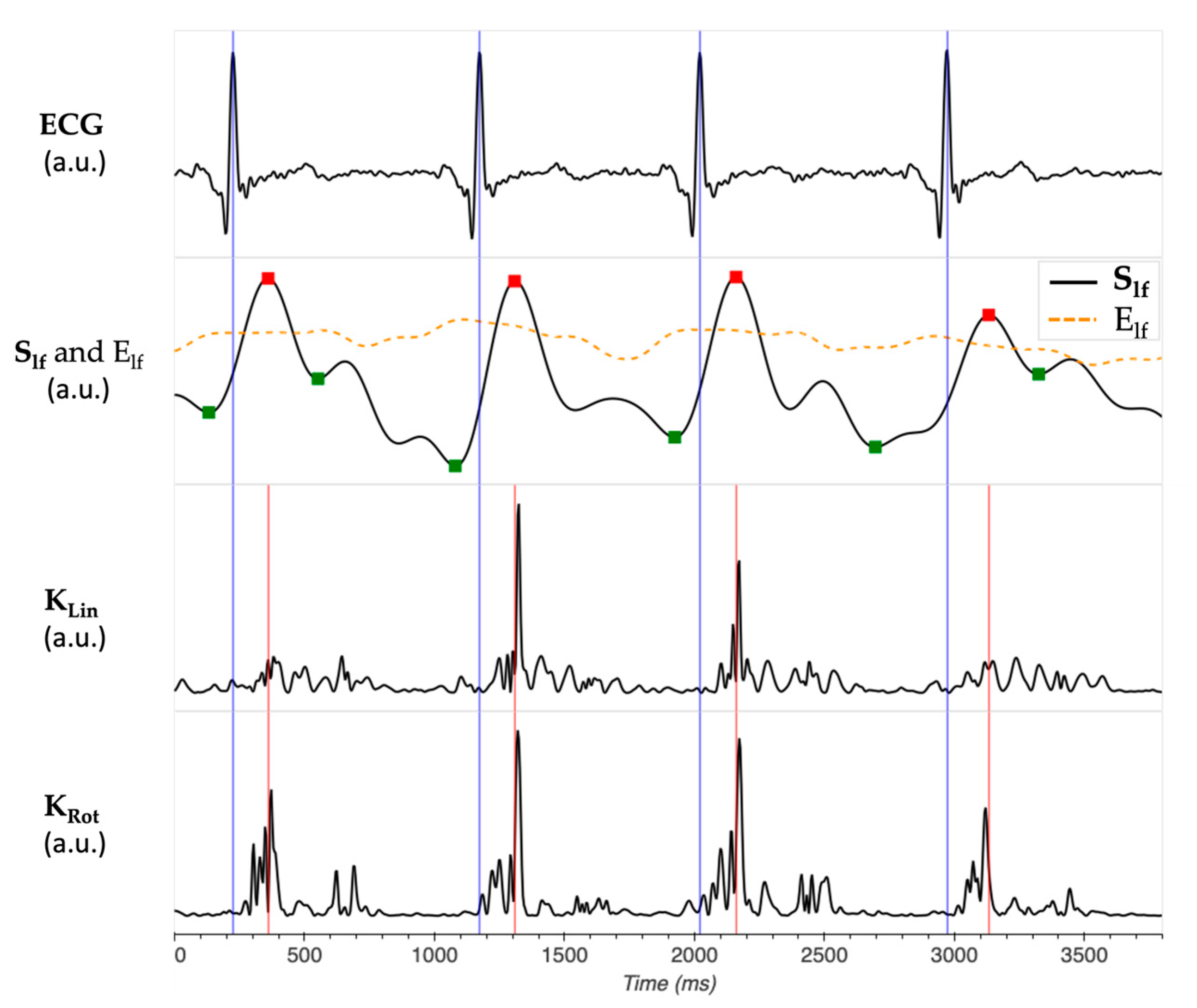
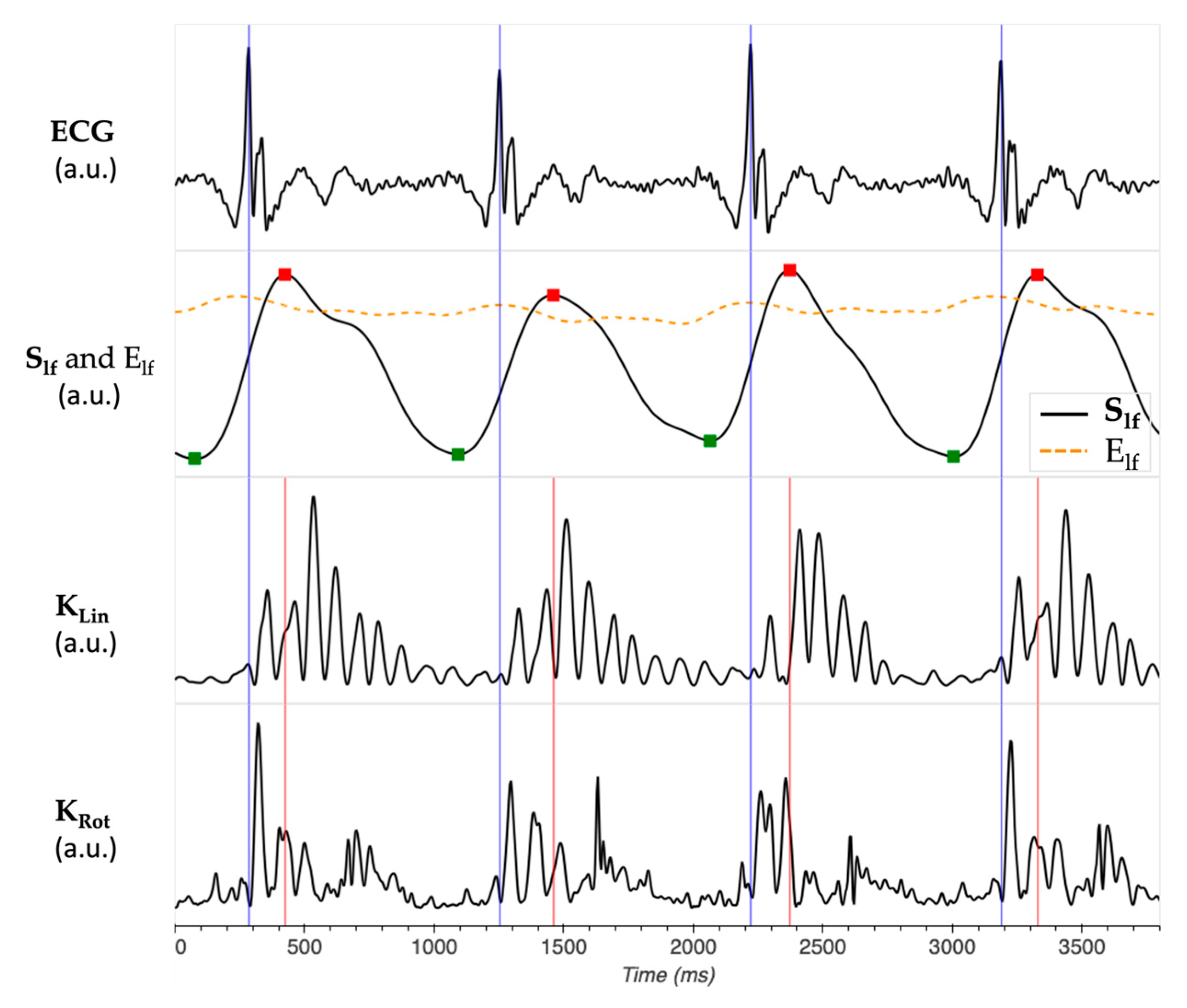
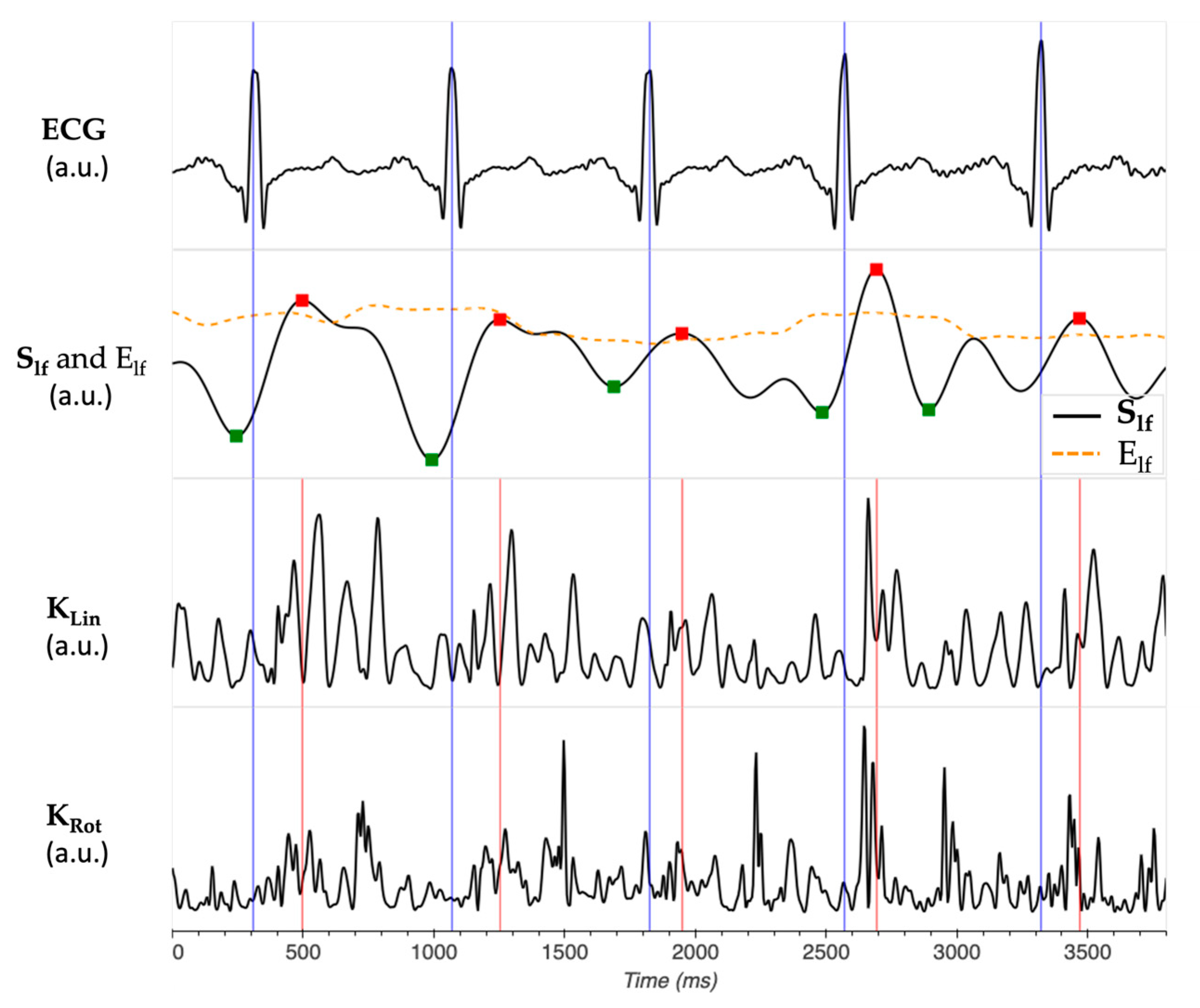
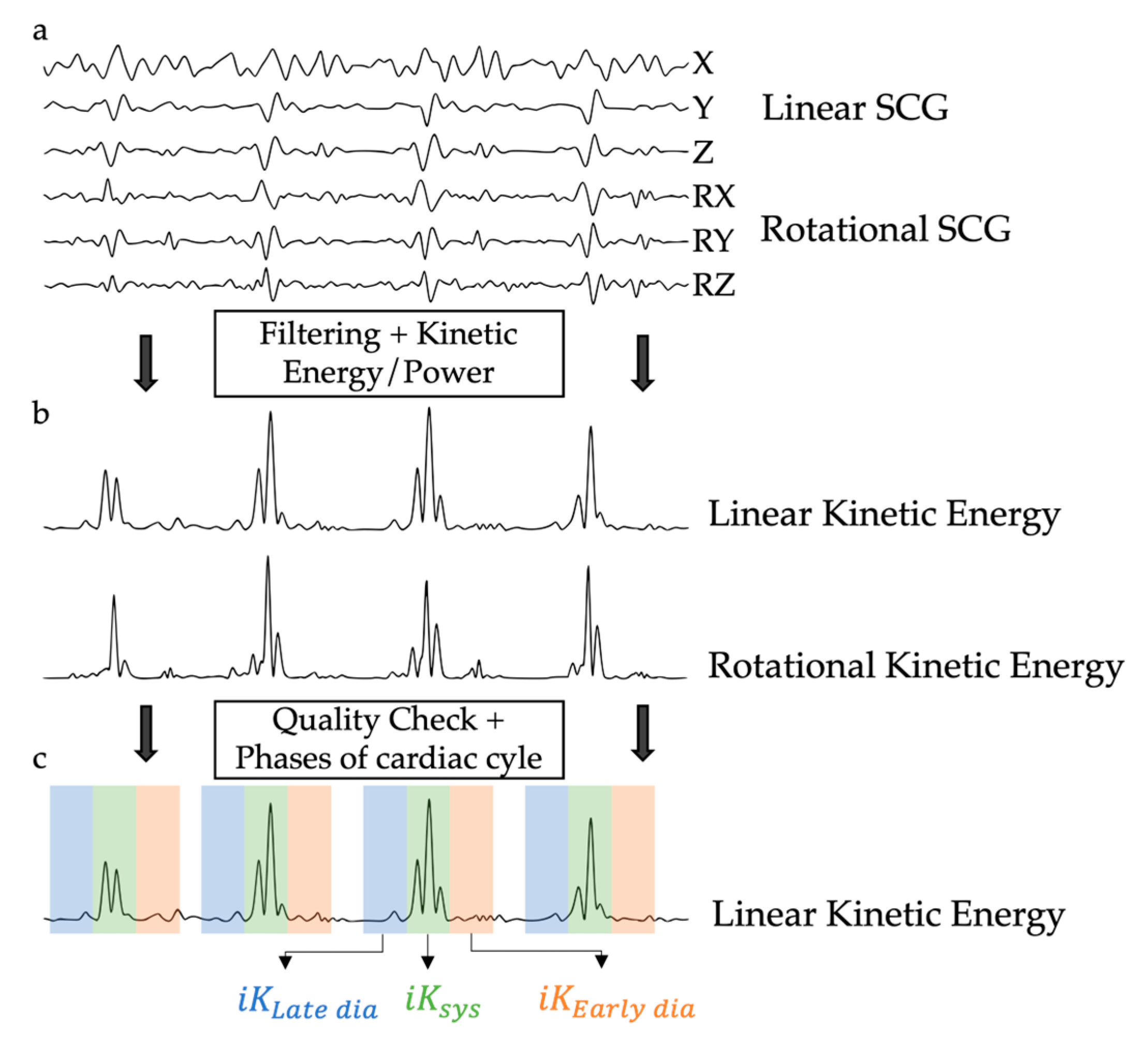
2.2. Kinetic Energy Data Analysis
- iKSys, which represented the cardiac activity during the systolic phase, and was computed between −100 ms and 100 ms around the reference point.
- iKLate dia, which represented the cardiac activity during the late diastolic phase, and was computed between −300 ms and −100 ms before the reference point.
- iKEarly dia, which represented the cardiac activity during the early diastolic phase, and was computed between 100 ms and 300 ms after the reference point.
