Recent Advances and Future Perspectives in the E-Nose Technologies Addressed to the Wine Industry
Abstract
1. Introduction
2. Review Methodology
3. Olfactory Evaluation of Wine: Wet Chemistry and Sensory Analysis
- Volatile Compound Selectivity: GC is highly effective in analyzing volatile compounds, but it may not capture all non-volatile or semi-volatile components in wine. This limitation can lead to an incomplete representation of the wine’s chemical composition [52].
- Matrix Effects: The complex matrix of wine, including various organic and inorganic components, can influence the separation and detection of compounds in GC [53]. Co-elution of compounds and interference from matrix components may occur, affecting the accuracy and specificity of the results.
- Quantification Challenges: While GC provides excellent qualitative information, quantifying compounds can be a challenge without the use of appropriate internal or external standards. Variability in detector response can also offer quantification difficulties [54].
- Need for Complementary Techniques: To achieve a comprehensive understanding of wine composition, GC is often coupled with other analytical methods such as Mass Spectrometry (GC-MS) [55] or Flame Ionization Detection (GC-FID) [56]. This integration adds complexity and may increase the cost of the analysis.
- Time-Consuming Sample Preparation: The preparation of wine samples for GC analysis involves extraction and concentration steps, which can be time consuming. Delicate handling of samples is essential to prevent changes in composition during preparation [57].
4. Application of Electronic Noses in Oenology: Principles, Use, and Main Issues
5. Case Study: E-Nose in Application to Detect Smoke Taint in Wines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGann, J.P. Poor human olfaction is a 19th-century myth. Science 2017, 356, eaam7263. [Google Scholar] [CrossRef] [PubMed]
- Omatu, S.; Yano, M.; Ikeda, Y. Smell classification of wines by the learning vector quantization method. In Proceedings of the 30th Annual ACM Symposium on Applied Computing (SAC ‘15), Salamanca Spain, 13–17 April 2015; Association for Computing Machinery: New York, NY, USA, 2015; pp. 195–200. [Google Scholar]
- Han, J.-K.; Kang, M.; Jeong, J.; Cho, I.; Yu, J.-M.; Yoon, K.-J.; Park, I.; Choi, Y.-K. Artificial Olfactory Neuron for an In-Sensor Neuromorphic Nose. Adv. Sci. 2022, 9, 2106017. [Google Scholar] [CrossRef]
- Zhou, G.; Lane, G.; Cooper, S.L.; Kahnt, T.; Zelano, C. Characterizing functional pathways of the human olfactory system. eLife 2019, 8, e47177. [Google Scholar] [CrossRef] [PubMed]
- Devanand, D.P.; Lee, S.; Manly, J.; Andrews, H.; Schupf, N.; Masurkar, A.; Stern, Y.; Mayeux, R.; Doty, R.L. Olfactory identification deficits and increased mortality in the community. Ann. Neurol. 2015, 78, 401–411. [Google Scholar] [CrossRef]
- Borowik, P.; Adamowicz, L.; Tarakowski, R.; Siwek, K.; Grzywacz, T. Odor Detection Using an E-Nose with a Reduced Sensor Array. Sensors 2020, 20, 3542. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, D.; Yan, K. Breath Analysis for Medical Applications; Springer: Singapore, 2017. [Google Scholar]
- Dragonieri, S.; Pennazza, G.; Carratu, P.; Resta, O. Electronic Nose Technology in Respiratory Diseases. Lung 2017, 195, 157–165. [Google Scholar] [CrossRef]
- Wilson, A.D. Application of Electronic-Nose Technologies and VOC-Biomarkers for the Noninvasive Early Diagnosis of Gastrointestinal Diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef]
- Wilson, A.D. Developments of Recent Applications for Early Diagnosis of Diseases Using Electronic-Nose and Other VOC-Detection Devices. Sensors 2023, 23, 7885. [Google Scholar] [CrossRef] [PubMed]
- Zarra, T.; Cimatoribus, C.; Naddeo, V.; Reiser, M.; Belgiorno, V.; Kranert, M. Environmental odour monitoring by electronic nose. Glob. NEST J. 2018, 20, 664–668. [Google Scholar]
- Prasad, P.; Raut, P.; Goel, S.; Barnwal, R.P.; Bodhe, G.L. Electronic nose and wireless sensor network for environmental monitoring application in pulp and paper industry: A review. Environ. Monit. Assess. 2022, 194, 855. [Google Scholar] [CrossRef]
- Sayago, I.; Aleixandre, M.; Santos, J.P. Development of Tin Oxide-Based Nanosensors for Electronic Nose Environmental Applications. Biosensors 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Oleneva, E.; Kuchmenko, T.; Drozdova, E.; Legin, A.; Kirsanov, D. Identification of plastic toys contaminated with volatile organic compounds using QCM gas sensor array. Talanta 2020, 211, 120701. [Google Scholar] [CrossRef] [PubMed]
- Zaytsev, V.; Fedorov, F.S.; Goikhman, B.; Maslennikov, A.; Mashukov, V.; Simonenko, N.P.; Simonenko, T.L.; Gabdullina, D.; Kovalenko, O.; Simonenko, E.P.; et al. Rapid and accurate quality assessment method of recycled food plastics VOCs by electronic nose based on Al-doped zinc oxide. J. Clean. Prod. 2023, 418, 138042. [Google Scholar] [CrossRef]
- Voss, H.G.J.; Ayub, R.A.; Stevan, S.L. E-nose Prototype to Monitoring the Growth and Maturation of Peaches in the Orchard. IEEE Sens. J. 2020, 20, 11741–11750. [Google Scholar] [CrossRef]
- Yavuzer, E. Determination of fish quality parameters with low cost electronic nose. Food Biosci. 2021, 41, 100948. [Google Scholar] [CrossRef]
- Andre, R.S.; Facure, M.H.; Mercante, L.A.; Correa, D.S. Electronic nose based on hybrid free-standing nanofibrous mats for meat spoilage monitoring. Sens. Actuators B Chem. 2022, 353, 131114. [Google Scholar] [CrossRef]
- Cascos, G.; Barea-Ramos, J.D.; Montero-Fernández, I.; Ruiz-Canales, A.; Lozano, J.; Martín-Vertedor, D. Burn Defect and Phenol Prediction for Flavoured Californian-Style Black Olives Using Digital Sensors. Foods 2023, 12, 1377. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Boselli, E.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. E-Nose Discrimination of Abnormal Fermentations in Spanish-Style Green Olives. Molecules 2021, 26, 5353. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K. Comparison of Cheese Aroma Intensity Measured Using an Electronic Nose (E-Nose) Non-Destructively with the Aroma Intensity Scores of a Sensory Evaluation: A Pilot Study. Sensors 2021, 21, 8368. [Google Scholar] [CrossRef]
- Vilela, A.; Bacelar, E.; Pinto, T.; Anjos, R.; Correia, E.; Gonçalves, B.; Cosme, F. Beverage and Food Fragrance Biotechnology, Novel Applications, Sensory and Sensor Techniques: An Overview. Foods 2019, 8, 643. [Google Scholar] [CrossRef]
- Balda, P.; de Toda, F.M. Variedades Minoritarias de vid en La Rioja, 1st ed.; Consejería de Agricultura; Ganadería y Medio Ambiente: Logroño, Spain, 2017. [Google Scholar]
- Naranjo, A.; Martínez-Lapuente, L.; Ayestarán, B.; Guadalupe, Z.; Pérez, I.; Canals, C.; Adell, E. Aromatic and Sensory Characterization of Maturana Blanca Wines Made with Different Technologies. Beverages 2021, 7, 10. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments, 3rd ed.; John Wiley & Sons: Chinchester, UK, 2021; p. 560. [Google Scholar]
- Petretto, G.L.; Mercenaro, L.; Urgeghe, P.P.; Fadda, C.; Valentoni, A.; Del Caro, A. Grape and Wine Composition in Vitis vinifera L. cv. Cannonau Explored by GC-MS and Sensory Analysis. Foods 2021, 10, 101. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. 7—Sensory analysis of wine. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Managing Wine Quality, 2nd ed.; Andrew, G., Reynolds, A.G., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 243–277. ISBN 9780081020678. [Google Scholar]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube -titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Wang, H.; Feng, X.; Suo, H.; Yuan, X.; Zhou, S.; Ren, H.; Jiang, Y.; Kan, J. Comparison of the performance of the same panel with different training levels: Flash profile versus descriptive analysis. Food Qual. Prefer. 2022, 99, 104582. [Google Scholar] [CrossRef]
- Njoman, M.F.; Nugroho, G.; Chandra, S.D.P.; Permana, Y.; Suhadi, S.; Mujiono, M.; Hermawan, A.D.; Sugiono, S. The vulnerability of human sensory evaluation and the promising senses instrumentation. Br. Food J. 2017, 119, 2145–2160. [Google Scholar] [CrossRef]
- Sipos, L.; Nyitrai, Á.; Hitka, G.; Friedrich, L.F.; Kókai, Z. Sensory Panel Performance Evaluation—Comprehensive Review of Practical Approaches. Appl. Sci. 2021, 11, 11977. [Google Scholar] [CrossRef]
- Fiszman, S.; Salgado, N.; Orrego, C.E.; Ares, G. Comparison of methods for generating sensory vocabulary with consumers: A case study with two types of satiating foods. Food Qual. Prefer. 2015, 44, 111–118. [Google Scholar] [CrossRef]
- Coulon-Leroy, C.; Symoneaux, R.; Lawrence, G.; Mehinagic, E.; Maitre, I. Mixed Profiling: A new tool of sensory analysis in a professional context. Application to wines. Food Qual. Prefer. 2017, 57, 8–16. [Google Scholar] [CrossRef]
- Larssen, W.E.; Monteleone, E.; Hersleth, M. Sensory description of marine oils through development of a sensory wheel and vocabulary. Food Res. Int. 2018, 106, 45–53. [Google Scholar] [CrossRef]
- Sharif, M.K.; Butt, M.S.; Sharif, H.R.; Nasir, M. Sensory Evaluation and Consumer Acceptability. In Handbook of Food Science and Technology; 2017, Volume 10, pp. 362–386. Cleveland: CRC Press.
- Robinson, J.; Harris, I.; King, N. Understanding Wines: Explaining Style and Quality; Wine & Spirit Education Trust: London, UK, 2016. [Google Scholar]
- Zraly, K. Windows on the World Complete Wine Course; Sterling Publishing Company: New York, NY, USA, 2016. [Google Scholar]
- MacNeil, K. The Wine Bible; Workman Publishing: New York, NY, USA, 2015. [Google Scholar]
- Nickles, J. Certified Wine Educator Manual for Candidates; Create Space Independent Publishing Platform: Scotts Valley, CA, USA, 2017. [Google Scholar]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- de-la-Fuente-Blanco, A.; Ferreira, V. Gas Chromatography Olfactometry (GC-O) for the (Semi)Quantitative Screening of Wine Aroma. Foods 2020, 9, 1892. [Google Scholar] [CrossRef]
- Hage, D.S. 1—Chromatography. In Principles and Applications of Clinical Mass Spectrometry; Rifai, N., Horvath, A.R., Wittwer, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–32. ISBN 9780128160633. [Google Scholar]
- Nolvachai, Y.; Kulsing, C.; Marriott, P.J. Multidimensional gas chromatography in food analysis. TrAC Trends Anal. Chem. 2017, 96, 124–137. [Google Scholar] [CrossRef]
- Ferreira, V.; Herrero, P.; Zapata, J.; Escudero, A. Coping with matrix effects in headspace solid phase microextraction gas chromatography using multivariate calibration strategies. J. Chromatogr. A 2015, 1407, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Tufariello, M.; Crupi, P.; Coletta, A.; Grieco, F.; Losito, I. Quantification of Volatile Compounds in Wines by HS-SPME-GC/MS: Critical Issues and Use of Multivariate Statistics in Method Optimization. Processes 2021, 9, 662. [Google Scholar] [CrossRef]
- Zhang, P.; Piergiovanni, M.; Franceschi, P.; Mattivi, F.; Vrhovsek, U.; Carlin, S. Application of Comprehensive 2D Gas Chromatography Coupled with Mass Spectrometry in Beer and Wine VOC Analysis. Analytica 2023, 4, 347–373. [Google Scholar] [CrossRef]
- Nicolli, K.P.; Biasoto, A.C.; Souza-Silva, É.A.; Guerra, C.C.; Dos Santos, H.P.; Welke, J.E.; Zini, C.A. Sensory, olfactometry and comprehensive two-dimensional gas chromatography analyses as appropriate tools to characterize the effects of vine management on wine aroma. Food Chem. 2018, 243, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Marín-San Román, S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Advancement in analytical techniques for the extraction of grape and wine volatile compounds. Food Res. Int. 2020, 137, 109712. [Google Scholar] [CrossRef]
- Parrilla Vázquez, P.; Hakme, E.; Uclés, S.; Cutillas, V.; Martínez Galera, M.; Mughari, A.R.; Fernández-Alba, A.R. Large multiresidue analysis of pesticides in edible vegetable oils by using efficient solid-phase extraction sorbents based on quick, easy, cheap, effective, rugged and safe methodology followed by gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2016, 1463, 20–31. [Google Scholar] [CrossRef]
- Chu, H.; Jo, J.; Son, Y.; Lee, J.Y.; Ahn, Y.G. Developing an Improved Strategy for the Analysis of Polychlorinated Dibenzo-p-Dioxins/Furans and Dioxin-like Polychlorinated Biphenyls in Contaminated Soils Using a Combination of a One-Step Cleanup Method and Gas Chromatography with Triple Quadrupole Mass Spectrometry. Toxics 2023, 11, 738. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, G.-J.; Wang, X.-J.; Kong, C.-L.; Liu, J.; Tao, Y.-S. Chemical profiles and aroma contribution of terpene compounds in Meili (Vitis vinifera L.) grape and wine. Food Chem. 2019, 284, 155–161. [Google Scholar] [CrossRef]
- Ling, M.; Zhou, Y.; Lan, Y.; Cheng, C.; Wu, G.; Duan, C.; Shi, Y. Modification of Sensory Expression of 3-Isobutyl-2-methoxypyrazine in Wines through Blending Technique. Molecules 2021, 26, 3172. [Google Scholar] [CrossRef] [PubMed]
- Timmins, J.J.B.; Kroukamp, H.; Paulsen, I.T.; Pretorius, I.S. The Sensory Significance of Apocarotenoids in Wine: Importance of Carotenoid Cleavage Dioxygenase 1 (CCD1) in the Production of β-Ionone. Molecules 2020, 25, 2779. [Google Scholar] [CrossRef] [PubMed]
- Capone, D.L.; Barker, A.; Williamson, P.O.; Francis, I.L. The role of potent thiols in Chardonnay wine aroma. Aust. J. Grape Wine Res. 2018, 24, 38–50. [Google Scholar] [CrossRef]
- Wang, J.; Abbey, T.; Kozak, B.; Madilao, L.L.; Tindjau, R.; Del Nin, J.; Castellarin, S.D. Evolution over the growing season of volatile organic compounds in Viognier (Vitis vinifera L.) grapes under three irrigation regimes. Food Res. Int. 2019, 125, 108512. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, M.; Pérez-Coello, M.S.; Díaz-Maroto, M.C.; Alañón, M.E.; Soriano, A. Effect of winery by-product extracts on oxidative stability, volatile organic compounds and aroma profile of cooked pork model systems during chilled storage. LWT 2021, 152, 112260. [Google Scholar] [CrossRef]
- Herderich, M.; Barter, S.; Black, C.A.; Bramley, R.; Capone, D.; Dry, P.; Siebert, T.; Zhang, P. Terroir Effects on Grape and Wine Aroma Compounds. In Advances in Wine Research; American Chemical Society: Washington, DC, USA, 2015; Volume 1203, pp. 131–146. [Google Scholar]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Agric. Food Chem. 2019, 99, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Van Leewen, C.; Roby, J.; de Resseguier, L. Soil-Related terroir factors: A review. OENO One 2018, 52, 173–188. [Google Scholar] [CrossRef]
- Chambers IV, E.; Koppel, K. Association of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef] [PubMed]
- Swain, K.K.; Mishra, P.M.; Behera, B.K. Semiconductor Metal Oxide Thin Films. In Metal Oxide Nanocomposite Thin Films for Optoelectronic Device Applications; Zargar, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar]
- Tian, X.; Hu, Z.; Jia, C.; Wang, H.; Wei, X. A review of advanced gas sensor based on sputtering SnO2 thin film—Challenges and opportunities. J. Environ. Chem. Eng. 2023, 11, 111516. [Google Scholar] [CrossRef]
- Boomashri, M.; Perumal, P.; Khan, A.; El-Toni, A.M.; Ansari, A.A.; Gupta, R.K.; Murahari, P.; Kumar, K.D.A. Zinc influence on nanostructured tin oxide (SnO2) films as ammonia sensor at room temperature. Surf. Interfaces 2021, 25, 101195. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Cheli, F.; Chiofalo, V. Fusion of electronic nose, electronic tongue and computer vision for animal source food authentication and quality assessment—A review. J. Food Eng. 2017, 210, 62–75. [Google Scholar] [CrossRef]
- Robbiani, S.; Lotesoriere, B.J.; Dellacà, R.L.; Capelli, L. Physical Confounding Factors Affecting Gas Sensors Response: A Review on Effects and Compensation Strategies for Electronic Nose Applications. Chemosensors 2023, 11, 514. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Miramirkhani, F.; Navarchian, A.H. Morphology, Structure, and Gas Sensing Performance of Conductive Polymers and Polymer/Carbon Black Composites Used for Volatile Compounds Detection. IEEE Sens. J. 2017, 17, 2992–3000. [Google Scholar] [CrossRef]
- Sorvin, M.; Belyakova, S.; Stoikov, I.; Shamagsumova, R.; Evtugyn, G. Solid-Contact Potentiometric Sensors and Multisensors Based on Polyaniline and Thiacalixarene Receptors for the Analysis of Some Beverages and Alcoholic Drinks. Front. Chem. 2018, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Graboski, A.M.; Galvagni, E.; Manzoli, A.; Shimizu, F.M.; Zakrzevski, C.A.; Weschenfelder, T.A.; Steffens, J.; Steffens, C. Lab-made electronic-nose with polyaniline sensor array used in classification of different aromas in gummy candies. Food Res. Int. 2018, 113, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Henrique, C.; Esteves, A.; Iglesias, B.A.; Ogawa, T.; Araki, K.; Hoehne, L.; Gruber, J. Identification of tobacco types and cigarette brands using an electronic nose based on conductive polymer/porphyrin composite sensors. ACS Omega 2018, 3, 6476–6482. [Google Scholar]
