High-Sensitivity MXene-Functionalized Photonic Crystal Fiber Surface Plasmon Resonance Sensor with Dual Rectangular Grooves for Cancer Detection
Abstract
1. Introduction
2. Materials and Methods
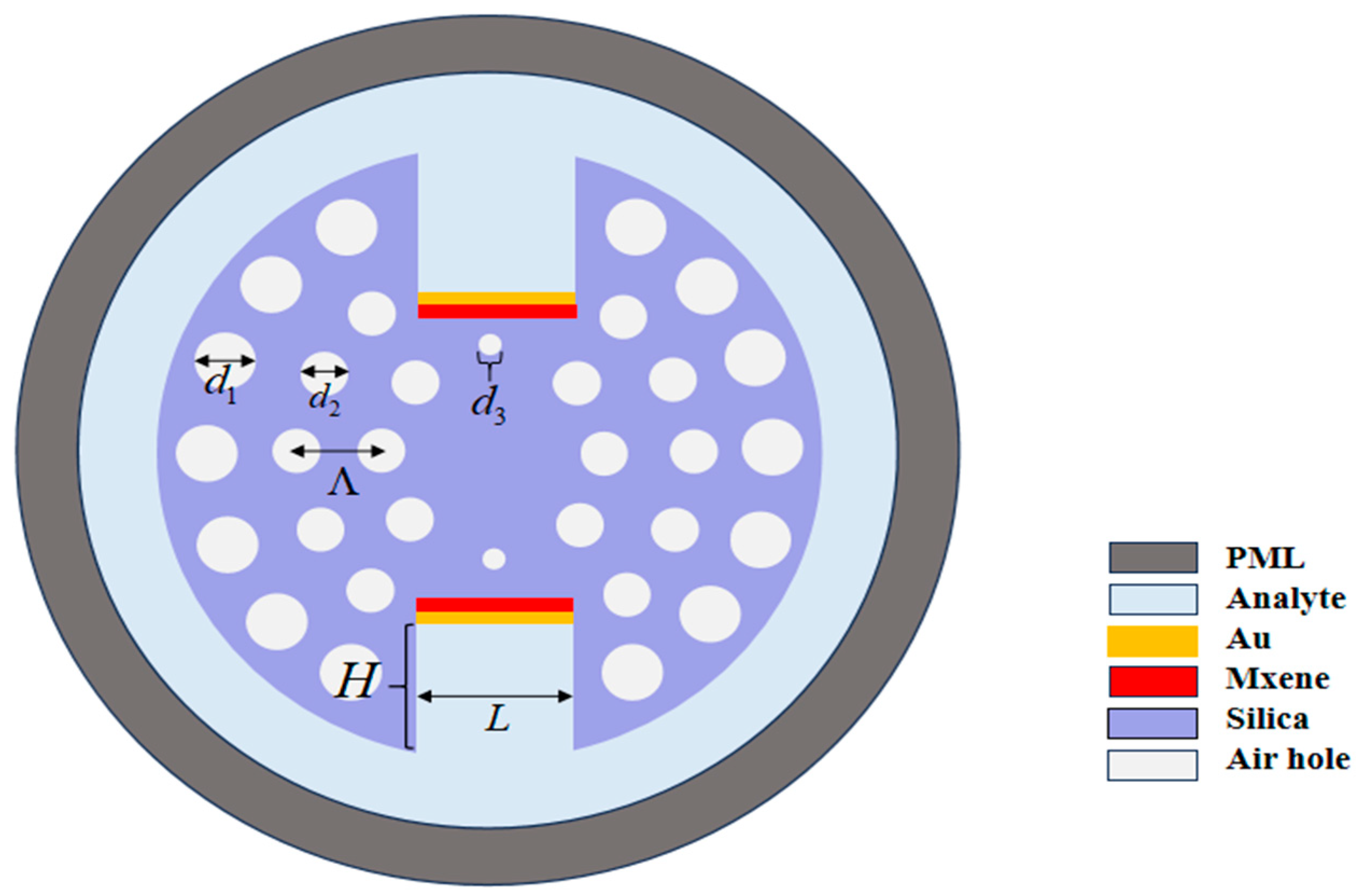
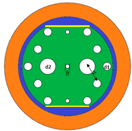
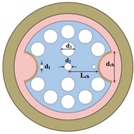
2.1. Sensor Model and Sensing Principle
2.2. Sensor Fabrication and Materials
3. Results and Discussion
3.1. Sensor Performance
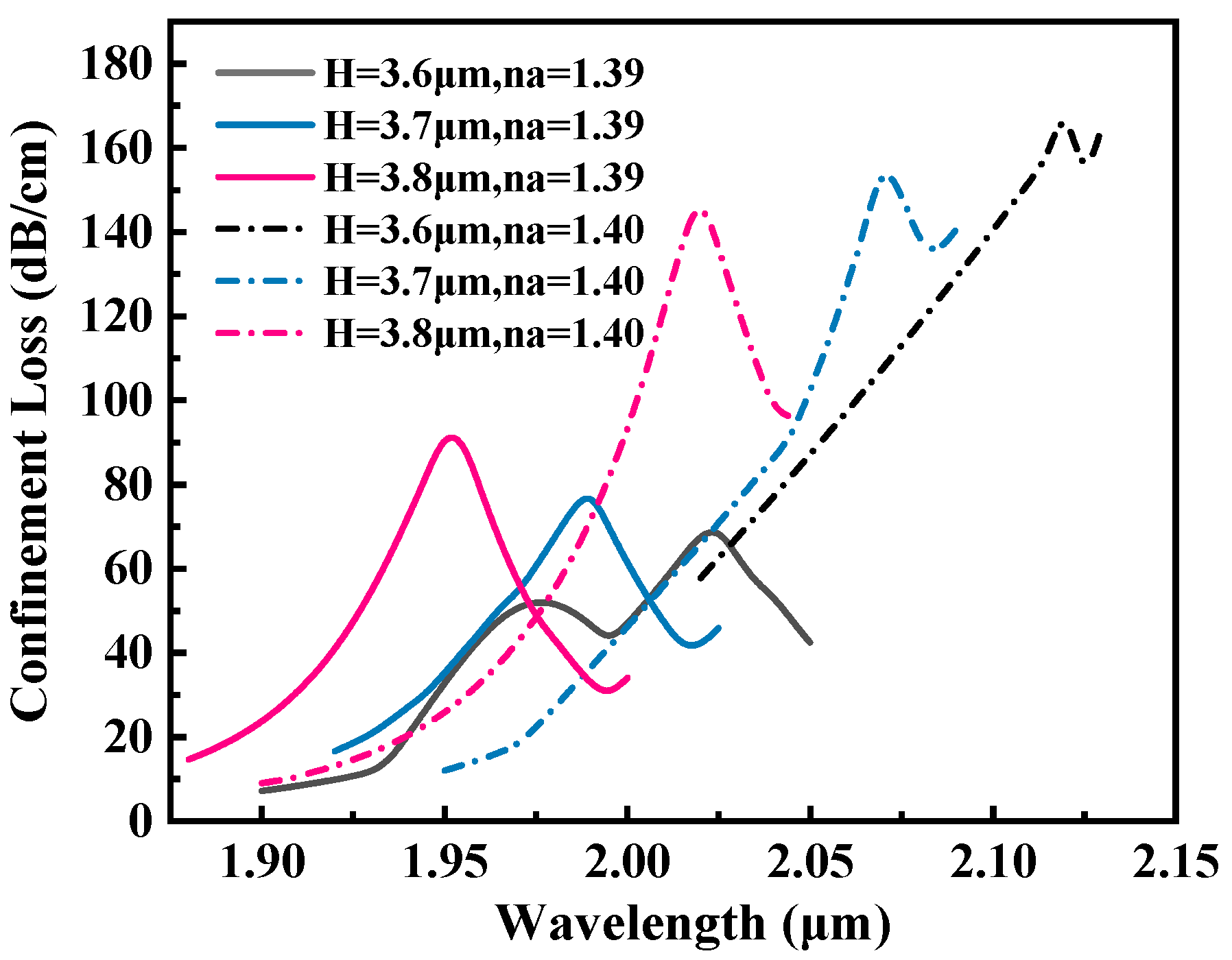
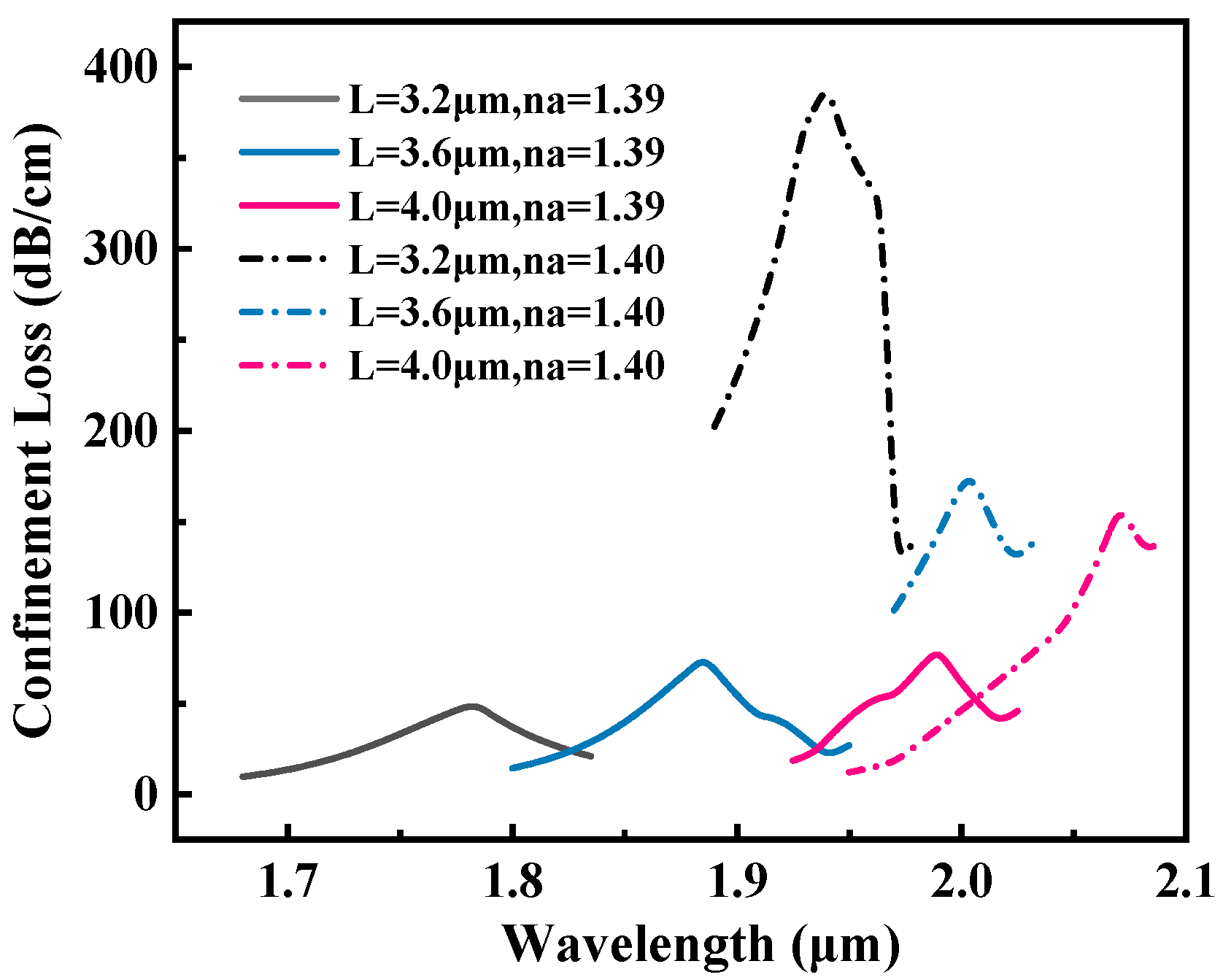
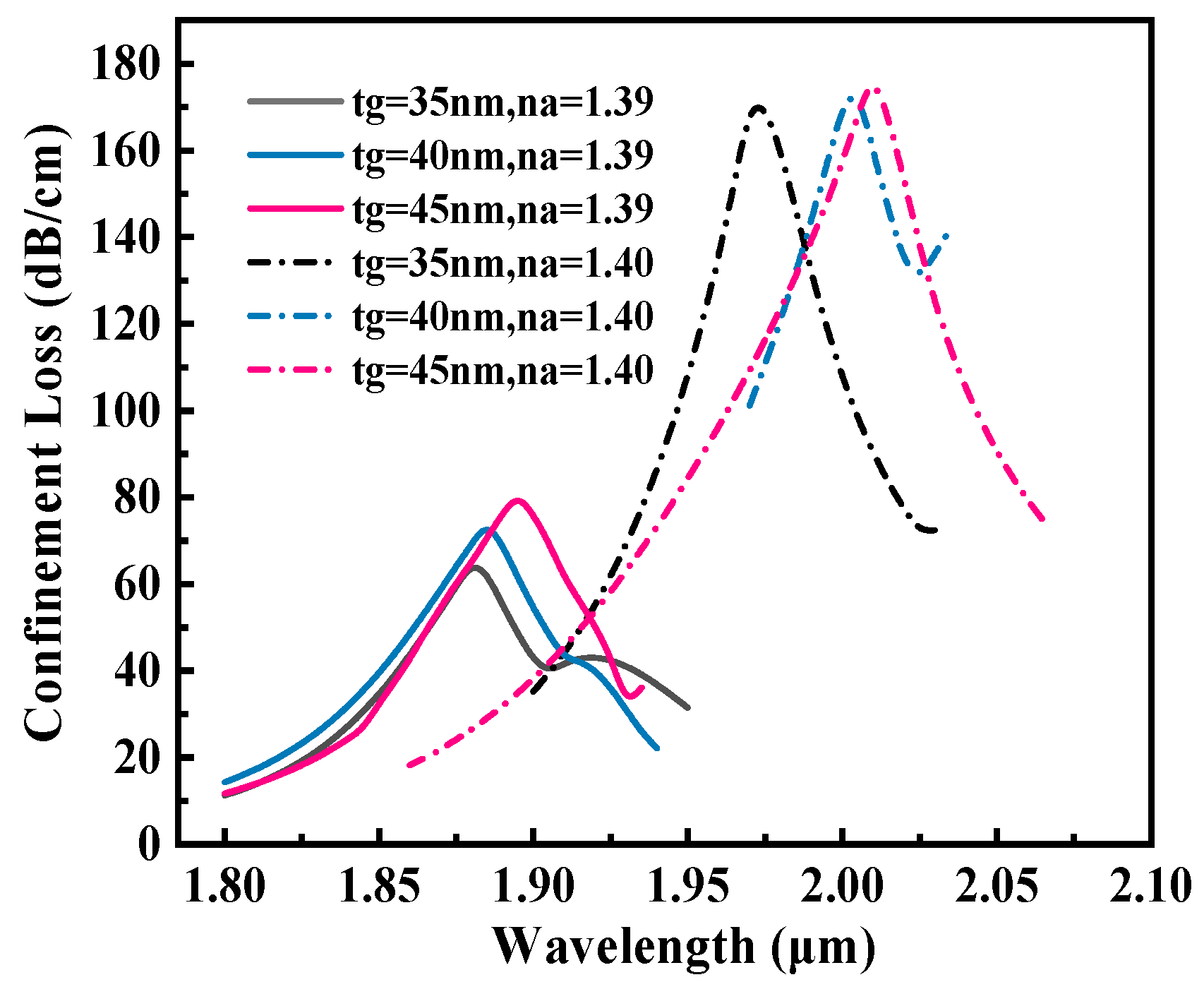
3.2. Optimization of Geometric Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Modha, S.; Castro, C.; Tsutsui, H. Recent developments in flow modeling and fluid control for paper-based microfluidic biosensors. Biosens. Bioelectron. 2021, 178, 113026. [Google Scholar] [CrossRef]