2.3. Statistics
2.3.1. Repeatability Analysis
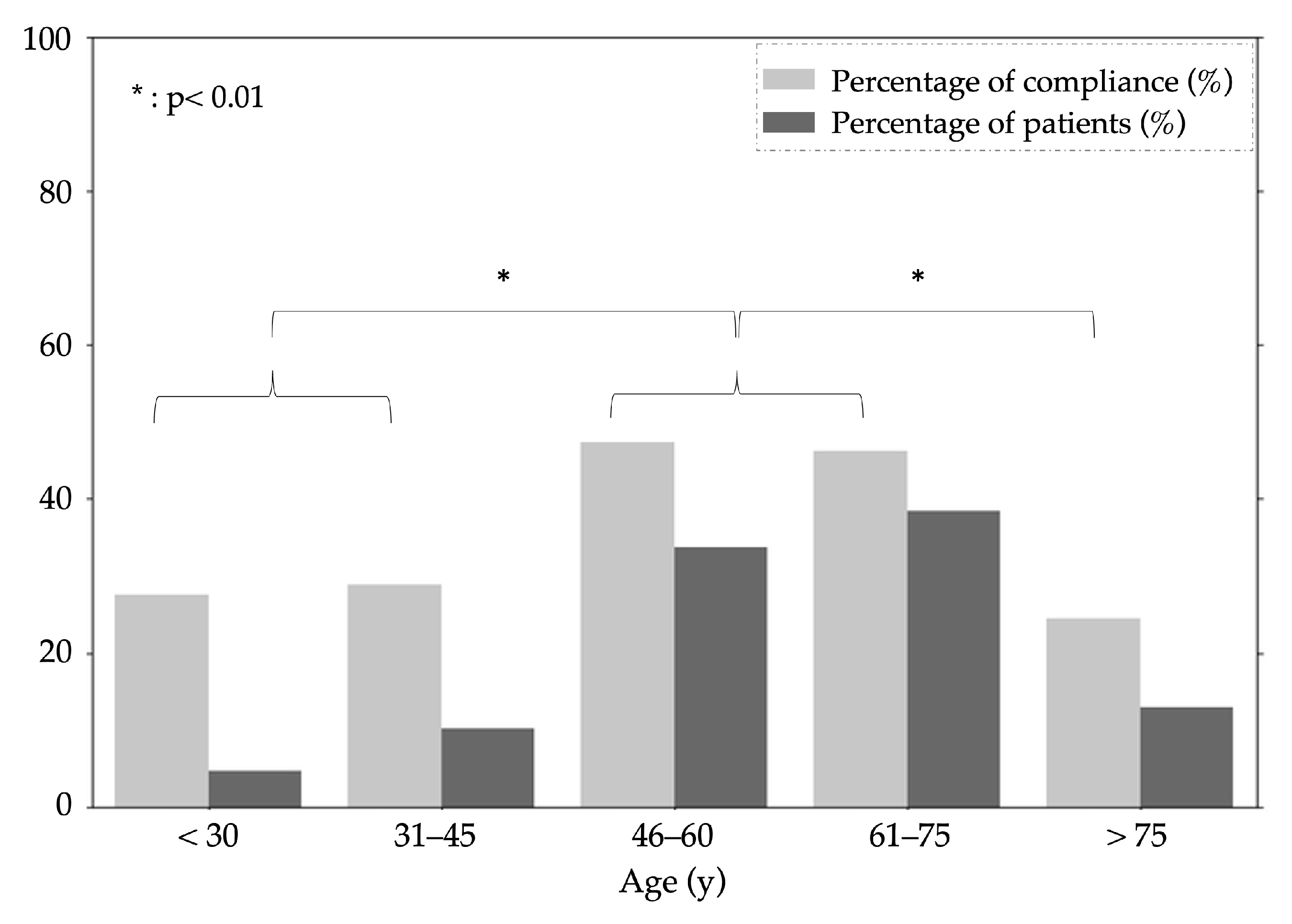
2.3.2. At-Home Measurements Patient Compliance
3. Results
3.1. Study Population
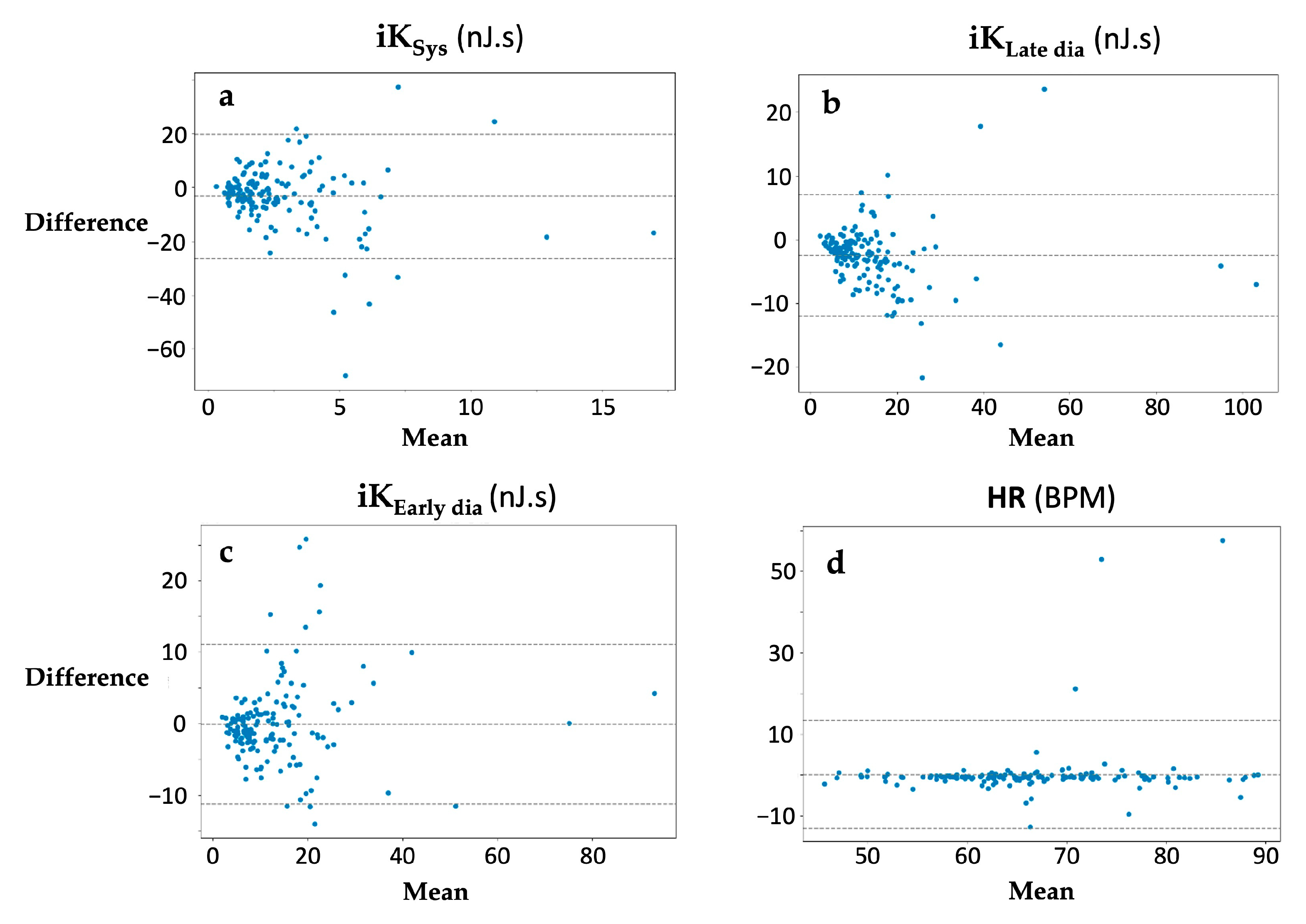
3.2. Assessments of Repeatability
3.3. Assessments of Patient Compliance
4. Discussion
4.1. Main Findings
4.2. Device vs. Commercial Smartphone Reliability and Reproducibility
4.3. Clinician vs. Patient Smartphone-Derived SCG Recording
4.4. Future Directions and Perspectives
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Measurements | Heart Failure | Control |
|---|---|---|
| iKSys (μJ·s) | 190 [85; 800] | 570 [80; 1000] |
| iKLate dia (%) | 0.205 [0.11; 0.35] | 0.135 [0.09; 0.15] |
| iKEarly dia (%) | 0.805 [0.65; 0.89] | 0.865 [0.85; 0.91] |
Appendix B
Appendix C
| Measurements | iKSys | iKLate dia | iKEarly dia | HR | |
|---|---|---|---|---|---|
| Device Comparison (A) | HF ([95% CI]) | 0.87 [0.77; 0.93] | 0.84 [0.60; 0.93] | 0.96 [0.94; 0.98] | 0.83 [0.77; 0.94] |
| HT ([95% CI]) | 0.90 [0.85; 0.93] | 0.78 [0.54; 0.89] | 0.80 [0.72; 0.86] | 0.81 [0.74; 0.87] | |
| VHD ([95% CI]) | 0.86 [0.79; 0.91] | 0.82 [0.57; 0.91] | 0.89 [0.85; 0.92] | 0.70 [0.60; 0.78] | |
| OTHER ([95% CI]) | 0.89 [0.83; 0.93] | 0.80 [0.52; 0.90] | 0.84 [0.77; 0.89] | 0.97 [0.95; 0.98] | |