- Wei, Z.; Zhou, Q.; Lu, Z.; Xu, L.; Gui, Y.; Tang, C. Morphology controllable synthesis of hierarchical WO3 nanostructures and C2H2 sensing properties. Phys. E Low-Dimens. Syst. Nanostructures 2019, 109, 253–260. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Chiofalo, V. 7—Electronic noses and tongues. In Chemical Analysis of Food, 2nd ed.; Yolanda Pico, Y., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 353–389. ISBN 9780128132661. [Google Scholar]
- Sierra-Padilla, A.; García-Guzmán, J.J.; López-Iglesias, D.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. E-Tongues/Noses Based on Conducting Polymers and Composite Materials: Expanding the Possibilities in Complex Analytical Sensing. Sensors 2021, 21, 4976. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.; Chang, Y.; Zhang, R.; Tao, J.; Qu, H.; Duan, X. Detection of volatile organic compunds by high-Q piezotransduced single-crystal silicon bulk acoustic resonator arrays. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Mujahid, A.; Dickert, F.L. Surface Acoustic Wave (SAW) for Chemical Sensing Applications of Recognition Layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.; Chang, Y.; Qu, H.; Pang, W.; Zhang, H.; Duan, X. Detection and discrimination of volatile organic compounds using a single multi-resonance mode piezotransduced silicon bulk acoustic wave resonator (PSBAR) as virtual sensor array. Sens. Actuators B Chem. 2018, 254, 1191–1199. [Google Scholar] [CrossRef]
- Gao, J.; Liu, G.; Li, J.; Li, G. Recent developments of film bulk acoustic resonators. Funct. Mater. Lett. 2016, 9, 1630002. [Google Scholar] [CrossRef]
- Okur, S.; Sarheed, M.; Huber, R.; Zhang, Z.; Heinke, L.; Kanbar, A.; Wöll, C.; Nick, P.; Lemmer, U. Identification of Mint Scents Using a QCM Based E-Nose. Chemosensors 2021, 9, 31. [Google Scholar] [CrossRef]
- Okur, S.; Zhang, Z.; Sarheed, M.; Nick, P.; Lemmer, U.; Heinke, L. Towards a MOF e-Nose: A SURMOF sensor array for detection and discrimination of plant oil scents and their mixtures. Sens. Actuators B Chem. 2020, 306, 127502. [Google Scholar] [CrossRef]
- Li, Q.; Gu, Y.; Wang, N.-F. Application of Random Forest Classifier by Means of a QCM-Based E-Nose in the Identification of Chinese Liquor Flavors. IEEE Sens. J. 2017, 17, 1788–1794. [Google Scholar] [CrossRef]
- Shuba, A.; Kuchmenko, T.; Umarkhanov, R. Piezoelectric Gas Sensors with Polycomposite Coatings in Biomedical Application. Sensors 2022, 22, 8529. [Google Scholar] [CrossRef] [PubMed]
- Ruangchaiwat, A.; Rannurags, N.; Phanichphant, S.; Liawruangrath, B.; Liawruangrath, S. Simple laboratory-made piezoelectric sensors for detection of selected gaseous and/or vapor sample. High Temp. 2016, 6, 7. [Google Scholar]
- Zhang, K.; Hu, R.; Fan, G.; Li, G. Graphene oxide/chitosan nanocomposite coated quartz crystal microbalance sensor for detection of amine vapors. Sens. Actuators B Chem. 2017, 243, 721–730. [Google Scholar] [CrossRef]
- Na Songkhla, S.; Nakamoto, T. Overview of Quartz Crystal Microbalance Behavior Analysis and Measurement. Chemosensors 2021, 9, 350. [Google Scholar] [CrossRef]
- Satapathy, A.; Mahimkar, K.; Mondal, S.; Pathaare, Y.; Kandasubramanian, B. Attenuation of electromagnetic waves in polymeric terahertz imbibers: Review. J. Mater. Sci. Mater. Electron. 2023, 34, 516. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Santos, J.P.; Suárez, J.I.; Cabellos, M.; Arroyo, T.; Horrillo, C. Restricted access Automatic Sensor System for the Continuous Analysis of the Evolution of Wine. Am. J. Enol. Vitic. 2015, 66, 148–155. [Google Scholar] [CrossRef]
- Martínez-García, R.; Moreno, J.; Bellincontro, A.; Centioni, L.; Puig-Pujol, A.; Peinado, R.A.; Mauricio, J.C.; García-Martínez, T. Using an electronic nose and volatilome analysis to differentiate sparkling wines obtained under different conditions of temperature, ageing time and yeast formats. Food Chem. 2021, 334, 127574. [Google Scholar] [CrossRef]
- Petrozziello, M.; Rosso, L.; Portesi, C.; Asproudi, A.; Bonello, F.; Nardi, T.; Rossi, A.M.; Schiavone, C.; Scuppa, S.; Cantamessa, S.; et al. Characterisation of Refined Marc Distillates with Alternative Oak Products Using Different Analytical Approaches. Appl. Sci. 2022, 12, 8444. [Google Scholar] [CrossRef]
- Gamboa, J.C.R.; Albarracin, E.S.A.; da Silva, A.; Ferreira, T.A.E. Electronic nose dataset for detection of wine spoilage thresholds. Data Brief 2019, 25, 104202. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.C.R.; da Silva, A.J.; Araujo, I.C.; Albarracin E., E.S.; Duran A., C.M. Validation of the rapid detection approach for enhancing the electronic nose systems performance, using different deep learning models and support vector machines. Sens. Actuators B Chem. 2021, 327, 128921. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, K.; Chen, X.; Wu, H.; Xiao, X.; Xie, L.; Wei, Z.; Xiong, R.; Zhou, X. Effects of Plant-Derived Polyphenols on the Antioxidant Activity and Aroma of Sulfur-Dioxide-Free Red Wine. Molecules 2023, 28, 5255. [Google Scholar] [CrossRef]
- Franceschi, D.; Cavalet, E.; Boatto, V.; Conte, G.; Bravi, M. Artificial diagnosis of sensory taints due to Brettanomyces spp. contamination in Valpolicella wines. Chem. Eng. Trans. 2016, 54, 343–348. [Google Scholar] [CrossRef]
- Meléndez, F.; Arroyo, P.; Herrero, J.L.; Fernández, J.A.; Carmona, P.; Rodríguez, S.; Lozano, J. Fast Detection of TCA in Cork Stoppers by Means of Electronic Noses. In Proceedings of the 2020 IEEE International Symposium on Circuits and Systems (ISCAS), Seville, Spain, 10–21 October 2020; pp. 1–4. [Google Scholar]
- Santos, J.P.; Sayago, I.; Sanjurjo, J.L.; Perez-Coello, M.S.; Díaz-Maroto, M.C. Rapid and Non-Destructive Analysis of Corky Off-Flavors in Natural Cork Stoppers by a Wireless and Portable Electronic Nose. Sensors 2022, 22, 4687. [Google Scholar] [CrossRef]
- Meléndez, F.; Arroyo, P.; Gómez-Suárez, J.; Palomeque-Mangut, S.; Suárez, J.I.; Lozano, J. Portable Electronic Nose Based on Digital and Analog Chemical Sensors for 2,4,6-Trichloroanisole Discrimination. Sensors 2022, 22, 3453. [Google Scholar] [CrossRef]
- Gamboa, J.C.R.; da Silva, A.J.; de Andrade Lima, L.L.; Ferreira, T.A.E. Wine quality rapid detection using a compact electronic nose system: Application focused on spoilage thresholds by acetic acid. LWT 2019, 108, 377–384. [Google Scholar] [CrossRef]
- Aleixandre, M.; Santos, J.P.; Sayago, I.; Cabellos, J.M.; Arroyo, T.; Horrillo, M.C. A Wireless and Portable Electronic Nose to Differentiate Musts of Different Ripeness Degree and Grape Varieties. Sensors 2015, 15, 8429–8443. [Google Scholar] [CrossRef] [PubMed]
- Baietto, M.; Wilson, A.D. Electronic-Nose Applications for Fruit Identification, Ripeness and Quality Grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Pan, L.; Chen, R. Characterization of flavor frame in grape wines detected by HS-SPME-GC-MS coupled with HPLC, electronic nose, and electronic tongue. Food Mater. Res. 2023, 3, 9. [Google Scholar] [CrossRef]
- Harris, N.; Viejo, C.G.; Barnes, C.; Pang, A.; Fuentes, S. Wine quality assessment for Shiraz vertical vintages based on digital technologies and machine learning modeling. Food Biosci. 2023, 56, 103354. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Harris, N.; Pang, A.; Barnes, C.; Fuentes, S. Quality Assessment of Wine Vertical Vintages Based on Electronic Nose Coupled with Machine Learning Modelling. In Proceedings of the 15th Pangborn Sensory Science Symposium-Meeting New Challenges in a Changing World (PSSS 2023), Nantes, France, 20–24 August 2023; p. E02. [Google Scholar]
- Paknahad, M.; Ahmadi, A.; Rousseau, J.; Nejad, H.R.; Hoorfar, M. On-Chip Electronic Nose For Wine Tasting: A Digital Microfluidic Approach. IEEE Sens. J. 2017, 17, 4322–4329. [Google Scholar] [CrossRef]
- Georgiadou, E.C.; Mina, M.; Neoptolemou, V.; Koundouras, S.; D’Onofrio, C.; Bellincontro, A.; Mencarelli, F.; Fotopoulos, V.; Manganaris, G.A. The beneficial effect of leaf removal during fruit set on physiological, biochemical, and qualitative indices and volatile organic compound profile of the Cypriot reference cultivar ‘Xynisteri’. J. Sci. Food Agric. 2023, 103, 3776–3786. [Google Scholar] [CrossRef] [PubMed]
- Antoce, A.O.; Cojocaru, G.A. Evaluation by Flash GC Electronic Nose of the Effect of Combinations of Yeasts and Nutrients on the Aromatic Profiles of Feteasca Regala Wines after Two Years of Storage. Fermentation 2021, 7, 223. [Google Scholar] [CrossRef]
- Antoce, A.O.; Cojocaru, G.A. The Use of GC-Electronic Nose for the Selection of a Winemaking Protocol Leading to an Enhanced Volatile Profile in Wines from Aromatic Grape Varietis; CABI Digital Library: Wallingford, UK, 2017; Scientific Papers. Series B, Horticulture. Volume LXI, 2017, Print ISSN 2285-5653, CD-ROM ISSN 2285-5661, Online ISSN 2286-1580, ISSN-L 2285-5653. [Google Scholar]
- Zhang, J.; Wang, T.; Zhao, N.; Xu, J.; Qi, Y.; Wei, X.; Fan, M. Performance of a novel β-glucosidase BGL0224 for aroma enhancement of Cabernet Sauvignon wines. LWT 2021, 144, 111244. [Google Scholar] [CrossRef]
- Bianchi, A.; Santini, G.; Piombino, P.; Pittari, E.; Sanmartin, C.; Moio, L.; Modesti, M.; Bellincontro, A.; Mencarelli, F. Nitrogen maceration of wine grape: An alternative and sustainable technique to carbonic maceration. Food Chem. 2023, 404 Pt A, 134138. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Modesti, M.; Moreno-García, J.; Rodríguez-Moreno, M.; Catini, A.; Capuano, R.; Di Natale, C.; Bellincontro, A.; Moreno, J. Differentiation through E-nose and GC-FID data modeling of rosé sparkling wines elaborated via traditional and Charmat methods. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef] [PubMed]
- Modesti, M.; Alfieri, G.; Chieffo, C.; Mencarelli, F.; Vannini, A.; Catalani, A.; Chilosi, G.; Bellincontro, A. Destructive and non-destructive early detection of postharvest noble rot (Botrytis cinerea) in wine grapes aimed at producing high-quality wine. J. Sci. Food Agric. 2023, 104, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, G.A.; Antoce, A.O. Influence of Glutathione and Ascorbic Acid Treatments during Vinification of Feteasca Regala Variety and Their Antioxidant Effect on Volatile Profile. Biosensors 2019, 9, 140. [Google Scholar] [CrossRef]
- Aleixandre, M.; Santos, J.P.; Sayago, I.; Horrillo, M.C.; Cabellos, J.M.; Arroyo, T. Use of an electronic nose as a tool to differentiate winemaking techniques. In Proceedings of the 2015 10th Spanish Conference on Electron Devices (CDE), Aranjuez, Madrid, Spain, 11–13 February 2015; pp. 1–4. [Google Scholar]
- Han, F.; Zhang, D.; Aheto, J.H.; Feng, F.; Duan, T. Integration of a low-cost electronic nose and a voltammetric electronic tongue for red wines identification. Food Sci. Nutr. 2020, 8, 4330–4339. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, D.; Vincenzi, S.; Boatto, V.L.; Bravi, M. Can sensory analysis and e-noses support the assessment work behind DOC and DOCG wines? Chem. Eng. Trans. 2017, 57, 1759–1764. [Google Scholar]
- Khorramifar, A.; Karami, H.; Wilson, A.D.; Sayyah, A.H.A.; Shuba, A.; Lozano, J. Grape Cultivar Identification and Classification by Machine Olfaction Analysis of Leaf Volatiles. Chemosensors 2022, 10, 125. [Google Scholar] [CrossRef]
- Antoce, A.O.; Stroe, M.V.; Cojocaru, G.A. Tentative Application of an Electronic Nose to the Study of the Parentage of Romanian Grape Varieties Sarba and Alb Aromat. Agric. Agric. Sci. Procedia 2015, 6, 110–117. [Google Scholar] [CrossRef][Green Version]
- Tarì, U.; Pettinelli, S.; Bianchi, A.; Pollon, M.; Alfieri, G.; Vitaggio, C.; Corona, O.; Modesti, M.; Fiorino, F.O.; Bellincontro, A.; et al. Identifying wine grape aromatic maturity using e-nose and GC-MS: The case of Nerello Mascalese grapes from two contrade of the Etna area. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Schroeder, L.C.; Pessenti, I.L.; Voss HG, J.; Ayub, R.A.; Farinelli, M.E.; Siqueira, H.V.; Stevan, S.L., Jr. Discriminant analysis of volatile compounds in wines obtained from different managements of vineyards obtained by e-nose. Smart Agric. Technol. 2023, 6, 100343. [Google Scholar] [CrossRef]
- Wotner, J.; Dorksen, H.; Pein-Hackelbusch, M. Key Indicators for the Discrimination of Wines by Electronic Noses. In Proceedings of the 2023 IEEE International Conference on Industrial Informatics (INDIN), Lemgo, Germany, 18–20 July 2023. [Google Scholar]
- Yu, D.; Wang, X.; Liu, H.; Gu, Y. A Multitask Learning Framework for Multi-Property Detection of Wine. IEEE Access 2019, 7, 123151–123157. [Google Scholar] [CrossRef]
- Qiao, J.; Su, G.; Yuan, L.; Wu, L.; Weng, X.; Liu, S.; Feng, Y.; Jiang, D.; Chen, Y.; Ma, Y. Effect of swelling agent treatment on grape fruit quality and the application of electronic nose identification detection. Front. Plant Sci. 2024, 14, 1292335. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S. Digital Assessment and Classification of Wine Faults Using a Low-Cost Electronic Nose, Near-Infrared Spectroscopy and Machine Learning Modelling. Sensors 2022, 22, 2303. [Google Scholar] [CrossRef]
- Aleixandre, M.; Cabellos, J.M.; Arroyo, T.; Horrillo, M.C. Quantification of Wine Mixtures with an Electronic Nose and a Human Panel. Front. Bioeng. Biotechnol. 2018, 6, 2018. [Google Scholar] [CrossRef]
- Gardner, D.M.; Duncan, S.E.; Zoecklein, B.W. Aroma Characterization of Petit Manseng Wines Using Sensory Consensus Training, SPME GC-MS, and Electronic Nose Analysis. Am. J. Enol. Vitic. 2017, 68, 112–119. [Google Scholar] [CrossRef]
- Celdrán, A.C.; Oates, M.J.; Molina Cabrera, C.; Pangua, C.; Tardaguila, J.; Ruiz-Canales, A. Low-Cost Electronic Nose for Wine Variety Identification through Machine Learning Algorithms. Agronomy 2022, 12, 2627. [Google Scholar] [CrossRef]
- Baniţă, C.; Antoce, O.A.; Cojocaru, G.A. Evaluation by a GC Electronic Nose of the Differences in Volatile Profile Induced by Stopping Fermentation with Octanoic and Decanoic Acid to Produce Sweet Wines. Chemosensors 2023, 11, 98. [Google Scholar] [CrossRef]
- Liu, L.; Na, N.; Yu, J.; Zhao, W.; Wang, Z.; Zhu, Y.; Hu, C. Sniffing Like a Wine Taster: Multiple Overlapping Sniffs (MOSS) Strategy Enhances Electronic Nose Odor Recognition Capability. Adv. Sci. 2023, 11, 2305639. [Google Scholar] [CrossRef] [PubMed]
- Hernández, E.; Pelegrí-Sebastiá, J.; Sogorb, T.; Chilo, J. Evaluation of Red Wine Acidification Using an E-Nose System with Venturi Tool Sampling. Sensors 2023, 23, 2878. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, X.-Z.; Liu, Y.-Y.; Tao, N.-P.; Lu, Y.; Wang, X.-C.; Lam, W.; Lin, L.; Xu, C.-H. Direct authentication and composition quantitation of red wines based on Tri-step infrared spectroscopy and multivariate data fusion. Food Chem. 2022, 372, 131259. [Google Scholar] [CrossRef]
- Paredes-Doig, A.L.; Cárcamo, H.; Hurtado Cotillo, M.; Sun Kou, R.; Doig-Camino, E.; Picasso, G.; La Rosa-Toro Gómez, A. Gas Sensors Modified with Zeolite Y for Assessing Wine Aroma Compounds. J. Chem. 2019, 2019, 5283208. [Google Scholar] [CrossRef]
- Paredes-Doig, A.L.; Doig-Camino, E.; Kou, R.S.; Picasso, G.; La Rosa-Toro, A. Use of Statistical Methods for the Interpretation of Measurement Results of Peruvian Wines with MOS E-Noses. 2021. Available online: https://osf.io/preprints/ecsarxiv/fybzx (accessed on 3 June 2023).
- Liu, H.; Li, Q.; Yan, B.; Zhang, L.; Gu, Y. Bionic Electronic Nose Based on MOS Sensors Array and Machine Learning Algorithms Used for Wine Properties Detection. Sensors 2019, 19, 45. [Google Scholar] [CrossRef]
- Yang, X.; Li, M.; Ji, X.; Chang, J.; Deng, Z.; Meng, G. Recognition algorithms in E-nose: A Review. IEEE Sens. J. 2023, 23, 20460–20472. [Google Scholar] [CrossRef]
- Giungato, P.; Di Gilio, A.; Palmisani, J.; Marzocca, A.; Mazzone, A.; Brattoli, M.; Giua, R.; de Gennaro, G. Synergistic approaches for odor active compounds monitoring and identification: State of the art, integration, limits and potentialities of analytical and sensorial techniques. TrAC Trends Anal. Chem. 2018, 107, 116–129. [Google Scholar] [CrossRef]
- Mastrangelo, N.; Bianchi, A.; Pettinelli, S.; Santini, G.; Merlani, G.; Bellincontro, A.; Baris, F.; Chinnici, F.; Mencarelli, F. Novelty of Italian Grape Ale (IGA) beer: Influence of the addition of Gamay macerated grape must or dehydrated Aleatico grape pomace on the aromatic profile. Heliyon 2023, 9, e20422. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.-L.; Ullah, N.; Tao, Y.-S. Aroma Glycosides in Grapes and Wine. J. Food Sci. 2017, 82, 248–259. [Google Scholar] [CrossRef] [PubMed]
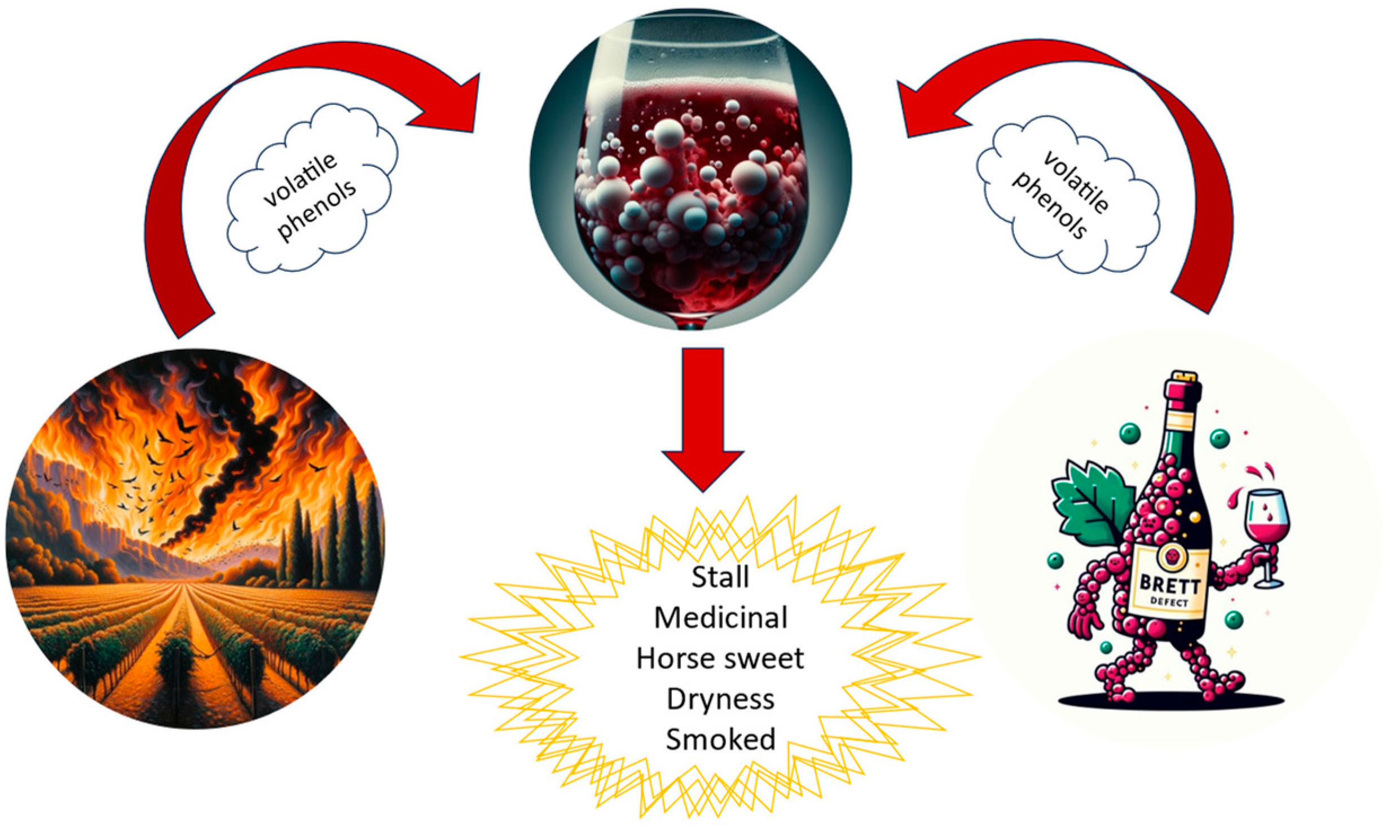
- Yang, R.; Qian, Y.L.; Feng, Y.; Huang, L.; Magana, A.A.; Qian, M.C. Volatile Phenols in Smoke-Exposed Pinot noir Wines-Biomarkers and Model Prediction. Am. J. Enol. Vitic. 2023, 74, 0740028. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M. Two Decades of “Horse Sweat” Taint and Brettanomyces Yeasts in Wine: Where do We Stand Now? Beverages 2018, 4, 32. [Google Scholar] [CrossRef]
- Kennison, K.R.; Wilkinson, K.L.; Williams, H.G.; Smith, J.H.; Gibberd, M.R. Smoke-derived taint in wine: Effect of postharvest smoke exposure of grapes on the chemical composition and sensory characteristics of wine. J. Agric. Food Chem. 2007, 55, 10897–10901. [Google Scholar] [CrossRef]
- Summerson, V.; Gonzalez Viejo, C.; Torrico, D.D.; Pang, A.; Fuentes, S. Digital Smoke Taint Detection in Pinot Grigio Wines Using an E-Nose and Machine Learning Algorithms Following Treatment with Activated Carbon and a Cleaving Enzyme. Fermentation 2021, 7, 119. [Google Scholar] [CrossRef]
- Høj, P.; Pretorius, I.; Blair, R. The Australian Wine Research Institute Annual Report; The Australian Wine Research Institute: Urrbrae, SA, Australia, 2003; pp. 37–39. [Google Scholar]
- Mirabelli-Montan, Y.A.; Marangon, M.; Graça, A.; Mayr Marangon, C.M.; Wilkinson, K.L. Techniques for Mitigating the Effects of Smoke Taint While Maintaining Quality in Wine Production: A Review. Molecules 2021, 26, 1672. [Google Scholar] [CrossRef]
- Oro, L.; Canonico, L.; Marinelli, V.; Ciani, M.; Comitini, F. Occurrence of Brettanomyces bruxellensis on Grape Berries and in Related Winemaking Cellar. Front. Microbiol. 2019, 10, 415. [Google Scholar] [CrossRef]
- AWRI Helpdesk. Stubble Burning—A Possible Source of Smoke Taint in Grapes. 2023. Available online: https://www.awri.com.au/wp-content/uploads/2018/05/Stubble-burning-fact-sheet.pdf (accessed on 3 June 2023).
- Otero, I.; Nielsen, J.Ø. Coexisting with wildfire? Achievements and challenges for a radical social-ecological transformation in Catalonia (Spain). Geoforum 2017, 85, 234–246. [Google Scholar] [CrossRef]
- Gilinsky, A.; Ford, J.; Newton, S.K.; Brown, D. An exploratory investigation into strategic resilience in the US wine industry. J. Wine Res. 2020, 31, 35–48. [Google Scholar] [CrossRef]
- Naulleau, A.; Gary, C.; Prévot, L.; Hossard, L. Evaluating Strategies for Adaptation to Climate Change in Grapevine Production–A Systematic Review. Front. Plant Sci. 2021, 11, 607859. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2020.607859 (accessed on 3 June 2023). [CrossRef]
- Brown, W. Climate Mitigation for Arid Region Vineyard. Ph.D. Thesis, The University of Arizona, Tucson, AZ, USA, 2021. [Google Scholar]
- Fuentes, S.; Summerson, V.; Gonzalez Viejo, C.; Tongson, E.; Lipovetzky, N.; Wilkinson, K.L.; Szeto, C.; Unnithan, R.R. Assessment of Smoke Contamination in Grapevine Berries and Taint in Wines due to Bushfires Using a Low-Cost E-Nose and an Artificial Intelligence Approach. Sensors 2020, 20, 5108. [Google Scholar] [CrossRef]
- Fuentes, S.; Summerson, V.; Viejo, C.G. Novel digital technologies to assess smoke taint in berries and wines due to bushfires. BIO Web Conf. 2022, 56, 01007. [Google Scholar] [CrossRef]
- Fuentes, S.; Summerson, V.; Tongson, E.; Viejo, C.G. Novel Digital Technologies to Assess Smoke Taint in Wine Using Non-Invasive Chemical Fingerprinting, a Low-Cost Electronic Nose, and Artificial Intelligence. Biol. Life Sci. Forum 2021, 6, 56. [Google Scholar] [CrossRef]
- Summerson, V.; Gonzalez Viejo, C.; Pang, A.; Torrico, D.D.; Fuentes, S. Review of the Effects of Grapevine Smoke Exposure and Technologies to Assess Smoke Contamination and Taint in Grapes and Wine. Beverages 2021, 7, 7. [Google Scholar] [CrossRef]
- Summerson, V.; Gonzalez Viejo, C.; Pang, A.; Torrico, D.D.; Fuentes, S. Assessment of Volatile Aromatic Compounds in Smoke Tainted Cabernet Sauvignon Wines Using a Low-Cost E-Nose and Machine Learning Modelling. Molecules 2021, 26, 5108. [Google Scholar] [CrossRef]
- Antolini, A.; Forniti, R.; Modesti, M.; Bellincontro, A.; Catelli, C.; Mencarelli, F. First Application of Ozone Postharvest Fumigation to Remove Smoke Taint from Grapes. Ozone Sci. Eng. 2021, 43, 54–262. [Google Scholar] [CrossRef]
| Category | Application | Sensor Arrays | Chemometrics | Classical Comparison | Reference |
|---|---|---|---|---|---|
| Wine aging | Evolution of wine over 9 months | MOS | PLS | GC-MS | [87] |
| Aging of sparkling wine | QMB | PLS-DA | GC-FID | [88] | |
| Characterization of refined marc distillates | MOS | PCA | GC-MS | [89] | |
| Wine defects | Electronic nose for detection of wine spoilage | MOS | [90] | ||
| Improving the performance of E-noses to evaluate defects | MOS | DL, SVM | [91] | ||
| Effects of plant-derived polyphenols on the antioxidant activity and aroma of sulfur-dioxide-free red wine | MOS | PCA, LDA | GC-MS, Sensory analysis | [92] | |
| Artificial diagnosis of Brettanomyces spp. in Valpolicella wines | QMB | PCA | Wet chemistry | [93] | |
| Fast detection of TCA | MOS | PCA | Wet chemistry | [94] | |
| Rapid and non-destructive analysis of corky off-flavors | MOS | PCA | Wet chemistry | [95] | |
| Portable Electronic Nose for 2,4,6-trichloroanisole | MOS | PCA | Wet chemistry | [96] | |
| Detection using Electronic Nose system: application focused on spoilage thresholds by acetic acid | MOS | PCA, SVM | Wet chemistry | [97] | |
| Wine and grape quality | Differentiate musts of different ripeness degree and grape varieties | MOS | PCA, PNN | Wet chemistry | [98] |
| Category | Application | Sensor Arrays | Chemometrics | Classical | Reference |
| Wine and grape quality | E-Nose applications for fruit identification, ripeness, and quality grading | CP | SAW | Wet chemistry | [99] |
