XMEA: A New Hybrid Diamond Multielectrode Array for the In Situ Assessment of the Radiation Dose Enhancement by Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
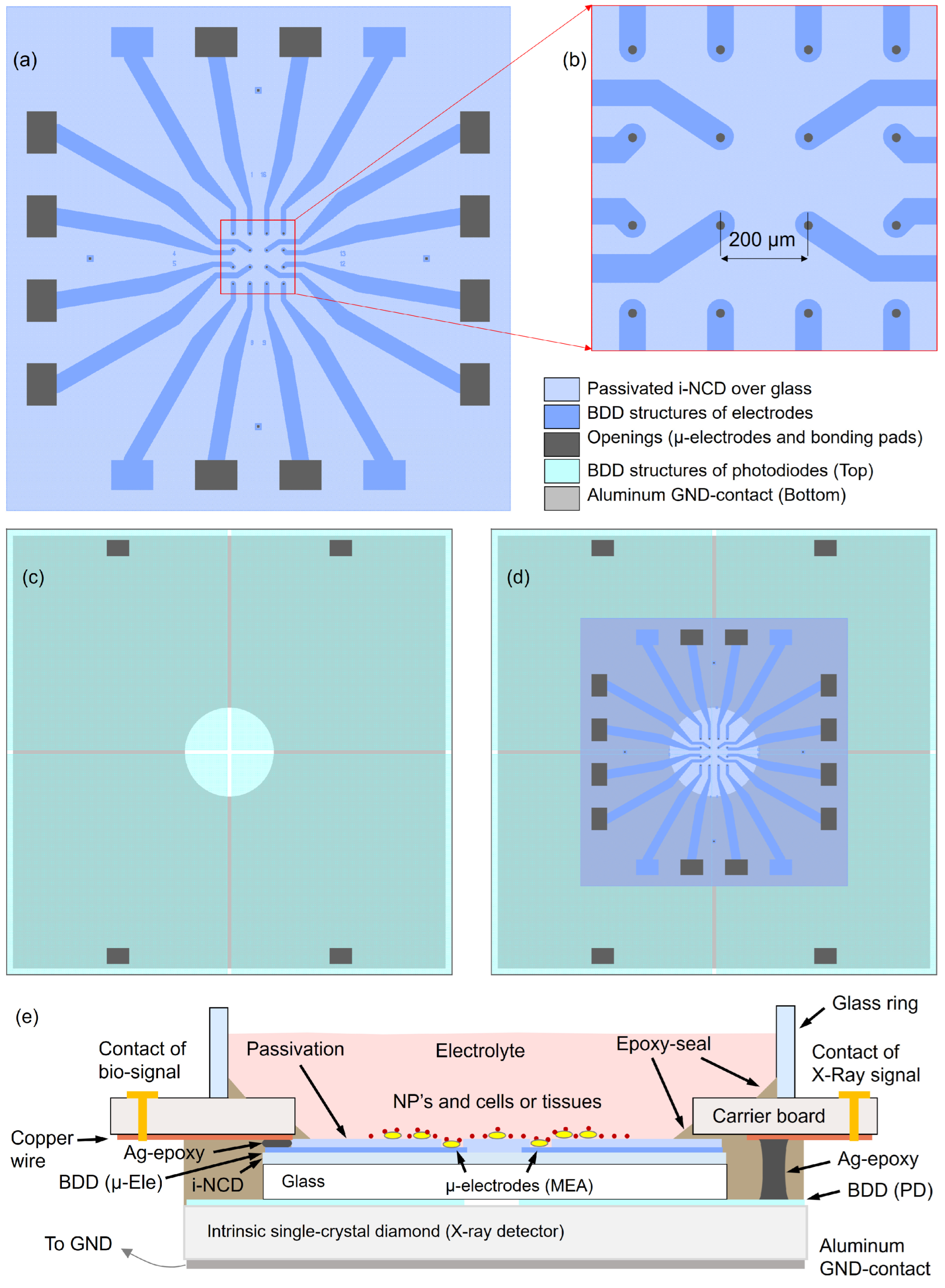
2.1. XMEA
2.2. Nanoparticles
2.3. Irradiations
2.4. Dose Enhancement Factor (DEF) and Response Enhancement Factor (REF)
3. Results
3.1. Characterization of the Nanoparticles
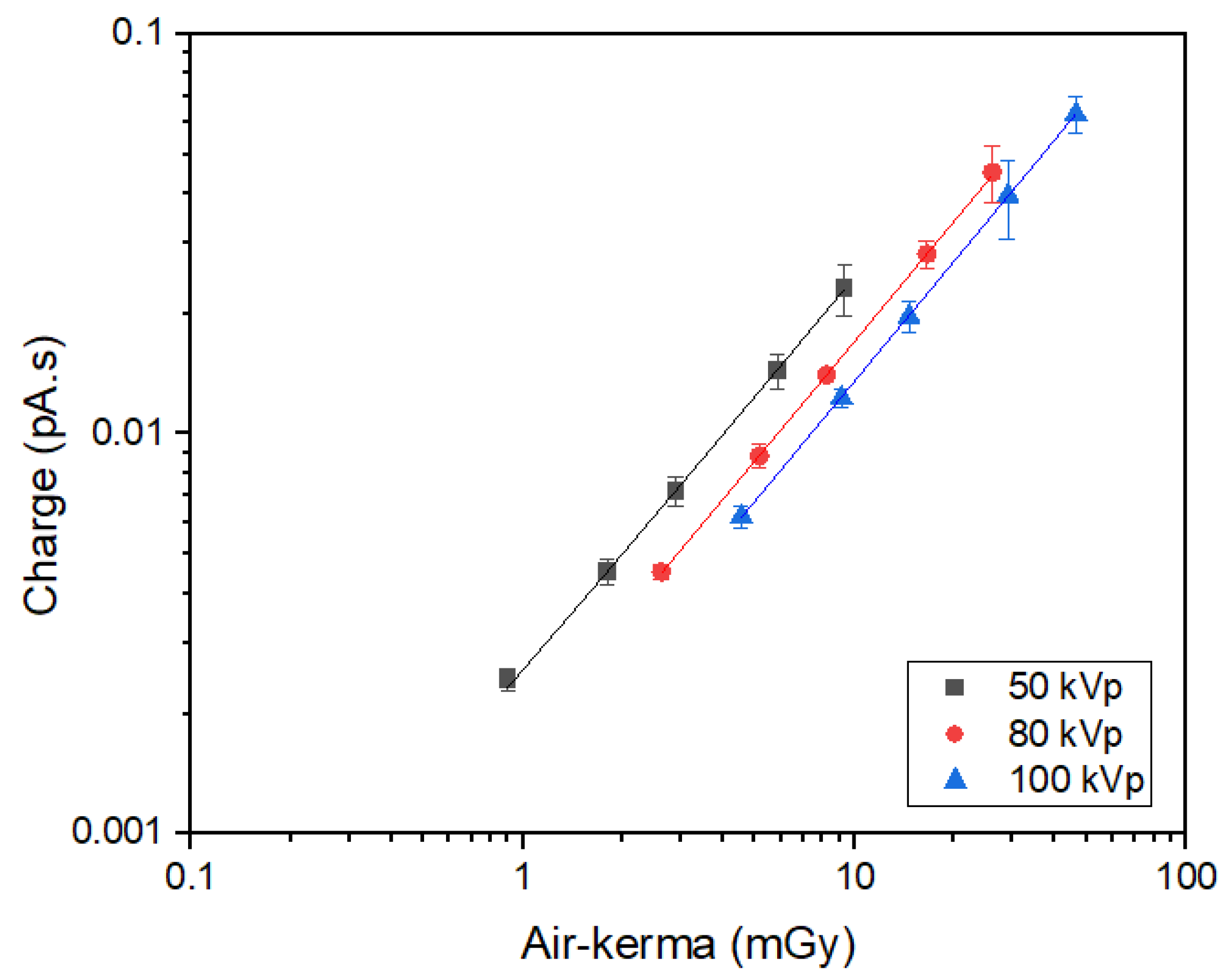
3.2. Response of the XMEA Detectors to X-rays
3.3. DEF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold nanoparticles as radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D.B.; Jelveh, S.; Jalali, F.; van Prooijen, M.; Allen, C.; Bristow, R.G.; Hill, R.P.; Jaffray, D.A. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Jones, B.L.; Krishnan, S. The dosimetric feasibility of gold nanoparticle-aided radiation therapy (gnrt) via brachytherapy using low-energy gamma-/x-ray sources. Phys. Med. Biol. 2009, 54, 4889–4905. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Krishnan, S. Dosimetric feasibility of gold nanoparticle-aided radiation therapy (gnrt) using a Yb-169 source. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, S639. [Google Scholar] [CrossRef]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02tr01. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaie, F.; Franich, R.; Feltis, B.; Geso, M. Oxidative damage to mitochondria enhanced by ionising radiation and gold nanoparticles in cancer cells. Int. J. Mol. Sci. 2022, 23, 6887. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Lo, C.Y.; Yu, S.Y.; Chen, F.H.; Huang, H.C.; Wang, L.K.; Liaw, J.W. Gold nanoparticles enhancing generation of ros for Cs-137 radiotherapy. Nanoscale Res. Lett. 2022, 17, 123. [Google Scholar] [CrossRef]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.D.; Bhattarai, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishnan, S. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1277–1283. [Google Scholar] [CrossRef]
- Abolaban, F.; Nisbet, A. Radiation dose enhancement in megavoltage radiation therapy using au, gd, pt, ag, and bi nanoparticles of various concentration level. Biointerface Res. Appl. Chem. 2022, 12, 2404–2414. [Google Scholar] [CrossRef]
- Lechtman, E.; Chattopadhyay, N.; Cai, Z.; Mashouf, S.; Reilly, R.; Pignol, J.P. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys. Med. Biol. 2011, 56, 4631–4647. [Google Scholar] [CrossRef] [PubMed]
- Rabus, H.; Li, W.B.; Villagrasa, C.; Schuemann, J.; Hepperle, P.A.; de la Fuente Rosales, L.; Beuve, M.; Di Maria, S.; Klapproth, A.P.; Li, C.Y.; et al. Intercomparison of monte carlo calculated dose enhancement ratios for gold nanoparticles irradiated by x-rays: Assessing the uncertainty and correct methodology for extended beams. Phys. Medica 2021, 84, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Sakata, D.; Kyriakou, I.; Tran, H.N.; Bordage, M.C.; Rosenfeld, A.; Ivanchenko, V.; Incerti, S.; Emfietzoglou, D.; Guatelli, S. Electron track structure simulations in a gold nanoparticle using geant4-DNA. Phys. Medica 2019, 63, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Vlastou, E.; Pantelis, E.; Efstathopoulos, E.P.; Karaiskos, P.; Kouloulias, V.; Platoni, K. Quantification of nanoscale dose enhancement in gold nanoparticle-aided external photon beam radiotherapy. Cancers 2022, 14, 2167. [Google Scholar] [CrossRef] [PubMed]
- Wälzlein, C.; Scifoni, E.; Krämer, M.; Durante, M. Simulations of dose enhancement for heavy atom nanoparticles irradiated by protons. Phys. Med. Biol. 2014, 59, 1441–1458. [Google Scholar] [CrossRef]
- Lima, I.S.; Guidelli, E.J.; Baffa, O. Dose enhancement factor caused by gold nanoparticles: Influence of the dosimetric sensitivity and radiation dose assessed by electron spin resonance dosimetry. Phys. Med. Biol. 2021, 66, 215013. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, T.; Guidelli, E.J.; Gómez, J.A.; Baffa, O.; Nicolucci, P. Experimental assessment of gold nanoparticle-mediated dose enhancement in radiation therapy beams using electron spin resonance dosimetry. Phys. Med. Biol. 2015, 60, 4465–4480. [Google Scholar] [CrossRef] [PubMed]
- Alyani Nezhad, Z.; Geraily, G.; Parwaie, W.; Hossein Nezhad, E. Evaluation of dose enhancement effect of bismuth oxide nanoparticles by means of magat and npag gel dosimeters. J. Radioanal. Nucl. Chem. 2022, 331, 1683–1689. [Google Scholar] [CrossRef]
- Dong, X.; Tian, Y.; Wang, F.; Chen, C.; Wang, Y.; Ma, J. Gold-nanoparticle-enhanced radio-fluorogenic hydrogel sensor for low radiation doses in clinical radiotherapy. Polymers 2022, 14, 4841. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, L.; Chen, H.; Hu, L. Recent advances in hydrogel-based sensors responding to ionizing radiation. Gels 2022, 8, 238. [Google Scholar] [CrossRef]
- Nakayama, M.; Akasaka, H.; Geso, M.; Morita, K.; Yada, R.; Uehara, K.; Sasaki, R. Utilisation of the chemiluminescence method to measure the radiation dose enhancement caused by gold nanoparticles: A phantom-based study. Radiat. Meas. 2020, 134, 106317. [Google Scholar] [CrossRef]
- Sem, D.; Lazenby, R.A. Electrochemical biosensor arrays for multiple analyte detection. Anal. Sens. 2023, 4, e202300047. [Google Scholar] [CrossRef]
- Yadav, N.; di Lisa, D.; Giacomozzi, F.; Cian, A.; Giubertoni, D.; Martinoia, S.; Lorenzelli, L. Development of multi-depth probing 3D microelectrode array to record electrophysiological activity within neural cultures. J. Micromech. Microeng. 2023, 33, 115002. [Google Scholar] [CrossRef]
- Lotfi, S.; Veisi, H.; Karmakar, B. A convenient strategy for the electrochemical evaluation of acetaminophen and caffeine in combined drugs and biological samples over TiO2@polymethyldopa/Pd nanocomposite functionalized glassy carbon electrodes. Measurement 2021, 186, 110156. [Google Scholar] [CrossRef]
- Falone, M.F.; Salamanca-Neto, C.A.R.; Moraes, J.T.; Sartori, E.R. Electrochemical evaluation and voltametric determination of laxative drug bisacodyl on boron-doped diamond electrode. Measurement 2019, 137, 464–469. [Google Scholar] [CrossRef]
- Mescola, A.; Canale, C.; Prtao, M.; Diaspro, A.; Berdondini, L.; Maccione, A.; Dante, S. Specific neuron placement on gold and silicon nitride-patterned substrates through a two-step functionalization method. Langmuir 2016, 32, 6319–6327. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, Y.; Li, H.; Xuan, X.; Xu, S.; Li, M. Flexible boron-doped diamond spiral electrode for application in brain-computer interface based on steady-state visual evoked potential. Measurement 2023, 211, 112673. [Google Scholar] [CrossRef]
- Kraft, A. Doped diamond: A compact review on a new, versatile electrode material. Int. J. Electrochem. Sci. 2007, 2, 355–385. [Google Scholar] [CrossRef]
- Almaviva, S.; Marinelli, M.; Milani, E.; Prestopino, G.; Tucciarone, A.; Verona, C.; Verona-Rinati, G.; Angelone, M.; Pillon, M. Extreme uv photodetectors based on cvd single crystal diamond in a p-type/intrinsic/metal configuration. Diam. Relat. Mater. 2009, 18, 101–105. [Google Scholar] [CrossRef]
- Almaviva, S.; Marinelli, M.; Milani, E.; Prestopino, G.; Tucciarone, A.; Verona, C.; Verona-Rinati, G.; Angelone, M.; Pillon, M.; Dolbnya, I.; et al. Chemical vapor deposition diamond based multilayered radiation detector: Physical analysis of detection properties. J. Appl. Phys. 2010, 107, 014511. [Google Scholar] [CrossRef]
- Almaviva, S.; Marinelli, M.; Milani, E.; Tucciarone, A.; Verona-Rinati, G.; Consorti, R.; Petrucci, A.; De Notaristefani, F.; Ciancaglioni, I. Synthetic single crystal diamond diodes for radiotherapy dosimetry. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2008, 594, 273–277. [Google Scholar] [CrossRef]
- Bohon, J.; Muller, E.; Smedley, J. Development of diamond-based x-ray detection for high-flux beamline diagnostics. J. Synchrotron Radiat. 2010, 17, 711–718. [Google Scholar] [CrossRef]
- Girolami, M.; Allegrini, P.; Conte, G.; Trucchi, D.M.; Ralchenko, V.G.; Salvatori, S. Diamond detectors for uv and X-ray source imaging. IEEE Electron Device Lett. 2012, 33, 224–226. [Google Scholar] [CrossRef]