| Patient vs. clinician (B) | HF ([95% CI]) | 0.81 [0.65; 0.90] | 0.78 [0.60; 0.89] | 0.80 [0.62; 0.90] | 0.75 [0.55; 0.87] |
| HT ([95% CI]) | 0.86 [0.80; 0.91] | 0.84 [0.77; 0.90] | 0.81 [0.73; 0.88] | 0.83 [0.76; 0.89] | |
| VHD ([95% CI]) | 0.84 [0.78; 0.89] | 0.83 [0.77; 0.88] | 0.81 [0.74; 0.87] | 0.83 [0.76; 0.88] | |
| OTHER ([95% CI]) | 0.83 [0.74; 0.89] | 0.85 [0.78; 0.91] | 0.82 [0.73; 0.89] | 0.80 [0.70; 0.87] |
References
- Maryam Alvandi, D.H.S. Telemedicine and Its Role in Revolutionizing Healthcare Delivery. Am. J. Accountable Care 2017, 5, e1–e5. [Google Scholar]
- Jin, Z.; Chen, Y. Telemedicine in the Cloud Era: Prospects and Challenges. IEEE Pervasive Comput. 2015, 14, 54–61. [Google Scholar] [CrossRef]
- Jamal, N.E.; Abi-Saleh, B.; Isma’eel, H. Advances in Telemedicine for the Management of the Elderly Cardiac Patient. J. Geriatr. Cardiol. 2021, 18, 759–767. [Google Scholar] [CrossRef]
- Salerno, D.M.; Zanetti, J. Seismocardiography for Monitoring Changes in Left Ventricular Function during Ischemia. Chest 1991, 100, 991–993. [Google Scholar] [CrossRef]
- Salerno, D.M.; Zanetti, J. Seismocardiography a New Technique for Recording Cardiac Vibrations Concept Method and Initial Observations. Available online: https://eurekamag.com/research/007/776/007776006.php (accessed on 21 November 2020).