| characterization of flavor frame in grape wines | MOS | PCA | HS-GC-MS | [100] | |
| Wine quality for Shiraz vertical vintages | MOS | ML | GC-MS | [101] | |
| Quality assessment of wine vertical vintages | MOS | ML | GC-MS | [102] | |
| On-chip Electronic Nose for wine tasting Effect of leaf removal on volatile organic compound | MOS | Wet chemistry | [103] | ||
| QMB | PCA | GC-MS | [104] | ||
| Winemaking techniques | Monitoring combinations of yeasts and nutrients on the aromatic profile wines GC-Electronic nose for the selection of winemaking protocol | MOS | PCA | Sensory Analysis Wet chemistry | [105] |
| MOS | PCA | [106] | |||
| Performance of a novel β-glucosidase for aroma enhancement of wines | MOS | PCA-PLSR | Sensory analysis, GC-MS | [107] | |
| An alternative and sustainable technique to carbonic maceration | QMB | PCA | GC-MS | [108] | |
| Differentiation through E-nose data modeling of rosé sparkling wines elaborated via traditional and Charmat methods | QMB | PLS-DA | GC-FID | [109] | |
| Early detection of postharvest noble rot in grapes | QMB | PLS-DA | Wet chemistry | [110] | |
| Category | Application | Sensor Arrays | Chemometrics | Classical | Reference |
| Winemaking techniques | Influence of glutathione and ascorbic acid treatments during vinification | MOS | Wet chemistry | [111] | |
| Use of an Electronic Nose as a tool to differentiate winemaking techniques | MOS | ANN, PCA | [112] | ||
| Wine and grape identification | Low-cost Electronic Nose for red wine identification. | Metalloporphyrin | PCA, ELM | Wet chemistry | [113] |
| Can sensory analysis and E-Noses support the assessment work | QMB | PCA | Sensory analysis | [114] | |
| Grape cultivar identification and classification by machine olfaction analysis | MOS | PCA, LDA, QDA, SVM, ANN | Wet chemistry | [115] | |
| Application of an Electronic Nose to the study of the parentage of Romanian grape varieties Identifying wine grape aromatic maturity | MOS | GC-FID | [116] | ||
| QMB | PCR, PCA | GC-MS | [117] | ||
| Wine and grape identification | Volatile compounds in wines obtained from different managements of vineyards | MOS | PCA, LDA | [118] | |
| Key indicators for the discrimination of wines by E-Nose | MOS-QMB | LDA | Wet chemistry | [119] | |
| A multitask learning framework for multi-property detection of wine | MOS | PCA | Wet chemistry | [120] | |
| Effect of swelling agent treatment on grape fruit quality and the application of electronic nose identification detection | MOS | LDA, SVM | GC-MS | [121] | |
| Wine characterization | Classification of wine faults using a low-cost electronic | MOS | ML | Wet chemistry | [122] |
| Category | Application | Sensor Arrays | Chemometrics | Classical Comparison | Reference |
| Wine characterization | Quantification of wine mixtures with an E-Nose | MOS | PLS, ANN | Sensory analysis | [123] |
| Aroma characterization of Petit Manseng | CP | ANOVA | GC-MS | [124] | |
| Low-cost E-Nose for wine variety identification | MOS | PCA | Wet chemistry | [125] | |
| Evaluation by a GC E-Nose of the differences in volatile profile sweet wines | MOS | PCA | GC-MS | [126] | |
| Sniffing like a wine taster: strategy enhances E-Nose odor recognition capability | MOS | SVM | [127] | ||
| Valuation of red wine acidification using an E-Nose system | MOS | [128] | |||
| Authentication and composition quantitation of red wines | MOS | PCA, PLS | Wet chemistry | [129] | |
| Gas sensors modified with zeolite for wine aroma compounds | MOS | PCA | Wet chemistry | [130] | |
| E-Nose for Peruvian wine classification | MOS | PCA | GC-HPLC-MS | [131] | |
| Bionic electronic nose based on MOS Sensor array and machine | MOS | ML, SVM | [132] |
| Application | Sensor Arrays | Chemometrics | Classical Comparison | Reference |
|---|---|---|---|---|
| Smoke contamination in grapevine berries and taint in wines due to bushfires using a low-cost E-Nose | MOS | ANN, Sensory analysis | HPLC-MS | [149] |
| Smoke taint detection in pinot grigio wines using an E-Nose and machine learning algorithms | MOS | ANN | [140] | |
| Novel digital technologies to assess smoke taint in berries and wines due to bushfires | MOS | ANN | [150] | |
| Novel digital technologies to assess smoke taint in wine | MOS | ML, ANN | [151] | |
| Effects of grapevine smoke exposure and technologies to assess smoke contamination and taint in grapes and wine | MOS | ML, ANN | [152] | |
| Volatile aromatic compounds in smoke-tainted cabernet sauvignon wines using a low-cost E-Nose | MOS | ANN | GC-MS | [153] |
| First application of ozone postharvest fumigation to remove smoke taint from grapes | QMB | PCA, PLS | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, G.; Modesti, M.; Riggi, R.; Bellincontro, A. Recent Advances and Future Perspectives in the E-Nose Technologies Addressed to the Wine Industry. Sensors 2024, 24, 2293. https://doi.org/10.3390/s24072293
Alfieri G, Modesti M, Riggi R, Bellincontro A. Recent Advances and Future Perspectives in the E-Nose Technologies Addressed to the Wine Industry. Sensors. 2024; 24(7):2293. https://doi.org/10.3390/s24072293
Chicago/Turabian StyleAlfieri, Gianmarco, Margherita Modesti, Riccardo Riggi, and Andrea Bellincontro. 2024. "Recent Advances and Future Perspectives in the E-Nose Technologies Addressed to the Wine Industry" Sensors 24, no. 7: 2293. https://doi.org/10.3390/s24072293
APA StyleAlfieri, G., Modesti, M., Riggi, R., & Bellincontro, A. (2024). Recent Advances and Future Perspectives in the E-Nose Technologies Addressed to the Wine Industry. Sensors, 24(7), 2293. https://doi.org/10.3390/s24072293