- Hu, O.; Li, Z.; Wu, J.; Tan, Y.; Chen, Z.; Tong, Y. A Multicomponent Nucleic Acid Enzyme-Cleavable Quantum Dot Nanobeacon for Highly Sensitive Diagnosis of Tuberculosis with the Naked Eye. ACS Sens. 2023, 8, 254–262. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Cheng, C.E.; Huang, C.; Liao, X.; Tang, J.; Hu, J.; Wang, Y.; Shao, W. Research on D-shape PCF temperature sensor with simple structure and high sensitivity. Optik 2025, 323, 172228. [Google Scholar] [CrossRef]
- Cheng, C.; Huang, C.; Zhang, Q.; Liao, X.; Yang, J.; Shao, W.; Tang, J.; Shao, L.; Hu, J.; Wang, Y. A highly sensitive dual-core PCF-SPR sensor based on ITO and Au for detecting refractive index and temperature. Phys. Scr. 2025, 100, 015509. [Google Scholar] [CrossRef]
- Yesudasu, V.; Pradhan, H.S.; Pandya, R.J. Recent progress in surface plasmon resonance based sensors: A comprehensive review. Heliyon 2021, 7, e06321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, D.; Qian, Y.; Yin, X.; Wang, L.; Zhang, S.; Wang, Y. Research on Fiber Optic Surface Plasmon Resonance Biosensors: A Review. Photonic Sens. 2024, 14, 240201. [Google Scholar] [CrossRef]
- Granizo, E.; Kriukova, I.; Escuderovilla, P.; Samokhvalov, P.; Nabiev, I. Microfluidics and Nanofluidics in Strong Light–Matter Coupling Systems. Nanomaterials 2024, 14, 1520. [Google Scholar] [CrossRef]
- Yang, H.; Liu, M.; Chen, Y.; Guo, L.; Yuan, L. Highly Sensitive Graphene-Au Coated Plasmon Resonance PCF Sensor. Sensors 2021, 21, 818. [Google Scholar] [CrossRef]
- Divya, J.; Selvendran, S. Surface Plasmon Resonance-Based Gold-Coated Hollow-Core Negative Curvature Optical Fiber Sensor. Biosensors 2023, 13, 148. [Google Scholar] [CrossRef]
- Haque, E.; Hossain, M.A.; Ahmed, F.; Namihira, Y. Surface Plasmon Resonance Sensor Based on Modified $D$ -Shaped Photonic Crystal Fiber for Wider Range of Refractive Index Detection. IEEE Sens. J. 2018, 18, 8287–8293. [Google Scholar] [CrossRef]
- Junbo, L.; Tonglei, C.; Shuguang, L.J. High-birefringence dual-wavelength single-polarization photonic crystal fibre polarizing filter based on surface plasmon resonance. J. Mod. Opt. 2018, 65, 2317–2325. [Google Scholar]
- Scheibe, B.; Wychowaniec, J.K.; Scheibe, M.; Peplińska, B.; Jarek, M.; Nowaczyk, G.; Przysiecka, Ł. Cytotoxicity Assessment of Ti–Al–C Based MAX Phases and Ti3C2Tx MXenes on Human Fibroblasts and Cervical Cancer Cells. ACS Biomater. Sci. Eng. 2019, 5, 6557–6569. [Google Scholar] [CrossRef]
- Aliqab, K.; Dave, K.; Sorathiya, V.; Alsharari, M.; Armghan, A. Numerical analysis of Phase change material and graphene-based tunable refractive index sensor for infrared frequency spectrum. Sci. Rep. 2023, 13, 7653. [Google Scholar] [CrossRef] [PubMed]
- Nagavel, B.; Dagar, H.; Krishnan, P. High-Performance Dual-Core Bilateral Surface Optimized PCF SPR Biosensor for Early Detection of Six Distinct Cancer Cells. Plasmonics 2025, 20, 4799–4809. [Google Scholar]
- Kumar, A.; Verma, P.; Jindal, P. Surface plasmon resonance biosensor based on a D-shaped photonic crystal fiber using Ti3C2TxMXene material. Opt. Mater. 2022, 128, 112397. [Google Scholar] [CrossRef]
- Ibrahimi, K.M.; Kumar, R.; Pakhira, W. C-grooved dual-core PCF SPR biosensor with graphene/au coating for enhanced early cancer cell detection. Appl. Phys. A-Mater. Sci. Process. 2024, 130, 439. [Google Scholar] [CrossRef]
- Abdelghaffar, M.; Gamal, Y.; El-Khoribi, R.A.; Soliman, W.; Badr, Y.; Hameed, M.F.O.; Obayya, S.S.A. Highly sensitive V-shaped SPR PCF biosensor for cancer detection. Opt. Quantum Electron. 2023, 55, 472. [Google Scholar] [CrossRef]
- Ibrahimi, K.M.; Kumar, R.; Pakhira, W. Enhance the Design and Performance Analysis of a Highly Sensitive Twin-Core PCF SPR Biosensor with Gold Plating for the Early Detection of Cancer Cells. Plasmonics 2023, 18, 995–1006. [Google Scholar] [CrossRef]
- Zhao, X.; Chu-Su, Y.; Tsai, W.-H.; Wang, C.-H.; Chuang, T.-L.; