Synergistic Applications of Graphene-Based Materials and Deep Eutectic Solvents in Sustainable Sensing: A Comprehensive Review
Abstract
1. Introduction
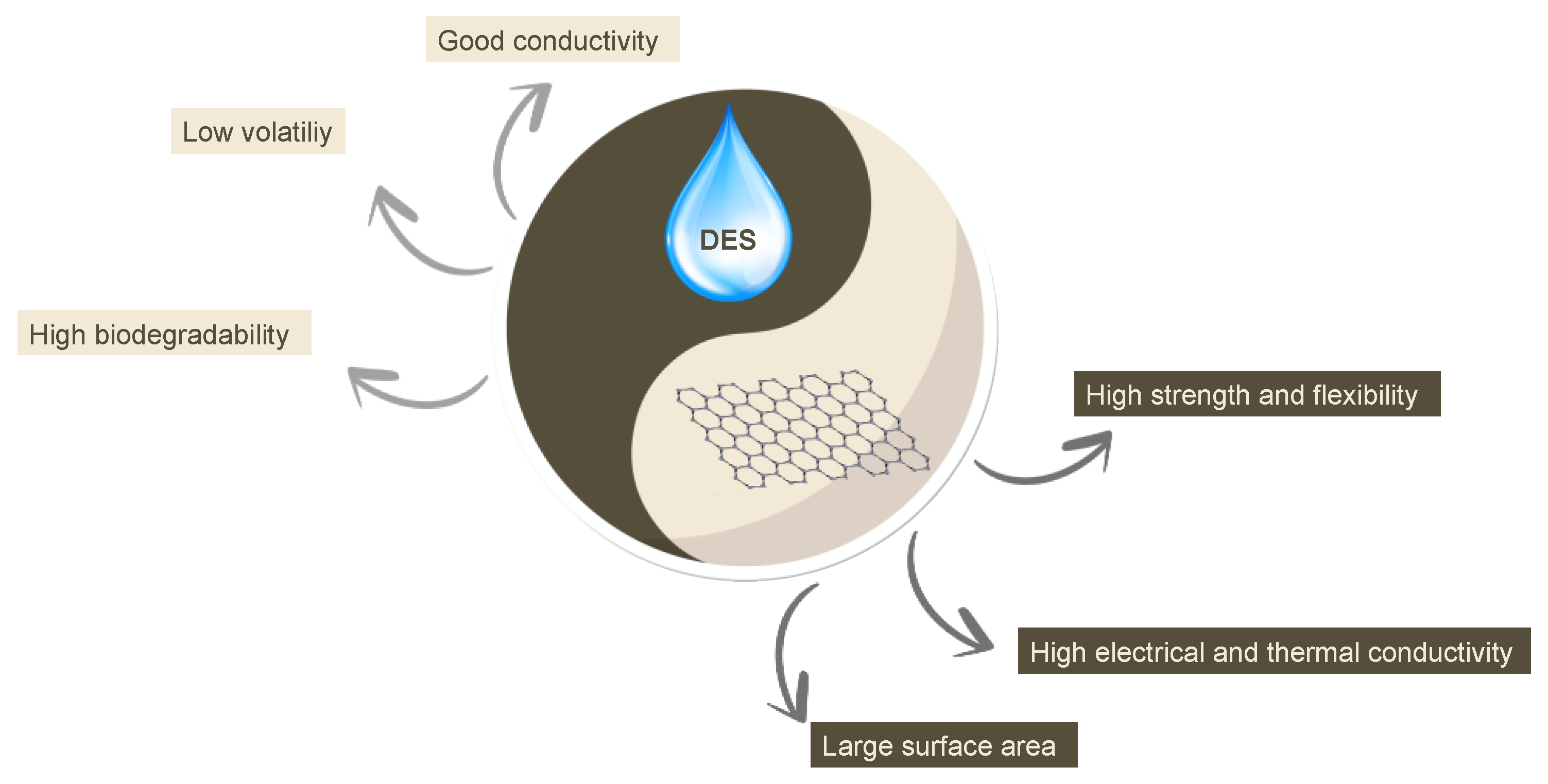
2. Enhancing Sensor Performance through DES–Graphene Integration
3. Conclusions and Outlook
- -
- It allows for the coupling of well-known high-performing semiconductor/conductors, such as graphene-related moieties, with chemically versatile solvents, like DESs. This opens numerous opportunities for the development of selective and high-performing sensors and biosensors;
- -
- DESs are truly eco-friendly, and they are really inexpensive (choline chloride, one of the most used building blocks for DESs, is produced in the range of about 150–170,000 tons/year);
- -
- The combination between the two materials allows for an unprecedented combination of advanced electronic and chemical properties at an extremely low cost, with a negligible environmental impact.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Craighead, H.G. Nanoelectromechanical Systems. Science 2000, 290, 1532–1535. [Google Scholar] [CrossRef]
- Arafat, M.M.; Dinan, B.; Akbar, S.A.; Haseeb, A.S.M.A. Gas Sensors Based on One Dimensional Nanostructured Metal-Oxides: A Review. Sensors 2012, 12, 7207–7258. [Google Scholar]
- Kasani, S.; Curtin, K.; Wu, N. A Review of 2D and 3D Plasmonic Nanostructure Array Patterns: Fabrication, Light Management and Sensing Applications. Nanophotonics 2019, 8, 2065–2089. [Google Scholar] [CrossRef]
- Mandler, D.; Turyan, I. Applications of Self-Assembled Monolayers in Electroanalytical Chemistry. Electroanalysis 1996, 8, 207–213. [Google Scholar] [CrossRef]
- Castellana, E.T.; Cremer, P.S. Solid Supported Lipid Bilayers: From Biophysical Studies to Sensor Design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef]
- Liu, D.; Lu, K.; Poon, C.; Lin, W. Metal–Organic Frameworks as Sensory Materials and Imaging Agents. Inorg. Chem. 2014, 53, 1916–1924. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A.U. Graphene Synthesis, Characterization and Its Applications: A Review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-Dimensional Gas of Massless Dirac Fermions in Graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van Der Waals Heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Akinwande, D.; Petrone, N.; Hone, J. Two-Dimensional Flexible Nanoelectronics. Nat. Commun. 2014, 5, 5678. [Google Scholar] [CrossRef]
- Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics Based on Two-Dimensional Materials. Nat. Nanotechnol. 2014, 9, 768–779. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing Graphene Quantum Dots and Carbon Dots: Properties, Syntheses, and Biological Applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, Related Two-Dimensional Crystals, and Hybrid Systems for Energy Conversion and Storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Jiang, H.; Lee, P.S.; Li, C. 3D Carbon Based Nanostructures for Advanced Supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. [Google Scholar] [CrossRef]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with Two-Dimensional Materials and Their Heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart Micro/Nanoparticles in Stimulus-Responsive Drug/Gene Delivery Systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef]
- Biju, V. Chemical Modifications and Bioconjugate Reactions of Nanomaterials for Sensing, Imaging, Drug Delivery and Therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-Based Nanomaterials for Drug Delivery and Tissue Engineering. J. Control Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Gu, Z.; Zhu, S.; Yan, L.; Zhao, F.; Zhao, Y. Graphene-Based Smart Platforms for Combined Cancer Therapy. Adv. Mater. 2019, 31, 1800662. [Google Scholar] [CrossRef]
- Lazăr, A.-I.; Aghasoleimani, K.; Semertsidou, A.; Vyas, J.; Roșca, A.-L.; Ficai, D.; Ficai, A. Graphene-Related Nanomaterials for Biomedical Applications. Nanomaterials 2023, 13, 1092. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A Review on Carbon Nanotubes and Graphene as Fillers in Reinforced Polymer Nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A New Emerging Lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical Properties of Graphene and Graphene-Based Nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Montazerian, A.; Arve Øverli, J.; Goutianos, S. Thermal Conductivity of Cementitious Composites Reinforced with Graphene-Based Materials: An Integrated Approach Combining Machine Learning with Computational Micromechanics. Constr. Build. Mater. 2023, 395, 132293. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.J.; Lin, L. A Review on Chemiresistive Room Temperature Gas Sensors Based on Metal Oxide Nanostructures, Graphene and 2D Transition Metal Dichalcogenides. Mikrochim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A Review on Graphene-Based Nanocomposites for Electrochemical and Fluorescent Biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and Its Sensor-Based Applications: A Review. Sens. Actuators A Phys. 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Beiranvand, B.; Sobolev, A.S. A Proposal for a Multi-Functional Tunable Dual-Band Plasmonic Absorber Consisting of a Periodic Array of Elliptical Grooves. J. Opt. 2020, 22, 105005. [Google Scholar] [CrossRef]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for Electrochemical Sensing and Biosensing. TrAC Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Hu, S. Nanocomposites of Graphene and Graphene Oxides: Synthesis, Molecular Functionalization and Application in Electrochemical Sensors and Biosensors. A Review. Microchim. Acta 2017, 184, 1–44. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-Based Gas Sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Ratinac, K.R.; Yang, W.; Ringer, S.P.; Braet, F. Toward Ubiquitous Environmental Gas Sensors—Capitalizing on the Promise of Graphene. Environ. Sci. Technol. 2010, 44, 1167–1176. [Google Scholar] [CrossRef]
- Ji, Q.; Honma, I.; Paek, S.-M.; Akada, M.; Hill, J.P.; Vinu, A.; Ariga, K. Layer-by-Layer Films of Graphene and Ionic Liquids for Highly Selective Gas Sensing. Angew. Chem. Int. Ed. Engl. 2010, 49, 9737–9739. [Google Scholar] [CrossRef]
- Mehta, J.; Vinayak, P.; Tuteja, S.K.; Chhabra, V.A.; Bhardwaj, N.; Paul, A.K.; Kim, K.-H.; Deep, A. Graphene Modified Screen Printed Immunosensor for Highly Sensitive Detection of Parathion. Biosens. Bioelectron. 2016, 83, 339–346. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of Individual Gas Molecules Adsorbed on Graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Basu, S.; Bhattacharyya, P. Recent Developments on Graphene and Graphene Oxide Based Solid State Gas Sensors. Sens. Actuators B Chem. 2012, 173, 1–21. [Google Scholar] [CrossRef]
- Demon, S.Z.N.; Kamisan, A.I.; Abdullah, N.; Noor, S.A.M.; Khim, O.K.; Kasim, N.A.M.; Yahya, M.Z.A.; Manaf, N.A.A.; Azmi, A.F.M.; Halim, N.A. Graphene-Based Materials in Gas Sensor Applications: A Review. Sens. Mater. 2020, 32, 759. [Google Scholar] [CrossRef]
- Alzate-Carvajal, N.; Luican-Mayer, A. Functionalized Graphene Surfaces for Selective Gas Sensing. ACS Omega 2020, 5, 21320–21329. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent Advances in Graphene-Based Biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef]
- Thakur, B.; Zhou, G.; Chang, J.; Pu, H.; Jin, B.; Sui, X.; Yuan, X.; Yang, C.-H.; Magruder, M.; Chen, J. Rapid Detection of Single, E. coli Bacteria Using a Graphene-Based Field-Effect Transistor Device. Biosens. Bioelectron. 2018, 110, 16–22. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Kumar, S.; Bukkitgar, S.D.; Singh, S.; Pratibha; Singh, V.; Reddy, K.R.; Shetti, N.P.; Venkata Reddy, C.; Sadhu, V.; Naveen, S. Electrochemical Sensors and Biosensors Based on Graphene Functionalized with Metal Oxide Nanostructures for Healthcare Applications. ChemistrySelect 2019, 4, 5322–5337. [Google Scholar] [CrossRef]
- Maio, A.; Pibiri, I.; Morreale, M.; Mantia, F.P.; Scaffaro, R. An Overview of Functionalized Graphene Nanomaterials for Advanced Applications. Nanomaterials 2021, 11, 1717. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic Liquids as Electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Tang, X.; Lv, S.; Jiang, K.; Zhou, G.; Liu, X. Recent Development of Ionic Liquid-Based Electrolytes in Lithium-Ion Batteries. J. Power Sources 2022, 542, 231792. [Google Scholar] [CrossRef]
- Maculewicz, J.; Świacka, K.; Stepnowski, P.; Dołżonek, J.; Białk-Bielińska, A. Ionic Liquids as Potentially Hazardous Pollutants: Evidences of Their Presence in the Environment and Recent Analytical Developments. J. Hazard. Mater. 2022, 437, 129353. [Google Scholar] [CrossRef]
- Mannu, A.; Blangetti, M.; Baldino, S.; Prandi, C. Promising Technological and Industrial Applications of Deep Eutectic Systems. Materials 2021, 14, 2494. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Chandran, K.; Kait, C.F.; Wilfred, C.D.; Zaid, H.F.M. A Review on Deep Eutectic Solvents: Physiochemical Properties and Its Application as an Absorbent for Sulfur Dioxide. J. Mol. Liq. 2021, 338, 117021. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jerome, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical Properties of Deep Eutectic Solvents: A Review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Yu, D.; Xue, Z.; Mu, T. Eutectics: Formation, Properties, and Applications. Chem. Soc. Rev. 2021, 50, 8596–8638. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, R.; Santana-Mayor, Á.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Recent Applications of Deep Eutectic Solvents in Environmental Analysis. Appl. Sci. 2021, 11, 4779. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of Improved Deep Eutectic Solvents Using Hole Theory. Chem. Eur. J. Chem. Phys. 2006, 7, 803–806. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, J.; Li, G.; Zhao, W.; Zhao, X.; Mu, T. The Electrochemical Stability of Ionic Liquids and Deep Eutectic Solvents. Sci. China Chem. 2016, 59, 571–577. [Google Scholar] [CrossRef]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green Solvents from Ionic Liquids and Deep Eutectic Solvents to Natural Deep Eutectic Solvents. Comptes Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef]
- Abolghasemi, M.M.; Piryaei, M.; Imani, R.M. Deep Eutectic Solvents as Extraction Phase in Head-Space Single-Drop Microextraction for Determination of Pesticides in Fruit Juice and Vegetable Samples. Microchem. J. 2020, 158, 105041. [Google Scholar] [CrossRef]
- Cai, T.; Qiu, H. Application of Deep Eutectic Solvents in Chromatography: A Review. Trends Anal. Chem. 2019, 9, 115623. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Rao, Z.; Dhanjai; Wu, J.; Shen, Y.; Boddula, R.; Huang, Y. Electrochemical (Bio) Sensors Go Green. Biosens. Bioelectron. 2020, 163, 112270. [Google Scholar] [CrossRef]
- Svigelj, R.; Dossi, N.; Grazioli, C.; Toniolo, R. Deep Eutectic Solvents (DESs) and Their Application in Biosensor Development. Sensors 2021, 21, 4263. [Google Scholar] [CrossRef]
- Abbott, A.P. Deep Eutectic Solvents and Their Application in Electrochemistry. Curr. Opin. Green Sustain. Chem. 2022, 36, 100649. [Google Scholar] [CrossRef]
- Brett, C.M.A. Deep Eutectic Solvents and Applications in Electrochemical Sensing. Curr. Opin. Electrochem. 2018, 10, 143–148. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M. Review on Hydrometallurgical Recovery of Metals with Deep Eutectic Solvents. Sustain. Chem. 2020, 1, 238–255. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of Alcohol-Based Deep Eutectic Solvent in Extraction and Determination of Flavonoids with Response Surface Methodology Optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Lomba, L.; García, C.B.; Ribate, M.P.; Giner, B.; Zuriaga, E. Applications of Deep Eutectic Solvents Related to Health, Synthesis, and Extraction of Natural Based Chemicals. Appl. Sci. 2021, 11, 10156. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of Phenolic Compounds from Virgin Olive Oil by Deep Eutectic Solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Jablonský, M.; Majová, V.; Šima, J.; Hroboňová, K.; Lomenová, A. Involvement of Deep Eutectic Solvents in Extraction by Molecularly Imprinted Polymers—A Minireview. Crystals 2020, 10, 217. [Google Scholar] [CrossRef]
- Tian, D.; Chandra, R.P.; Lee, J.-S.; Lu, C.; Saddler, J.N. A Comparison of Various Lignin-Extraction Methods to Enhance the Accessibility and Ease of Enzymatic Hydrolysis of the Cellulosic Component of Steam-Pretreated Poplar. Biotechnol. Biofuels 2017, 10, 157. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Spychaj, T.; Mąka, H. Imidazole-Based Deep Eutectic Solvents for Starch Dissolution and Plasticization. Carbohydr. Polym. 2016, 140, 416–423. [Google Scholar] [CrossRef]
- Svigelj, R.; Bortolomeazzi, R.; Dossi, N.; Giacomino, A.; Bontempelli, G.; Toniolo, R. An Effective Gluten Extraction Method Exploiting Pure Choline Chloride-Based Deep Eutectic Solvents (ChCl-DESs). Food Anal. Methods 2017, 10, 4079–4085. [Google Scholar] [CrossRef]
- Tan, T.; Zhang, M.; Wan, Y.; Qiu, H. Utilization of Deep Eutectic Solvents as Novel Mobile Phase Additives for Improving the Separation of Bioactive Quaternary Alkaloids. Talanta 2016, 149, 85–90. [Google Scholar] [CrossRef]
- Toniolo, R.; Dossi, N.; Giannilivigni, E.; Fattori, A.; Svigelj, R.; Bontempelli, G.; Giacomino, A.; Daniele, S. Modified Screen Printed Electrode Suitable for Electrochemical Measurements in Gas Phase. Anal. Chem. 2020, 92, 3689–3696. [Google Scholar] [CrossRef]
- Zuliani, I.; Fattori, A.; Svigelj, R.; Dossi, N.; Grazioli, C.; Bontempelli, G.; Toniolo, R. Amperometric Detection of Ethanol Vapors by Screen Printed Electrodes Modified by Paper Crowns Soaked with Room Temperature Ionic Liquids. Electroanalysis 2022, 35, e202200150. [Google Scholar] [CrossRef]
- Svigelj, R.; Dossi, N.; Pizzolato, S.; Toniolo, R.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J. Truncated Aptamers as Selective Receptors in a Gluten Sensor Supporting Direct Measurement in a Deep Eutectic Solvent. Biosens. Bioelectron. 2020, 165, 112339. [Google Scholar] [CrossRef]
- Svigelj, R.; Dossi, N.; Grazioli, C.; Toniolo, R. Paper-Based Aptamer-Antibody Biosensor for Gluten Detection in a Deep Eutectic Solvent (DES). Anal. Bioanal. Chem. 2021, 414, 3341–3348. [Google Scholar] [CrossRef]
- De Miguel Turullois, I.; Herradón García, B.; Mann Morales, E.A.; Morales Bergas, E. Exfoliation of Graphite with Deep Eutectic. Solvents. Patent EP3050844, 3 August 2016. [Google Scholar]
- Prasad, K.; Sharma, M.; Mondal, D.; Saha, A.; Singh, N. Process for the Production of Graphene Sheets with Tunable Functionalities from Seaweed Promoted by Deep Eutectic Solvents. U.S. Patent 10549997, 4 February 2020. [Google Scholar]
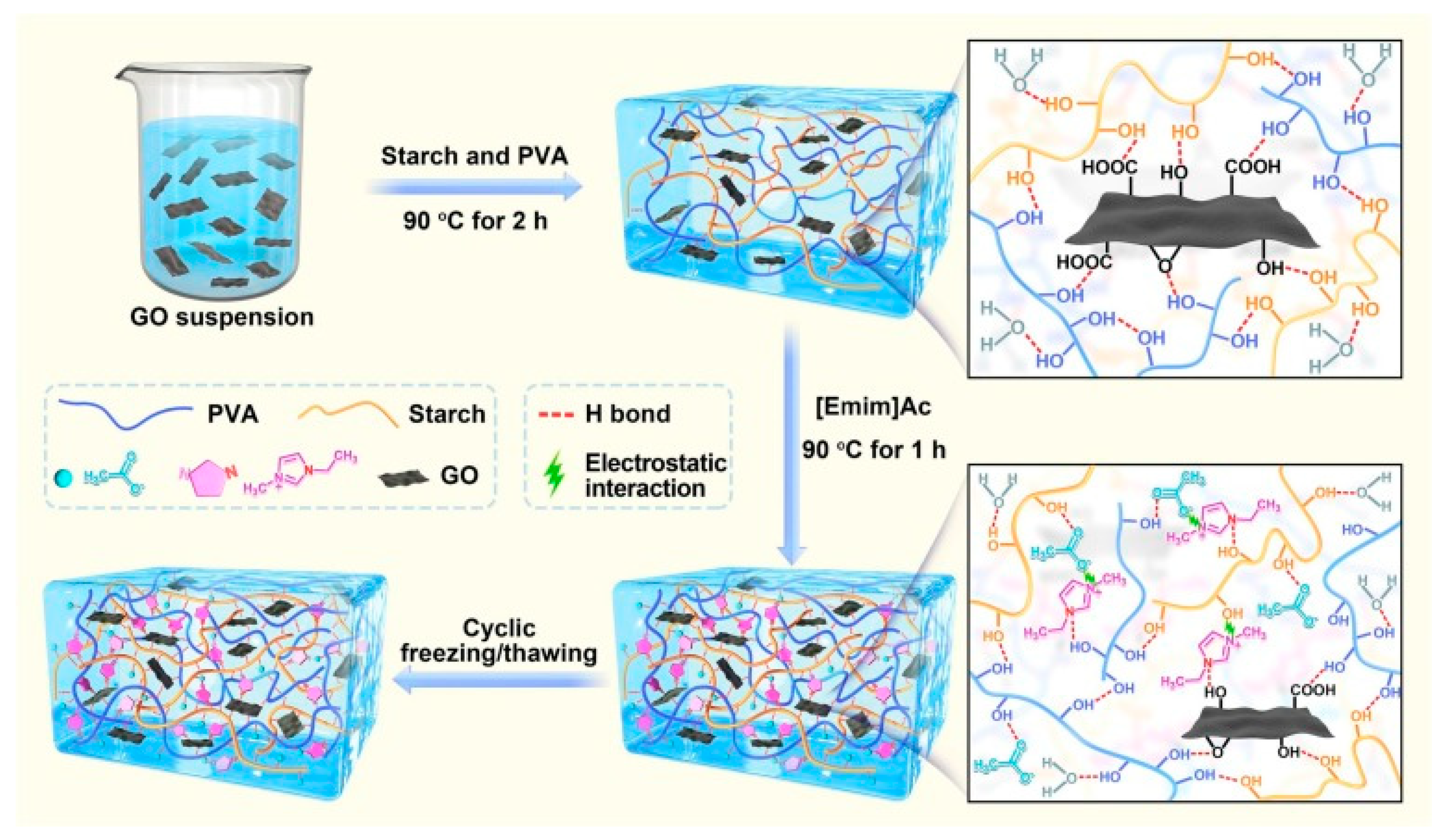
- Li, X.; Zhang, S.; Li, X.; Lu, L.; Cui, B.; Yuan, C.; Guo, L.; Yu, B.; Chai, Q. Starch/Polyvinyl Alcohol with Ionic Liquid/Graphene Oxide Enabled Highly Tough, Conductive and Freezing-Resistance Hydrogels for Multimodal Wearable Sensors. Carbohydr. Polym. 2023, 320, 121262. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, W.; Chang, M.; Wang, Y.; Li, Y. Development of Rutin Sensor Based on Graphene Quantum Dots@nano-Carbon Ionic Liquid Electrode. Ionics 2023, 29, 3385–3392. [Google Scholar] [CrossRef]
- Shahinfard, H.; Shabani-Nooshabadi, M.; Reisi-Vanani, A.; Darabi, R. Electrochemical Sensor Based on CuO/Reduced Graphene Nanoribbons and Ionic Liquid for Simultaneous Determination of Tramadol, Olanzapine and Acetaminophen. Carbon Lett. 2023, 33, 1433–1444. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, F.; Zeng, B. Preparation and Application of Sunset Yellow Imprinted Ionic Liquid Polymer—Ionic Liquid Functionalized Graphene Composite Film Coated Glassy Carbon Electrodes. Electrochim. Acta 2014, 115, 247–254. [Google Scholar] [CrossRef]
- Hayyan, M.; Abo-Hamad, A.; AlSaadi, M.A.; Hashim, M.A. Functionalization of Graphene Using Deep Eutectic Solvents. Nanoscale Res. Lett. 2015, 10, 324. [Google Scholar] [CrossRef]
- Ni, Y.; Xu, J.; Liu, H.; Shao, S. Fabrication of RGO-NiCo2O4 Nanorods Composite from Deep Eutectic Solvents for Nonenzymatic Amperometric Sensing of Glucose. Talanta 2018, 185, 335–343. [Google Scholar] [CrossRef]
- Ding, M.; Niu, H.; Zhang, N.; Hou, T.; Guan, P.; Hu, X. Facile Fabrication of Electrochemically Reduced Graphene Oxide/Polythionine-Methylene Blue and Its Use as a Platform for Detection of Nicotinamide Adenine Dinucleotide in the Artificial Urine Sample. Electrochim. Acta 2022, 425, 140715. [Google Scholar] [CrossRef]
- Hosu, O.; Bârsan, M.M.; Cristea, C.; Săndulescu, R.; Brett, C.M.A. Nanostructured Electropolymerized Poly(Methylene Blue) Films from Deep Eutectic Solvents. Optimization and Characterization. Electrochim. Acta 2017, 232, 285–295. [Google Scholar] [CrossRef]
- Ding, M.; Hou, T.; Niu, H.; Zhang, N.; Guan, P.; Hu, X. Electrocatalytic Oxidation of NADH at Graphene-Modified Electrodes Based on Electropolymerized Poly(Thionine-Methylene Blue) Films from Nature Deep Eutectic Solvents. J. Electroanal. Chem. 2022, 920, 116602. [Google Scholar] [CrossRef]
- Fuchs, D.; Bayer, B.C.; Gupta, T.; Szabo, G.L.; Wilhelm, R.A.; Eder, D.; Meyer, J.C.; Steiner, S.; Gollas, B. Electrochemical Behavior of Graphene in a Deep Eutectic Solvent. ACS Appl. Mater. Interfaces 2020, 12, 40937–40948. [Google Scholar] [CrossRef]
- Chavda, V.; Hirpara, D.; Kumar, S. GO/Ionic Surfactant Inspired Photophysical Modulation of Rhodamine B in Reline with or without Additives. J. Mol. Liq. 2022, 368, 120614. [Google Scholar] [CrossRef]
- Mahyari, M.; Hooshmand, S.E.; Sepahvand, H.; Gholami, S.; Rezayan, A.H.; Zarei, M.A. Gold Nanoparticles Anchored onto Covalent Poly Deep Eutectic Solvent Functionalized Graphene: An Electrochemical Aptasensor for the Detection of C-Reactive Protein. Mater. Chem. Phys. 2021, 269, 124730. [Google Scholar] [CrossRef]
- Gong, L.; Fu, H.; Liu, L.; Li, Z.; Guo, J.; Cao, Z.; Yao, J. Construction and Performance of a Nanocellulose–Graphene-Based Humidity Sensor with a Fast Response and Excellent Stability. ACS Appl. Polym. Mater. 2022, 4, 3656–3666. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Li, Q.; Wan, C. Upgrading Lignocellulose to Porous Graphene Enabled by Deep Eutectic Solvent Pretreatment: Insights into the Role of Lignin and Pseudo-Lignin. ACS Sustain. Chem. Eng. 2022, 10, 11501–11511. [Google Scholar] [CrossRef]
- Arab, N.; Fotouhi, L.; Salis, A.; Dorraji, P.S. An Amplified Electrochemical Sensor Employing a Polymeric Film and Graphene Quantum Dots/Multiwall Carbon Nanotubes in a Deep Eutectic Solvent for Sensitive Analysis of Paracetamol and 4-Aminophenol. New J. Chem. 2020, 44, 15742–15751. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Thi Pham, N.; Ngo, D.-H.; Kumar, S.; Cao, X.T. Covalently Functionalized Graphene with Molecularly Imprinted Polymers for Selective Adsorption and Electrochemical Detection of Chloramphenicol. ACS Omega 2023, 8, 25385–25391. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Spisso, A.; Fernanda Silva, M. Pencil Graphite Electrodes for Improved Electrochemical Detection of Oleuropein by the Combination of Natural Deep Eutectic Solvents and Graphene Oxide. Electrophoresis 2017, 38, 2704–2711. [Google Scholar] [CrossRef]



| Target Analyte | Type of DES | Type of DES-Based Graphene System | Role and Function of the DES–Graphene Composite System | Sensing Method | Linear Range | LOD | Stability–Reproducibility–Repeatability | Reference |
|---|---|---|---|---|---|---|---|---|
| Glucose | ChCl–urea mixture (CU-DESs) | Reduced graphene oxide-supported nickel cobaltate nanorod composite (rGO-NiCo2O4 nanorods) | Electrocatalytic activity toward glucose oxidation in alkaline media; enhanced electrical conductivity. | Amperometry (nonenzymatic) | 1 μM–25 mM | 0.35 μM | Stability: sensor current response to 1 mM glucose in NaOH solution was stable above 90% of the initial response up to 1800 s. Reproducibility: anodic peak currents of four independently prepared RGO-NiCo2O4/Nafion/GCE electrodes showed 1.92% relative standard deviation (RSD). Repeatability: ten anodic peak current measurements on the modified electrode showed RSD of 1.98%. | [95] |
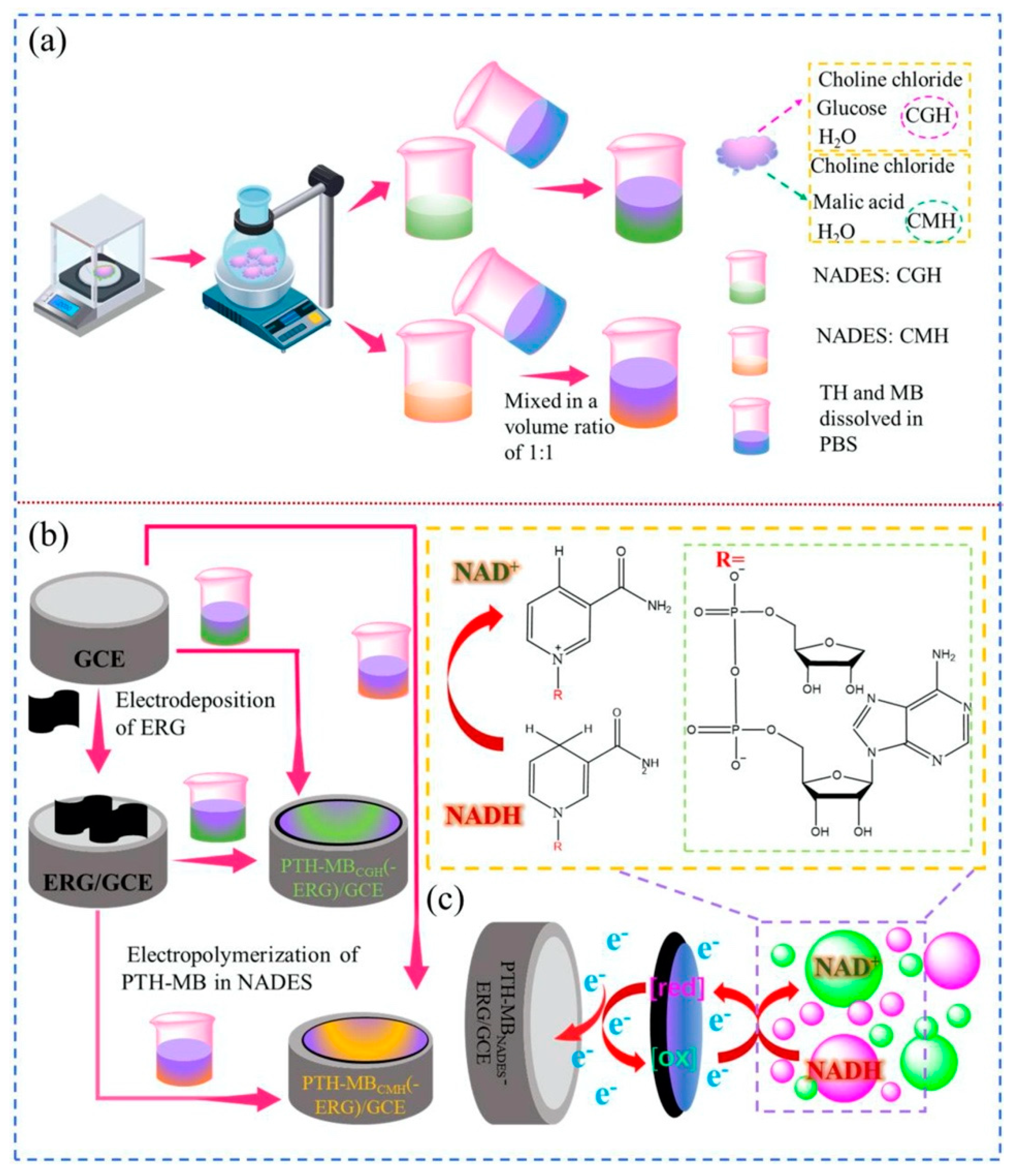
| Nicotinamide adenine dinucleotide (NADH) | Choline chloride:ethylene glycol (ChCl-EG, CE) | Polythionine–methylene blue (PTH-MB) electropolymerized in deep eutectic solvent (CE)–electrochemically reduced graphene oxide (ERG)-modified GCE glassy carbon electrode (PTH-MBCE-ERG/GCE) | Electropolymerization of PTH-MB film in CE provides improved stability and sensitivity and a significant reduction in LOD value; ERG film facilitates electron transfer. | Cyclic voltammetry (CV) | 1.52 μM–3.33 mM | 0.51 nM | Stability: the sensor response to 1.0 mM NADH showed a loss in sensitivity by ca. 12% after 28 days. | [96] |
| Nicotinamide adenine dinucleotide (NADH) | Natural DES (NADES) | Composite electrode based on electrochemically reduced graphene oxide (ERG)/poly(thionine–methylene blue) (PTH-MB) | NADES was used for the electropolymerization of PTH-MB polymer films, while ERG increased not only the charge transfer rate but also the surface area of the polymer. | Cyclic voltammetry (CV) | 0.51–3.3 mM | 0.159 nM | Stability: the sensor current response to 1.0 mM NADH decreased by ca. 10% of the initial current response after 28 days. | [98] |
| Amperometry | 1.78 μM–0.3 mM | 0.13 μM | ||||||
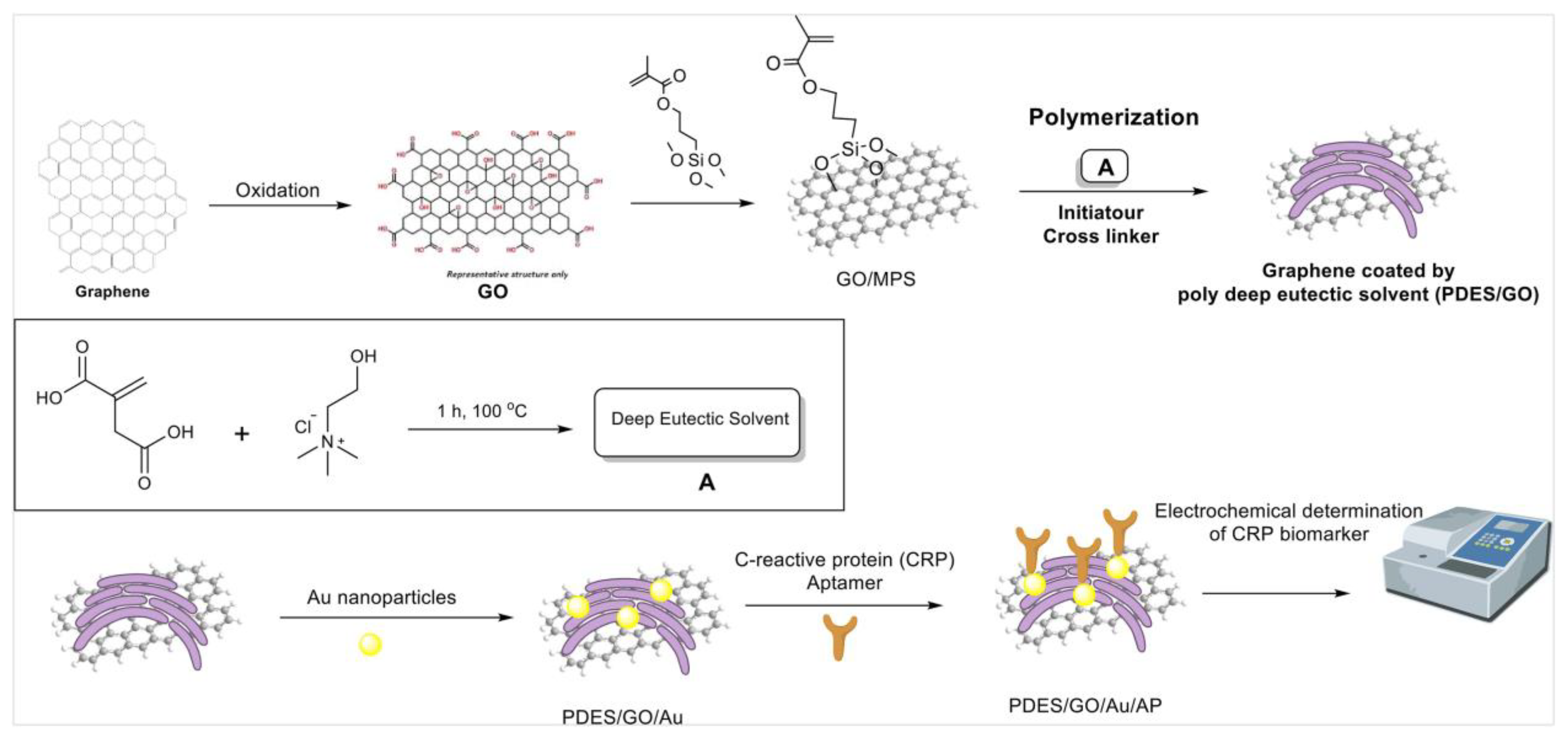
| C-reactive protein (CRP) as ring-shaped pentameric protein found in blood plasma | Polymerized deep eutectic solvent (PDES) | DNA aptamer immobilized on a graphene nanocomposite functionalized with PDES and coated with gold nanoparticles (AuNPs-PDES-GO) | Covalent functionalization of graphene with PDES boosted its dispersity in several solvents, particularly in aqueous media. | Electrochemical impedance spectroscopy (EIS) | 0.001–50 ng mL−1 | 0.0003 ng mL−1 | Stability: sensor response decreased at 96% of the initial response once operated several times over 10 days. Reproducibility: RSD of 4.6% across five aptasensors. | [101] |
| Humidity | Betaine/oxalic acid deep eutectic solvent (DES) | Cellulose nanofiber-dispersed graphene (CNF/Gr) | DES was used to extract and esterify the CNF with the aid of ultrasonic treatment. | Resistance | 15–99% RH | -- | Stability/reproducibility: samples operated under 99% RH for 15 days showed ΔR/R0 changes by less than 1%. | [102] |
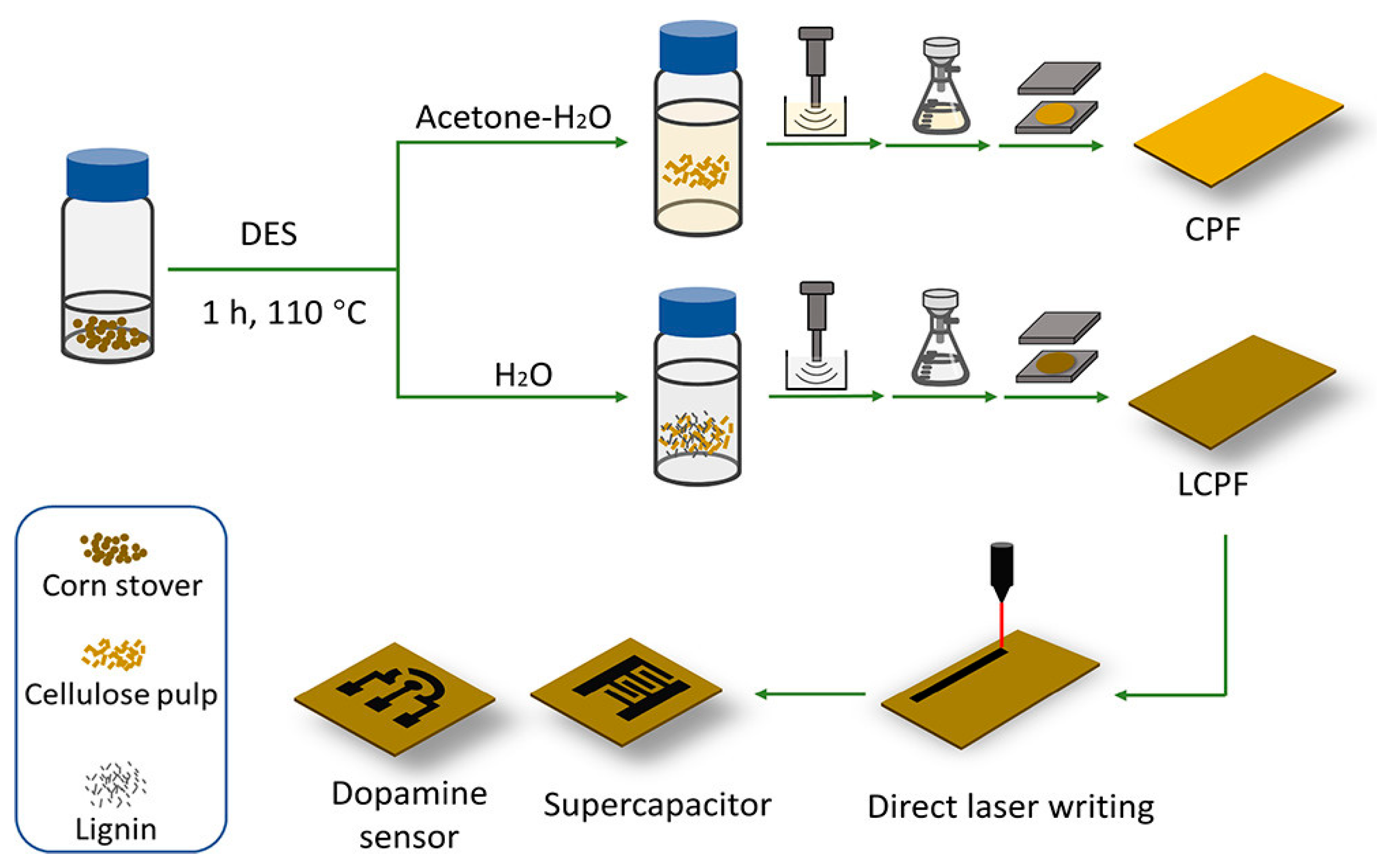
| Dopamine | Choline chloride mixed with an oxalic acid anhydride (ChCl:OA) | Regenerated lignin-incorporated cellulose pulp films scribed with CO2 laser at 25% of max. laser power for laser-induced graphene (LCPF-LIG-25) | ChCl:OA used for cellulose pretreatment to promote hemicellulose hydrolysis; scribed LIG acted as the working electrode and counter electrode. | Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) | 1 μM–40 μM | 0.659 μM | -- | [103] |
| Paracetamol (PA) | Choline chloride–urea | Nanocomposite consisting of graphene quantum dots, a deep eutectic solvent, and carboxyl-functionalized multiwall carbon nanotubes (GQDs + DES + MWCNTs-COOH) | Increased anodic peak currents attributed to the high electrical conductivity of the MWCNTs-COOH and DES, and to the high surface area given by the GQDs and the pores generated by the DES. | Differential pulse voltammetry (DPV) | 0.030–110 mmol L−1 | 0.010 mmol L−1 | Stability: peak currents at 96.5% of initial current after 30 h. Repeatability: RSD of 2.7% for six successive determinations of 20.0 mmol L−1. Reproducibility: RSDs were 2.90% and 4.09% for intraday and six interday experiments, respectively. | [104] |
| 4-aminophenol (4-AP) | 0.050–100 mmol L−1 | 0.017 mmol L−1 | Stability: peak currents at 96.1% of initial current after 30 h. Repeatability: RSD of 3.1% for six successive determinations of 20.0 mmol L−1. Reproducibility: RSDs were 3.22% and 4.17% for intraday and 6 interday experiments, respectively. | |||||
| Chloramphenicol | DES prepared from a mixture of ZnCl2 and ChCl | Nanocomposite based on covalently functionalizing molecularly imprinted polymers (MIPs) onto the surface of reduced graphene oxide (rGO), which was pretreated with maleic anhydride (MA) via the Diels−Alder reaction in the DES | DES was used as environmentally friendly medium in the Diels−Alder reaction for rGO surface modification in ambient conditions; high electrical conductivity of MIP-functionalized rGO to enhance efficiency as electrochemical sensing materials. | Chronoamperometry, amperometry | 0.05–8.0 μM | 0.204 μM | Repeatability: testing the same MIP-rGO-based sensor for five subsequent experiments showed a retention at 96.2% of the initial current density response with a relative standard deviation (RSD) of 2.4%. Reproducibility: current responses given by five MIP-rGO-based sensor devices tested for CAP detection, varied with an RSD of 2.35%. | [105] |
| Oleuropein (OLE) | Natural deep eutectics solvent (NADES) | Graphene oxide (GO) and pencil graphite electrode (PGE) in combination with a buffer modified with a NADES, containing 10% (v/v) of lactic acid, glucose, and H2O (LGH) | NADES enhances electrochemical detection, while PGE is used as low-cost, mechanically stable, carbonaceous electrode material. | Differential pulse voltammetry (DPV) | 0.10–37 μM | 0.030 μM | Reproducibility: RSD of the oxidation peak current to 18 μM of OLE was 3.16% over five electrodes. | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svigelj, R.; Toniolo, R.; Bertoni, C.; Fraleoni-Morgera, A. Synergistic Applications of Graphene-Based Materials and Deep Eutectic Solvents in Sustainable Sensing: A Comprehensive Review. Sensors 2024, 24, 2403. https://doi.org/10.3390/s24082403
Svigelj R, Toniolo R, Bertoni C, Fraleoni-Morgera A. Synergistic Applications of Graphene-Based Materials and Deep Eutectic Solvents in Sustainable Sensing: A Comprehensive Review. Sensors. 2024; 24(8):2403. https://doi.org/10.3390/s24082403
Chicago/Turabian StyleSvigelj, Rossella, Rosanna Toniolo, Cristina Bertoni, and Alessandro Fraleoni-Morgera. 2024. "Synergistic Applications of Graphene-Based Materials and Deep Eutectic Solvents in Sustainable Sensing: A Comprehensive Review" Sensors 24, no. 8: 2403. https://doi.org/10.3390/s24082403
APA StyleSvigelj, R., Toniolo, R., Bertoni, C., & Fraleoni-Morgera, A. (2024). Synergistic Applications of Graphene-Based Materials and Deep Eutectic Solvents in Sustainable Sensing: A Comprehensive Review. Sensors, 24(8), 2403. https://doi.org/10.3390/s24082403