- Liu, J.; Chang, J.; Zhang, J.; Zhong, G.; Liu, X.; Pang, X.; Jia, J. Design, fabrication and testing of cvd diamond detectors with high performance. AIP Adv. 2019, 9, 045205. [Google Scholar] [CrossRef]
- Liu, L.; Ouyang, X.; Zhang, J.; Zhang, X.; Zhong, Y. Polycrystalline cvd diamond detector: Fast response and high sensitivity with large area. AIP Adv. 2014, 4, 017114. [Google Scholar] [CrossRef]
- Schirru, F.; Kisielewicz, K.; Nowak, T.; Marczewska, B. Single crystal diamond detector for radiotherapy. J. Phys. D Appl. Phys. 2010, 43, 265101. [Google Scholar] [CrossRef]
- Agosteo, S. Detectors for measurement of microdosimetric quantities. Radiat. Meas. 2022, 156, 106807. [Google Scholar] [CrossRef]
- Rosenfeld, A.B.; Biasi, G.; Petasecca, M.; Lerch, M.L.F.; Villani, G.; Feygelman, V. Semiconductor dosimetry in modern external-beam radiation therapy. Phys. Med. Biol. 2020, 65, 16tr01. [Google Scholar] [CrossRef] [PubMed]
- Solevi, P.; Magrin, G.; Moro, D.; Mayer, R. Monte carlo study of microdosimetric diamond detectors. Phys. Med. Biol. 2015, 60, 7069–7083. [Google Scholar] [CrossRef]
- Carabelli, V.; Gosso, S.; Marcantoni, A.; Xu, Y.; Colombo, E.; Gao, Z.; Vittone, E.; Kohn, E.; Pasquarelli, A.; Carbone, E. Nanocrystalline diamond microelectrode arrays fabricated on sapphire technology for high-time resolution of quantal catecholamine secretion from chromaffin cells. Biosens. Bioelectron. 2010, 26, 92–98. [Google Scholar] [CrossRef]
- Picollo, F.; Tomagra, G.; Bonino, V.; Carabelli, V.; Mino, L.; Olivero, P.; Pasquarelli, A.; Truccato, M. Triggering neurotransmitters secretion from single cells by X-ray nanobeam irradiation. Nano Lett. 2020, 20, 3889–3894. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhang, W.; Zhang, H.; Chen, Y.; Rehman, F.; Jiang, H.; Strehle, S.; Pasquarelli, A.; Wang, X.D. Highly sensitive electrochemical detection of living cells based on diamond microelectrode arrays. Chin. Chem. Lett. 2017, 29, 919–921. [Google Scholar] [CrossRef]
- Pasquarelli, A.; Marcantoni, A.; Gavello, D.; Battiato, A.; Picollo, F.; Olivero, P.; Carbone, E.; Carabelli, V. Simultaneous fluorescent and amperometric detection of catecholamine release from neuroendocrine cells with transparent diamond meas. Front. Neurosci. 2016. [Google Scholar] [CrossRef]
- Gosso, S.; Marcantoni, A.; Vandael, D.; Turturici, M.; Alsawafi, S.; Izadi, I.; Colombo, E.; Pasquarelli, A.; Carbone, E.; Carabelli, V. Multi-purpose nanocrystalline boron-doped diamond meas for amperometric, potentiometric and ph recordings from excitable cells. In Proceedings of the 8th International Meeting on Substrate-Integrated Microelectrode Arrays, Reutlingen, Germany, 10–13 July 2012; pp. 323–324. [Google Scholar]
- González Brito, R.; Montenegro, P.; Méndez, A.; Carabelli, V.; Tomagra, G.; Shabgahi, R.E.; Pasquarelli, A.; Borges, R. Multielectrode arrays as a means to study exocytosis in human platelets. Biosensors 2023, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Stehl, C.; Fischer, M.; Gsell, S.; Berdermann, E.; Rahman, M.S.; Traeger, M.; Klein, O.; Schreck, M. Efficiency of dislocation density reduction during heteroepitaxial growth of diamond for detector applications. Appl. Phys. Lett. 2013, 103, 151905. [Google Scholar] [CrossRef]
- Isberg, J.; Hammersberg, J.; Johansson, E.; Wikström, T.; Twitchen, D.J.; Whitehead, A.J.; Coe, S.E.; Scarsbrook, G.A. High carrier mobility in single-crystal plasma-deposited diamond. Science 2002, 297, 1670–1672. [Google Scholar] [CrossRef] [PubMed]
- Berdermann, E.; Afanaciev, K.; Ciobanu, M.; Fischer, M.; Gsell, S.; Kiš, M.; Lagomarsino, S.; Lohmann, W.; Mayr, M.; Pomorski, M.; et al. Progress in detector properties of heteroepitaxial diamond grown by chemical vapor deposition on ir/ysz/si(001) wafers. Diam. Relat. Mater. 2019, 97, 107420. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Ramos, A.P.; Zaniquelli, M.E.; Nicolucci, P.; Baffa, O. Synthesis and characterization of silver/alanine nanocomposites for radiation detection in medical applications: The influence of particle size on the detection properties. Nanoscale 2012, 4, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Guidelli, E.J.; Baffa, O. Influence of photon beam energy on the dose enhancement factor caused by gold and silver nanoparticles: An experimental approach. Med. Phys. 2014, 41, 032101. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, H.; Bazuin, C.G.; Peng, W.; Masson, J.-F. Polymer-templated gold nanoparticles on optical fibers for enhanced-sensitivity localized surface plasmon resonance biosensors. ACS Sens. 2019, 4, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Ganjeh, Z.; Salehi, Z. Monte carlo study of nanoparticles effectiveness on the dose enhancement when irradiated by protons. AIP Adv. 2023, 13, 035018. [Google Scholar] [CrossRef]
- Martelli, S.; Chow, J.C.L. Dose enhancement for the flattening-filter-free and flattening-filter photon beams in nanoparticle-enhanced radiotherapy: A monte carlo phantom study. Nanomaterials 2020, 10, 637. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, B.; Zaker, Y.; Atnagulov, A.; Yoon, B.; Landman, U.; Bigioni, T.P. Chemistry and structure of silver molecular nanoparticles. Acc. Chem. Res. 2018, 51, 3104–3113. [Google Scholar] [CrossRef]
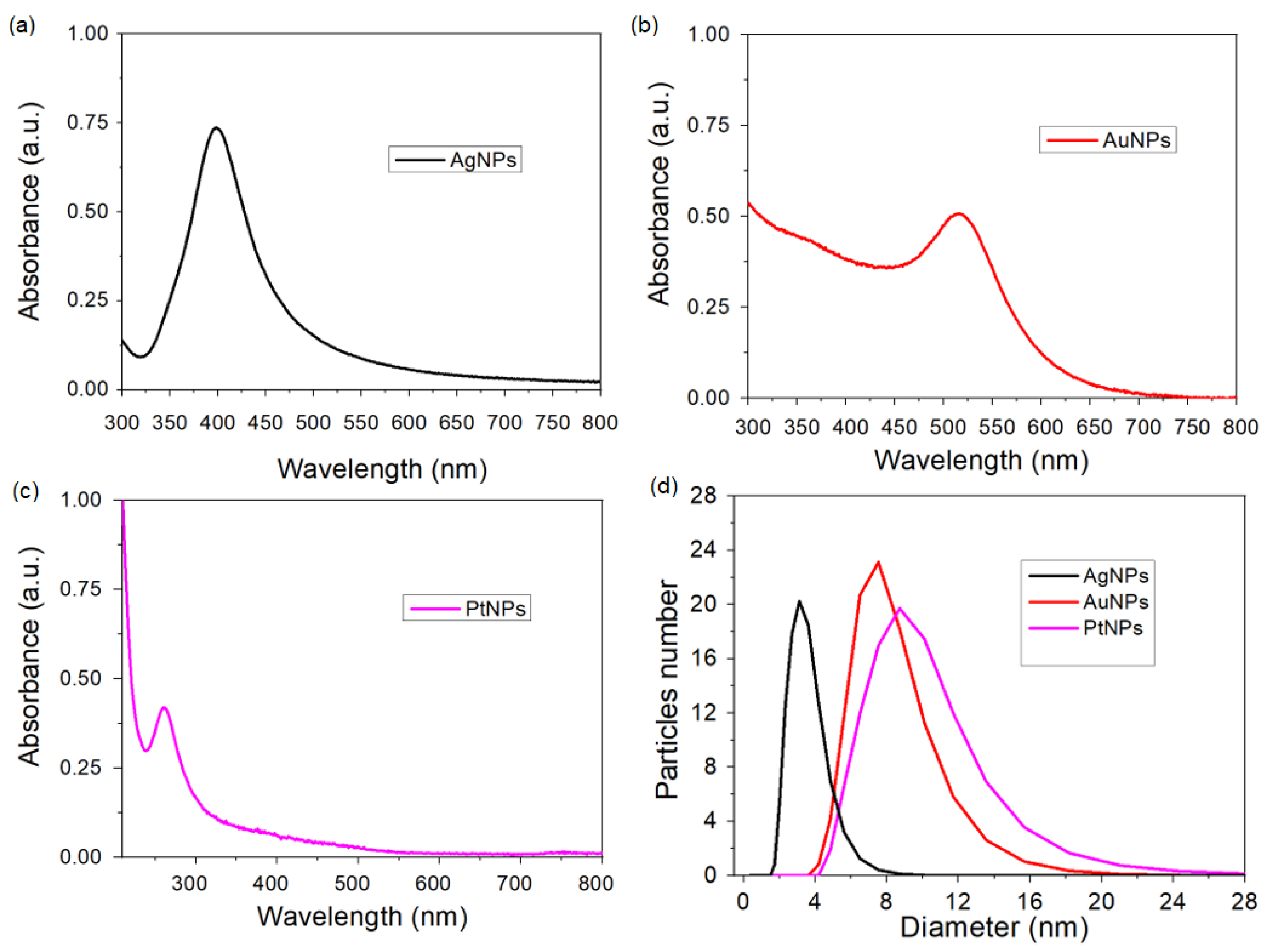




| Material | Concentration (µg/mL) | Molar Concentration (mol/mL) | Mass Percentage (% w/w) |
|---|---|---|---|
| Ag | 5 | 4.63 × 10−8 | 0.0005 |
| 10 | 9.27 × 10−8 | 0.001 | |
| 20 | 1.85 × 10−7 | 0.002 | |
| 40 | 3.71 × 10−7 | 0.004 | |
| 60 | 5.56 × 10−7 | 0.006 | |
| 100 | 9.27 × 10−7 | 0.01 | |
| 200 | 1.85 × 10−6 | 0.02 | |
| 1000 | 9.27 × 10−6 | 0.1 | |
| Pt | 5 | 2.56 × 10−8 | 0.0005 |
| 10 | 5.13 × 10−8 | 0.001 | |
| 20 | 1.03 × 10−7 | 0.002 | |
| 40 | 2.05 × 10−7 | 0.004 | |
| 60 | 3.08 × 10−7 | 0.006 | |
| 100 | 5.13 × 10−7 | 0.01 | |
| 200 | 1.03 × 10−6 | 0.02 | |
| 1000 | 5.13 × 10−6 | 0.1 | |
| Au | 5 | 2.54 × 10−8 | 0.0005 |
| 10 | 5.08 × 10−8 | 0.001 | |
| 20 | 1.02 × 10−7 | 0.002 | |
| 40 | 2.03 × 10−7 | 0.004 | |
| 60 | 3.05 × 10−7 | 0.006 | |
| 100 | 5.08 × 10−7 | 0.01 | |
| 200 | 1.02 × 10−6 | 0.02 | |
| 1000 | 5.08 × 10−6 | 0.1 |
| 50 kVp | 80 kVp | 100 kVp | |
|---|---|---|---|
| Sensitivity (pA/mGy) ×10−4 | |||
| 24.6 ± 0.14 | 17.0 ± 0.09 | 13.5 ± 0.03 |
| Material | Concentration (µg/mL) | DEF |
|---|---|---|
| Ag | 5 | 0.919 ± 0.074 |
| 10 | 0.918 ± 0.086 | |
| 20 | 0.906 ± 0.082 | |
| 40 | 0.897 ± 0.108 | |
| 60 | 0.862 ± 0.126 | |
| 100 | 0.923 ± 0.081 | |
| 200 | 0.932 ± 0.058 | |
| 1000 | 0.631 ± 0.297 | |
| Pt | 5 | 1.907 ± 0.741 |
| 10 | 1.518 ± 0.440 | |
| 20 | 1.486 ± 0.393 | |
| 40 | 1.419 ± 0.306 | |
| 60 | 1.423 ± 0.320 | |
| 100 | 1.406 ± 0.275 | |
| 200 | 1.401 ± 0.330 | |
| 1000 | 1.788 ± 0.605 | |
| Au | 5 | 1.256 ± 0.039 |
| 10 | 1.896 ± 0.103 | |
| 20 | 2.054 ± 0.129 | |
| 40 | 2.881 ± 0.183 | |
| 60 | 3.013 ± 0.304 | |
| 100 | 3.067 ± 0.315 | |
| 200 | 3.068 ± 0.316 | |
| 1000 | 3.068 ± 0.316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolucci, P.; Gambaro, G.; Araujo Silva, K.M.; Souza Lima, I.; Baffa, O.; Pasquarelli, A. XMEA: A New Hybrid Diamond Multielectrode Array for the In Situ Assessment of the Radiation Dose Enhancement by Nanoparticles. Sensors 2024, 24, 2409. https://doi.org/10.3390/s24082409
Nicolucci P, Gambaro G, Araujo Silva KM, Souza Lima I, Baffa O, Pasquarelli A. XMEA: A New Hybrid Diamond Multielectrode Array for the In Situ Assessment of the Radiation Dose Enhancement by Nanoparticles. Sensors. 2024; 24(8):2409. https://doi.org/10.3390/s24082409
Chicago/Turabian StyleNicolucci, Patricia, Guilherme Gambaro, Kyssylla Monnyelle Araujo Silva, Iara Souza Lima, Oswaldo Baffa, and Alberto Pasquarelli. 2024. "XMEA: A New Hybrid Diamond Multielectrode Array for the In Situ Assessment of the Radiation Dose Enhancement by Nanoparticles" Sensors 24, no. 8: 2409. https://doi.org/10.3390/s24082409






