Experimental Study on SPR Array Sensing Chip Integrated with Microvalves
Abstract
1. Introduction
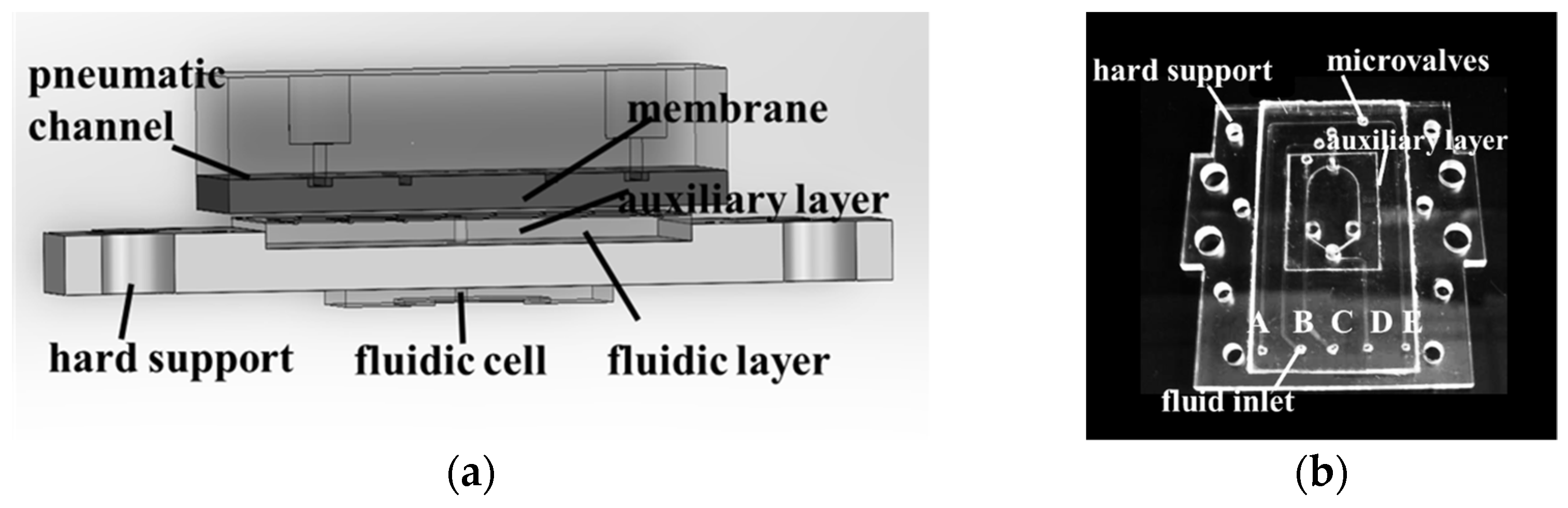
2. Design and Fabrication of Microfluidic Systems
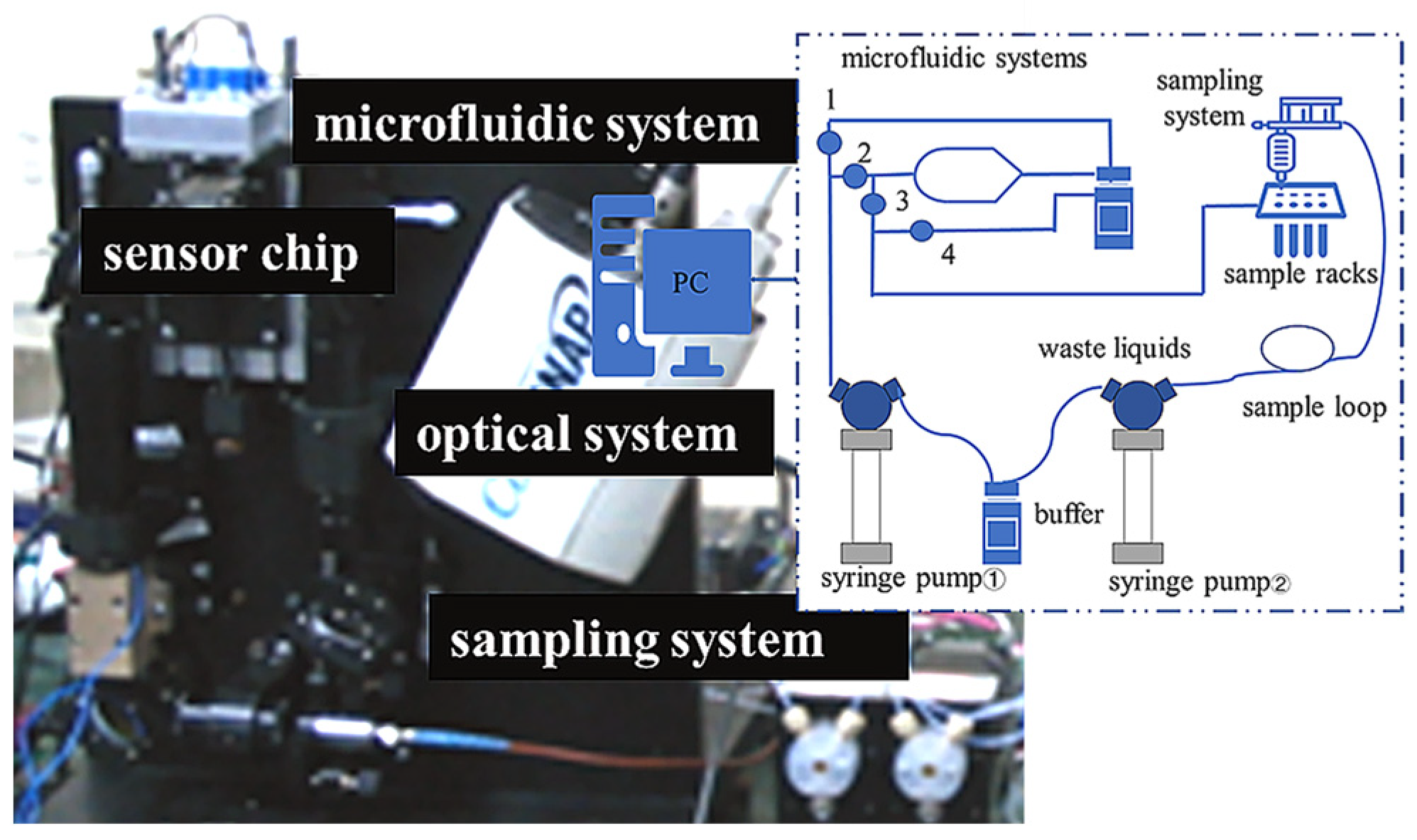
2.1. Structure of the SPR Array Detection System
2.2. Design of Microvalves
2.2.1. Materials for Microfluidic Systems
2.2.2. Microvalve Structure Design
- (1)
- Within one experimental cycle, solution changeover can be accomplished by simply alternating the switching of Valves 1 and 3 and 2 and 4. By controlling the actuation pressure via a computer, simple and reliable sampling automation can be achieved.
- (2)
- The mixing path of the buffer and sample solutions is just a short microchannel before entering the microfluidic pool, thereby preventing the mixing of sample