- Ramos-Castro, J.; Moreno, J.; Miranda-Vidal, H.; García-González, M.A.; Fernández-Chimeno, M.; Rodas, G.; Capdevila, L. Heart Rate Variability Analysis Using a Seismocardiogram Signal. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; Volume 2012, pp. 5642–5645. [Google Scholar] [CrossRef]
- Sieciński, S.; Kostka, P. Heart Beat Detection from Smartphone SCG Signals: Comparison with Previous Study on HR Estimation. In Proceedings of the Innovations in Biomedical Engineering, Katowice, Poland, 18–20 October 2018; Tkacz, E., Gzik, M., Paszenda, Z., Piętka, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 123–130. [Google Scholar]
- Elgendi, M.; Wu, W.; Guan, C.; Menon, C. Revolutionizing Smartphone Gyrocardiography for Heart Rate Monitoring: Overcoming Clinical Validation Hurdles. Front. Cardiovasc. Med. 2023, 10, 1237043. [Google Scholar] [CrossRef]
- Wu, W.; Elgendi, M.; Fletcher, R.R.; Bomberg, H.; Eichenberger, U.; Guan, C.; Menon, C. Detection of Heart Rate Using Smartphone Gyroscope Data: A Scoping Review. Front. Cardiovasc. Med. 2023, 10, 1329290. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jung, Y.H.; Jeung, K.W.; Lee, D.H.; Lee, B.K.; Jang, G.Y.; Oh, T.I.; Mamadjonov, N.; Heo, T. Discrimination between the Presence and Absence of Spontaneous Circulation Using Smartphone Seismocardiography: A Preliminary Investigation. Resuscitation 2021, 166, 66–73. [Google Scholar] [CrossRef]
- Mehrang, S.; Jafari Tadi, M.; Kaisti, M.; Lahdenoja, O.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. Machine Learning Based Classification of Myocardial Infarction Conditions Using Smartphone-Derived Seismo- and Gyrocardiography. In Proceedings of the 2018 Computing in Cardiology Conference (CinC), Maastricht, The Netherlands, 23–26 September 2018; Volume 45, pp. 1–4. [Google Scholar]
- Jaakkola, J.; Jaakkola, S.; Lahdenoja, O.; Hurnanen, T.; Koivisto, T.; Pänkäälä, M.; Knuutila, T.; Kiviniemi, T.O.; Vasankari, T.; Airaksinen, K.E.J. Mobile Phone Detection of Atrial Fibrillation with Mechanocardiography. Circulation 2018, 137, 1524–1527. [Google Scholar] [CrossRef]
- Mehrang, S.; Lahdenoja, O.; Kaisti, M.; Tadi, M.J.; Hurnanen, T.; Airola, A.; Knuutila, T.; Jaakkola, J.; Jaakkola, S.; Vasankari, T.; et al. Classification of Atrial Fibrillation and Acute Decompensated Heart Failure Using Smartphone Mechanocardiography: A Multilabel Learning Approach. IEEE Sens. J. 2020, 20, 7957–7968. [Google Scholar] [CrossRef]
- Albrecht, U.-V.; Lawin, D.; Kuhn, S.; Kulau, U. Seismocardiography with Smartphones: No Leap from Bench to Bedside (Yet). Stud. Health Technol. Inf. 2022, 295, 271–275. [Google Scholar] [CrossRef]
- Mehrang, S.; Tadi, M.J.; Hurnanen, T.; Knuutila, T.; Lahdenoja, O.; Jaakkola, J.; Jaakkola, S.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; et al. Reliability of Self-Applied Smartphone Mechanocardiography for Atrial Fibrillation Detection. IEEE Access 2019, 7, 146801–146812. [Google Scholar] [CrossRef]
- Hossein, A.; Rabineau, J.; Gorlier, D.; Pinki, F.; van de Borne, P.; Nonclercq, A.; Migeotte, P.-F. Effects of Acquisition Device, Sampling Rate, and Record Length on Kinocardiography during Position-Induced Haemodynamic Changes. BioMed. Eng. OnLine 2021, 20, 3. [Google Scholar] [CrossRef]
- Jafari Tadi, M.; Lehtonen, E.; Saraste, A.; Tuominen, J.; Koskinen, J.; Teräs, M.; Airaksinen, J.; Pänkäälä, M.; Koivisto, T. Gyrocardiography: A New Non-Invasive Monitoring Method for the Assessment of Cardiac Mechanics and the Estimation of Hemodynamic Variables. Sci. Rep. 2017, 7, 6823. [Google Scholar] [CrossRef]
- Sieciński, S.; Kostka, P.S.; Tkacz, E.J. Gyrocardiography: A Review of the Definition, History, Waveform Description, and Applications. Sensors 2020, 20, 6675. [Google Scholar] [CrossRef]
- Castiglioni, P.; Meriggi, P.; Rizzo, F.; Vaini, E.; Faini, A.; Parati, G.; Merati, G.; Di Rienzo, M. Cardiac Sounds from a Wearable Device for Sternal Seismocardiography. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 4283–4286. [Google Scholar]
- Yeh, C.-C.M.; Zhu, Y.; Ulanova, L.; Begum, N.; Ding, Y.; Dau, H.A.; Silva, D.F.; Mueen, A.; Keogh, E. Matrix Profile I: All Pairs Similarity Joins for Time Series: A Unifying View That Includes Motifs, Discords and Shapelets. In Proceedings of the 2016 IEEE 16th International Conference on Data Mining (ICDM), Barcelona, Spain, 12–15 December 2016; IEEE: Barcelona, Spain, 2016; pp. 1317–1322. [Google Scholar]
- Law, S.M. STUMPY: A Powerful and Scalable Python Library for Time Series Data Mining. J. Open Source Softw. 2019, 4, 1504. [Google Scholar] [CrossRef]
- De Keyzer, E.; Hossein, A.; Rabineau, J.; Morissens, M.; Almorad, A.; van de Borne, P. Non-Invasive Cardiac Kinetic Energy Distribution: A New Marker of Heart Failure with Impaired Ejection Fraction (KINO-HF). Front. Cardiovasc. Med. 2023, 10, 1096859. [Google Scholar] [CrossRef]
- Herkert, C.; Migeotte, P.-F.; Hossein, A.; Spee, R.F.; Kemps, H.M.C. The Kinocardiograph for Assessment of Changes in Haemodynamic Load in Patients with Chronic Heart Failure with Reduced Ejection Fraction. ESC Heart Fail. 2021, 8, 4925–4932. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman Analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- McGraw, K.O.; Wong, S.P. Forming Inferences about Some Intraclass Correlation Coefficients. Psychol. Methods 1996, 1, 30–46. [Google Scholar] [CrossRef]
- Carlsson, M.; Heiberg, E.; Toger, J.; Arheden, H. Quantification of Left and Right Ventricular Kinetic Energy Using Four-Dimensional Intracardiac Magnetic Resonance Imaging Flow Measurements. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H893–H900. [Google Scholar] [CrossRef]
- Garg, P.; Crandon, S.; Swoboda, P.P.; Fent, G.J.; Foley, J.R.J.; Chew, P.G.; Brown, L.A.E.; Vijayan, S.; Hassell, M.E.C.J.; Nijveldt, R.; et al. Left Ventricular Blood Flow Kinetic Energy after Myocardial Infarction—Insights from 4D Flow Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2018, 20, 61. [Google Scholar] [CrossRef]
- Eriksson, J.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.-J. Four-Dimensional Blood Flow-Specific Markers of LV Dysfunction in Dilated Cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 417–424. [Google Scholar] [CrossRef]
- Hossein, A.; Mirica, D.C.; Rabineau, J.; Rio, J.I.D.; Morra, S.; Gorlier, D.; Nonclercq, A.; van de Borne, P.; Migeotte, P.-F. Accurate Detection of Dobutamine-Induced Haemodynamic Changes by Kino-Cardiography: A Randomised Double-Blind Placebo-Controlled Validation Study. Sci. Rep. 2019, 9, 10479. [Google Scholar] [CrossRef]
- Morra, S.; Hossein, A.; Rabineau, J.; Gorlier, D.; Racape, J.; Migeotte, P.-F.; Borne, P. Assessment of Left Ventricular Twist by 3D Ballistocardiography and Seismocardiography Compared with 2D STI Echocardiography in a Context of Enhanced Inotropism in Healthy Subjects. Sci. Rep. 2021, 11, 683. [Google Scholar] [CrossRef]
- Subasi, A.; Kontio, E.; Jafaritadi, M. Chapter 7—Deep Learning Approaches for the Cardiovascular Disease Diagnosis Using Smartphone. In 5G IoT and Edge Computing for Smart Healthcare; Bhoi, A.K., de Albuquerque, V.H.C., Sur, S.N., Barsocchi, P., Eds.; Intelligent Data-Centric Systems; Academic Press: Cambridge, MA, USA, 2022; pp. 163–193. ISBN 978-0-323-90548-0. [Google Scholar]
- Hossein, A.; Rabineau, J.; Gorlier, D.; Del Rio, J.I.J.; van de Borne, P.; Migeotte, P.-F.; Nonclercq, A. Kinocardiography Derived from Ballistocardiography and Seismocardiography Shows High Repeatability in Healthy Subjects. Sensors 2021, 21, 815. [Google Scholar] [CrossRef]
- Profant, J.; Dimsdale, J.E. Race and Diurnal Blood Pressure Patterns. Hypertension 1999, 33, 1099–1104. [Google Scholar] [CrossRef]
- Fagard, R.H. Dipping Pattern of Nocturnal Blood Pressure in Patients with Hypertension. Expert Rev. Cardiovasc. Ther. 2009, 7, 599–605. [Google Scholar] [CrossRef]
- Herkert, C.; De Lathauwer, I.; van Leunen, M.; Spee, R.F.; Balali, P.; Migeotte, P.-F.; Hossein, A.; Lu, Y.; Kemps, H.M.C. The Kinocardiograph for Assessment of Fluid Status in Patients with Acute Decompensated Heart Failure. ESC Heart Fail. 2023, 10, 3446–3453. [Google Scholar] [CrossRef]
- Rabineau, J.; Nonclercq, A.; Leiner, T.; van de Borne, P.; Migeotte, P.-F.; Haut, B. Closed-Loop Multiscale Computational Model of Human Blood Circulation. Applications to Ballistocardiography. Front. Physiol. 2021, 12, 734311. [Google Scholar] [CrossRef]
- Centracchio, J.; Parlato, S.; Esposito, D.; Bifulco, P.; Andreozzi, E. ECG-Free Heartbeat Detection in Seismocardiography Signals via Template Matching. Sensors 2023, 23, 4684. [Google Scholar] [CrossRef]
- Parlato, S.; Centracchio, J.; Esposito, D.; Bifulco, P.; Andreozzi, E. Heartbeat Detection in Gyrocardiography Signals without Concurrent ECG Tracings. Sensors 2023, 23, 6200. [Google Scholar] [CrossRef]
- Skoric, J.; D’Mello, Y.; Aboulezz, E.; Hakim, S.; Clairmonte, N.; Lortie, M.A.; Plant, D.V. Respiratory Modulation of Sternal Motion in the Context of Seismocardiography. IEEE Sens. J. 2022, 22, 13055–13066. [Google Scholar] [CrossRef]
- Balali, P.; Rabineau, J.; Hossein, A.; Tordeur, C.; Debeir, O.; van de Borne, P. Investigating Cardiorespiratory Interaction Using Ballistocardiography and Seismocardiography—A Narrative Review. Sensors 2022, 22, 9565. [Google Scholar] [CrossRef]
- Taebi, A.; Solar, B.E.; Bomar, A.J.; Sandler, R.H.; Mansy, H.A. Recent Advances in Seismocardiography. Vibration 2019, 2, 64–86. [Google Scholar] [CrossRef]
- Ashouri, H.; Hersek, S.; Inan, O.T. Universal Pre-Ejection Period Estimation Using Seismocardiography: Quantifying the Effects of Sensor Placement and Regression Algorithms. IEEE Sens. J. 2018, 18, 1665–1674. [Google Scholar] [CrossRef]

| Measurements | iKSys (nJ·s) | iKLate dia (nJ·s) | iKEarly dia (nJ·s) | HR (bpm) |
|---|---|---|---|---|
| Kinocardiograph (A) | 18.6 [5.9; 55.7] | 7.4 [2.6; 26.9] | 10.1 [3.1; 32.2] | 64.7 [51.3; 85.0] |
| Reference Smartphone (A) | 21.6 [7.9; 70.0] | 10.0 [2.9; 39.4] | 10.0 [3.8; 27.7] | 65.6 [51.5; 82.9] |
| Patient’s Smartphone by HCP (B) | 11.2 [0.6; 70.7] | 4.3 [0.2; 27.4] | 6.8 [0.2; 29.7] | 66.1 [54.2; 84.0] |
| Patient’s Smartphone by Patient (B) | 13.5 [0.6; 59.9] | 4.9 [0.2; 30.5] | 6.2 [0.2; 31.3] | 65.9 [54.7; 83.4] |
| Features | iKSys | iKLate dia | iKEarly dia | HR | |
|---|---|---|---|---|---|
| Device Comparison (A) | BA Bias ([95% CI]) (nJ·s) | 0.01 [−0.05; 0.015] | −0.08 [−0.13; 0.01] | −0.01 [−0.04; 0.013] | 0.03 [−0.04; 0.09] |
| BA LoA [Lower; Upper] (nJ·s) | [−22; 22] | [−12; 11] | [−11; 11] | [−10; 11] | |
| ([95% CI]) | 0.86 [0.81; 0.90] | 0.77 [0.61; 0.85] | 0.88 [0.84; 0.91] | 0.77 [0.7; 0.83] | |
| Patient vs. clinician (B) | BA Bias ([95% CI]) (nJ·s) | −2 [−2.50; −0.50] | −3 [−4.20; −2.10] | −0.01 [−0.03; 0.011] | 0.03 [−0.03; 0.10] |
| BA LoA [Lower; Upper] (nJ·s) | [−25; 20] | [−12; 7] | [−11; 10] | [−13; 13] | |
| ([95% CI]) | 0.85 [0.80; 0.89] | 0.83 [0.77; 0.87] | 0.83 [0.77; 0.87] | 0.83 [0.77; 0.88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossein, A.; Abdessater, E.; Balali, P.; Cosneau, E.; Gorlier, D.; Rabineau, J.; Almorad, A.; Faoro, V.; van de Borne, P. Smartphone-Derived Seismocardiography: Robust Approach for Accurate Cardiac Energy Assessment in Patients with Various Cardiovascular Conditions. Sensors 2024, 24, 2139. https://doi.org/10.3390/s24072139
Hossein A, Abdessater E, Balali P, Cosneau E, Gorlier D, Rabineau J, Almorad A, Faoro V, van de Borne P. Smartphone-Derived Seismocardiography: Robust Approach for Accurate Cardiac Energy Assessment in Patients with Various Cardiovascular Conditions. Sensors. 2024; 24(7):2139. https://doi.org/10.3390/s24072139
Chicago/Turabian StyleHossein, Amin, Elza Abdessater, Paniz Balali, Elliot Cosneau, Damien Gorlier, Jérémy Rabineau, Alexandre Almorad, Vitalie Faoro, and Philippe van de Borne. 2024. "Smartphone-Derived Seismocardiography: Robust Approach for Accurate Cardiac Energy Assessment in Patients with Various Cardiovascular Conditions" Sensors 24, no. 7: 2139. https://doi.org/10.3390/s24072139