solutions with the buffer before they enter the microfluidic pool and ensuring the accurate measurement of the kinetic constant.
2.3. Microfluidic System Assembly Procedure
- (1)
- Two pieces of PMMA are sealed with AR pressure-sensitive adhesive to form the air channel and then set aside for 18 h before use;
- (2)
- Using the oxygen plasma bonding method, the PDMS membrane on the Plexiglass plate is bonded with the auxiliary layer using oxygen plasma, and then demolded from the Plexiglass plate;
- (3)
- Using a punch, holes are created corresponding to the auxiliary layer’s inlet on the membrane;
- (4)
- Similar to Step 2, the other side of the membrane is bonded to the fluidic layer using oxygen plasma, but the microvalve positions need to be aligned during bonding;
- (5)
- After plasma treating the other side of the auxiliary layer, AR double-sided adhesive is applied. Then, the air channel layer and the other side of the double-sided adhesive undergo oxygen plasma bonding and are set aside for 18 h;
- (6)
- A PEEK compression block is pressed directly onto the auxiliary layer of PDMS substrate. After aligning their inlet holes, the PEEK compression block is fixed to the PMMA support with screw connections, resulting in a complete microfluidic system.
3. Microvalve Performance Testing
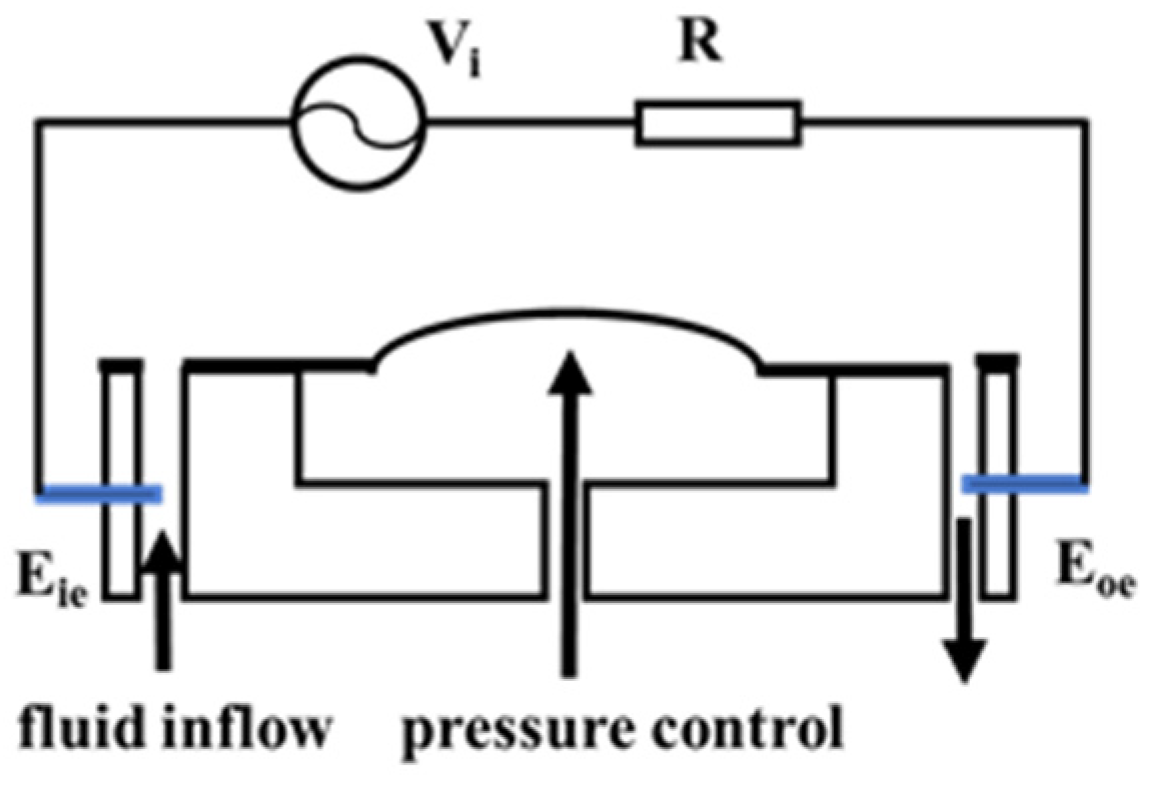
3.1. Principle of Conductivity Detection
3.2. Conductivity Measurement System
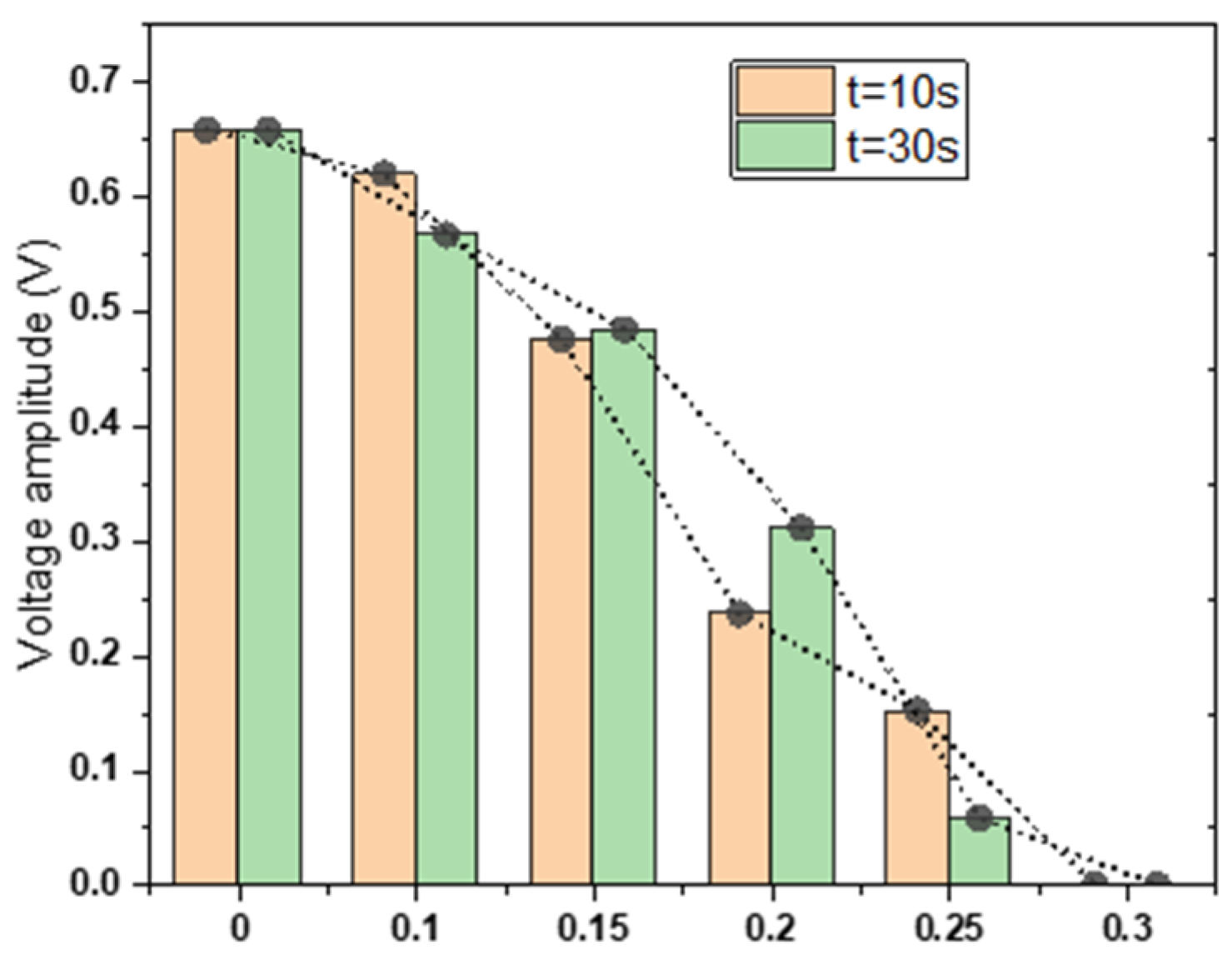
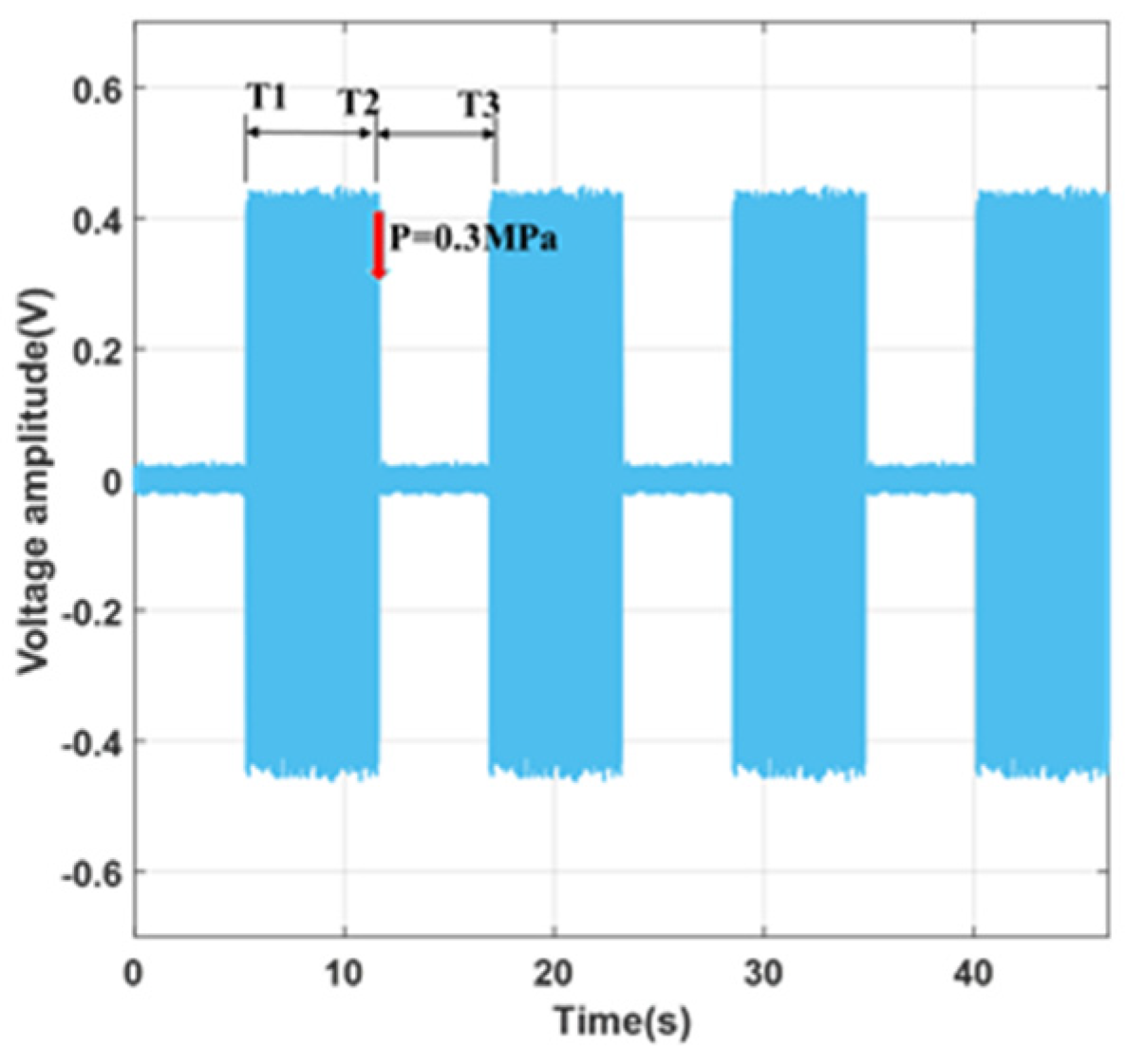
3.3. Microvalve Characteristic Testing Experiment
4. Microfluidic System Application in SPR Array Sensing
4.1. Pre-Experiment for Cleaning the Tubing
4.2. SPR Array Detection Experiments
4.2.1. Automatic Sampling Experiments and Results
- (1)
- Deionized water is injected for 60 s to establish the baseline. This step is crucial for SPR experiments as it provides a reference point against which changes in the refractive index can be measured;
- (2)
- The microvalve is opened to introduce the NaCl solution for 240 s. This longer duration ensures that the NaCl solution has sufficient time to interact with the sensing area, facilitating stable refractive index measurements;
- (3)
- Deionized water is reintroduced to flush the system and conclude the experiment. This step helps to return the system to its initial state and verifies the reversibility of the interaction on the sensing surface.
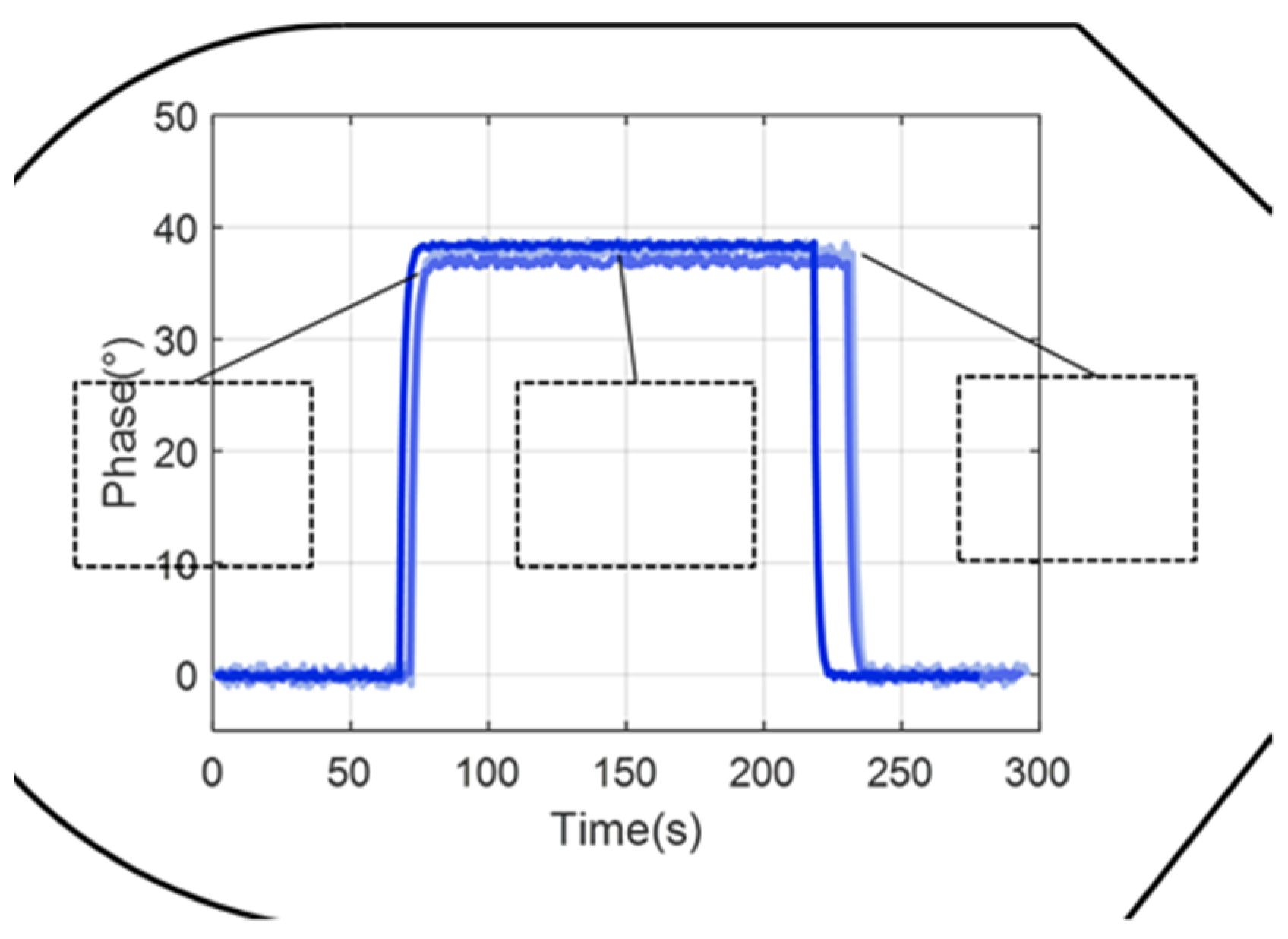
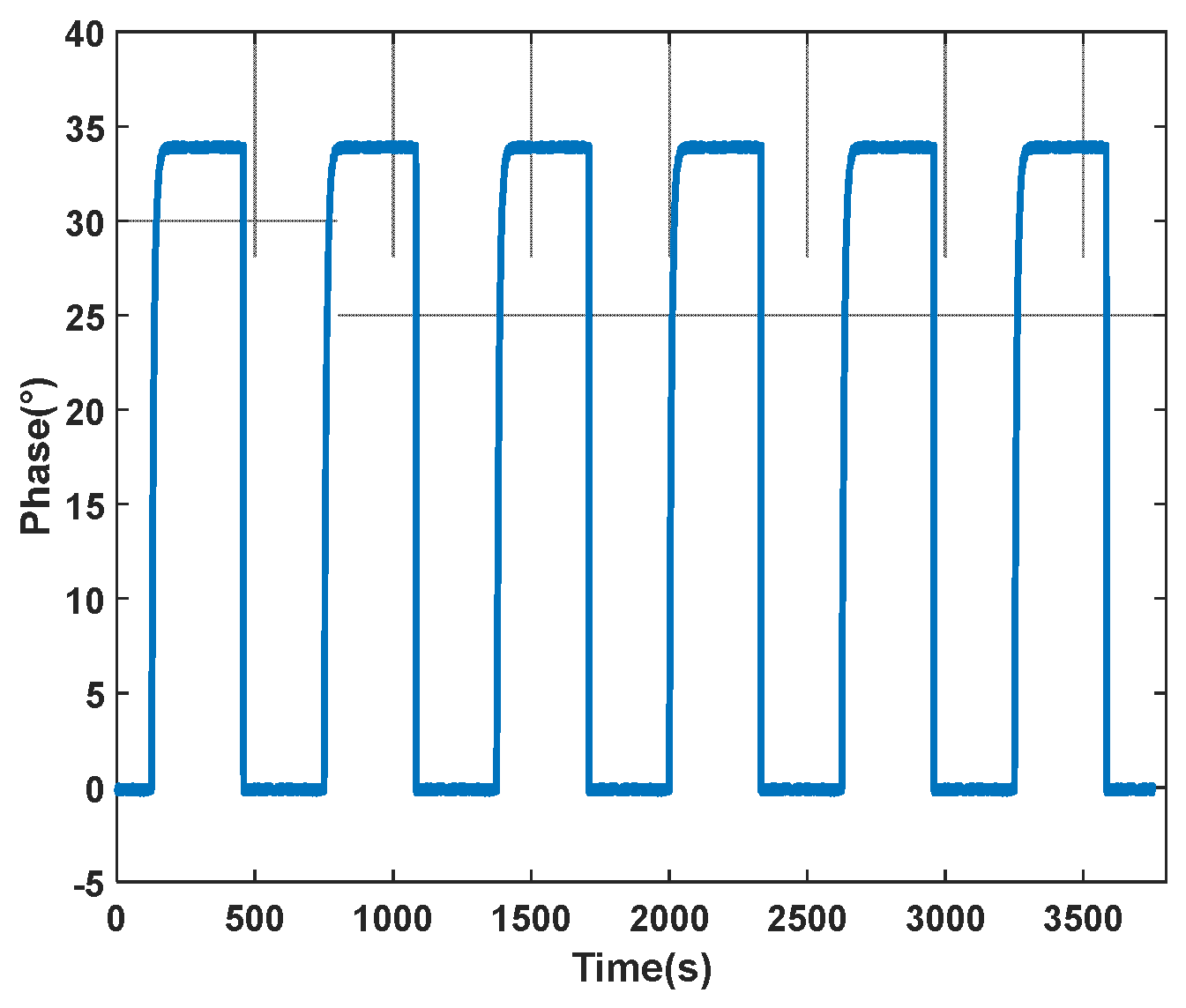
4.2.2. Analysis and Results of Microvalve Repeatability Experiments
5. Conclusions
- (1)
- The closing status of the microvalve can be influenced by the control pressure; the microvalve can achieve complete closure at 0.3 MPa with reliable reproducibility;
- (2)
- The microvalve detection method is capable of characterizing both individual and multiple microvalves simultaneously and demonstrating effective parallel detection;
- (3)
- In SPR array sensing tasks, microvalves enable the automation of flow direction and injection processes;
- (4)
- This detection method is versatile, suitable for a range of microfluidic systems equipped with integrated microvalves.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tyers, M.; Mann, M. From Genomics to Proteomics. Nature 2003, 422, 193–197. [Google Scholar] [CrossRef]
- Blow, N. Proteins and Proteomics: Life on the Surface. Nat. Methods 2009, 6, 389–393. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Shaegh, S.A.M.; Wang, Z.; Ng, S.H.; Wu, R.; Nguyen, H.T.; Chan, L.C.Z.; Toh, A.G.G.; Wang, Z. Plug-and-Play Microvalve and Micropump for Rapid Integration with Microfluidic Chips. Microfluid. Nanofluid. 2015, 19, 557–564. [Google Scholar] [CrossRef]
- Fu, H.; Liu, X.; Li, S. Mixing Indexes Considering the Combination of Mean and Dispersion Information from Intensity Images for the Performance Estimation of Micromixing. RSC Adv. 2017, 7, 10906–10914. [Google Scholar] [CrossRef]
- Hess, J.F.; Zehnle, S.; Juelg, P.; Hutzenlaub, T.; Zengerle, R.; Paust, N. Review on Pneumatic Operations in Centrifugal Microfluidics. Lab Chip 2019, 19, 3745–3770. [Google Scholar] [CrossRef]
- Xiang, J.; Cai, Z.; Zhang, Y.; Wang, W. Wedge Actuated Normally-Open and Normally-Closed Valves for Centrifugal Microfluidic Applications. Sens. Actuators B Chem. 2017, 243, 542–548. [Google Scholar] [CrossRef]
- Chen, S.; Lu, S.; Liu, Y.; Wang, J.; Tian, X.; Liu, G.; Yang, Z. A Normally-Closed Piezoelectric Micro-Valve with Flexible Stopper. AIP Adv. 2016, 6, 045112. [Google Scholar] [CrossRef]
- Nam, S.-W.; Noort, D.V.; Yang, Y.; Park, S. A Biological Sensor Platform Using a Pneumatic-Valve Controlled Microfluidic Device Containing Tetrahymena Pyriformis. Lab Chip 2007, 7, 638. [Google Scholar] [CrossRef]
- Bae, B.; Han, J.; Masel, R.I.; Shannon, M.A. A Bidirectional Electrostatic Microvalve With Microsecond Switching Performance. J. Microelectromech. Syst. 2007, 16, 1461–1471. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Hou, C.-W.; Li, X.-J.; Jin, Z.-J. Actuation Mechanism of Microvalves: A Review. Micromachines 2020, 11, 172. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic Microfabricated Valves and Pumps by Multilayer Soft Lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic Large-Scale Integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef]
- Bates, S.R.; Quake, S.R. Highly Parallel Measurements of Interaction Kinetic Constants with a Microfabricated Optomechanical Device. Appl. Phys. Lett. 2009, 95, 073705. [Google Scholar] [CrossRef]
- Araci, I.E.; Pop, P.; Chakrabarty, K. Microfluidic Very Large-Scale Integration for Biochips: Technology, Testing and Fault-Tolerant Design. In Proceedings of the 2015 20th IEEE European Test Symposium (ETS), Cluj-Napoca, Romania, 25–29 May 2015; IEEE: New York, NY, USA, 2015; pp. 1–8. [Google Scholar]
- Lee, C.-C.; Sui, G.; Elizarov, A.; Shu, C.J.; Shin, Y.-S.; Dooley, A.N.; Huang, J.; Daridon, A.; Wyatt, P.; Stout, D.; et al. Multistep Synthesis of a Radiolabeled Imaging Probe Using Integrated Microfluidics. Science 2005, 310, 1793–1796. [Google Scholar] [CrossRef]
- El Hasni, A.; Pfirrmann, S.; Kolander, A.; Yacoub-George, E.; König, M.; Landesberger, C.; Voigt, A.; Grützner, G.; Schnakenberg, U. Six-Layer Lamination of a New Dry Film Negative-Tone Photoresist for Fabricating Complex 3D Microfluidic Devices. Microfluid. Nanofluid. 2017, 21, 41. [Google Scholar] [CrossRef]
- Mukherjee, P.; Nebuloni, F.; Gao, H.; Zhou, J.; Papautsky, I. Rapid Prototyping of Soft Lithography Masters for Microfluidic Devices Using Dry Film Photoresist in a Non-Cleanroom Setting. Micromachines 2019, 10, 192. [Google Scholar] [CrossRef]
- Thangawng, A.L.; Ruoff, R.S.; Swartz, M.A.; Glucksberg, M.R. An Ultra-Thin PDMS Membrane as a Bio/Micro–Nano Interface: Fabrication and Characterization. Biomed. Microdevices 2007, 9, 587–595. [Google Scholar] [CrossRef]
- Grover, W.H.; Skelley, A.M.; Liu, C.N.; Lagally, E.T.; Mathies, R.A. Monolithic Membrane Valves and Diaphragm Pumps for Practical Large-Scale Integration into Glass Microfluidic Devices. Sens. Actuators B Chem. 2003, 89, 315–323. [Google Scholar] [CrossRef]
- Jensen, E.C.; Grover, W.H.; Mathies, R.A. Micropneumatic Digital Logic Structures for Integrated Microdevice Computation and Control. J. Microelectromech. Syst. 2007, 16, 1378–1385. [Google Scholar] [CrossRef]
- Berlanda, S.F.; Breitfeld, M.; Dietsche, C.L.; Dittrich, P.S. Recent Advances in Microfluidic Technology for Bioanalysis and Diagnostics. Anal. Chem. 2021, 93, 311–331. [Google Scholar] [CrossRef]
- Li, H.Q.; Roberts, D.C.; Steyn, J.L.; Turner, K.T.; Yaglioglu, O.; Hagood, N.W.; Spearing, S.M.; Schmidt, M.A. Fabrication of a High Frequency Piezoelectric Microvalve. Sens. Actuators A Phys. 2004, 111, 51–56. [Google Scholar] [CrossRef]
- Studer, V.; Hang, G.; Pandolfi, A.; Ortiz, M.; French Anderson, W.; Quake, S.R. Scaling Properties of a Low-Actuation Pressure Microfluidic Valve. J. Appl. Phys. 2004, 95, 393–398. [Google Scholar] [CrossRef]
- Galas, J.-C.; Studer, V.; Chen, Y. Characterization of Pneumatically Activated Microvalves by Measuring Electrical Conductance. Microelectron. Eng. 2005, 78–79, 112–117. [Google Scholar] [CrossRef]

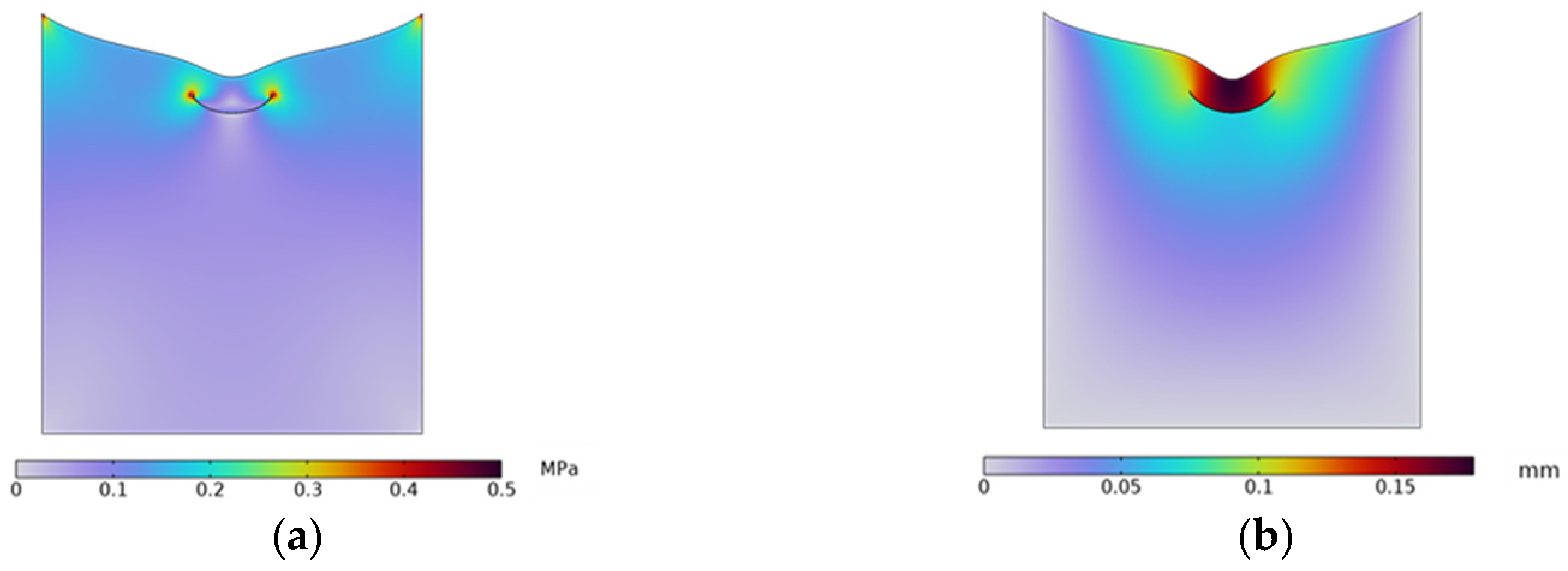



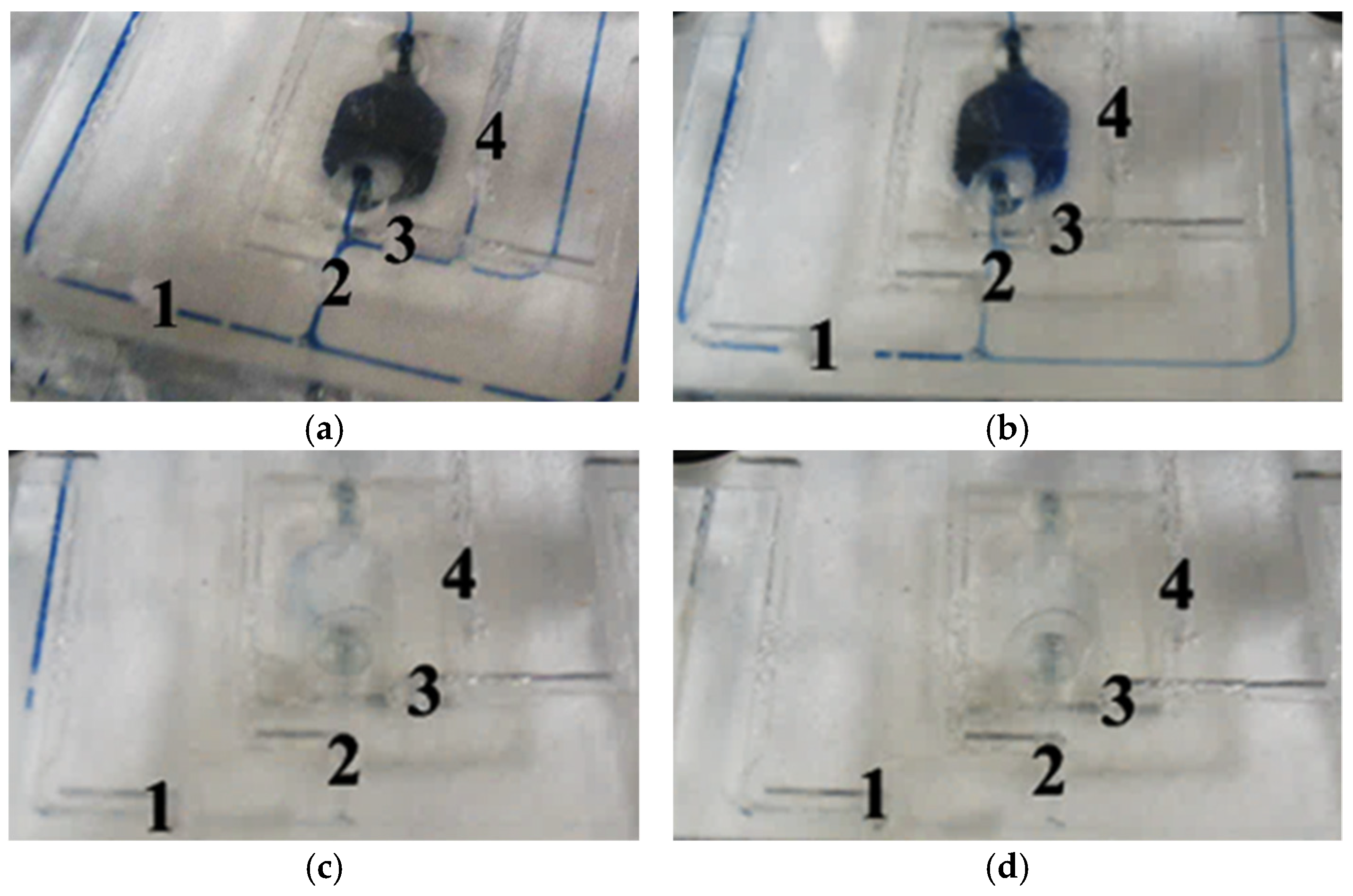
| Cross-Sectional Shape | NO.1 | NO.2 | NO.3 | NO.4 |
|---|---|---|---|---|
| Cross-sectional area (mm2) | 0.0157 | 0.0344 | 0.0217 | 0.0257 |
| Maximum required pressure (MPa) | 0.35 | 0.2 | 0.25 | 0.42 |
| Maximum shear stress at connection (MPa) | 0.46 | 0.23 | 0.32 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Wang, P.; Li, B. Experimental Study on SPR Array Sensing Chip Integrated with Microvalves. Sensors 2024, 24, 2540. https://doi.org/10.3390/s24082540
Chen W, Wang P, Li B. Experimental Study on SPR Array Sensing Chip Integrated with Microvalves. Sensors. 2024; 24(8):2540. https://doi.org/10.3390/s24082540
Chicago/Turabian StyleChen, Wanwan, Peng Wang, and Bin Li. 2024. "Experimental Study on SPR Array Sensing Chip Integrated with Microvalves" Sensors 24, no. 8: 2540. https://doi.org/10.3390/s24082540
APA StyleChen, W., Wang, P., & Li, B. (2024). Experimental Study on SPR Array Sensing Chip Integrated with Microvalves. Sensors, 24(8), 2540. https://doi.org/10.3390/s24082540





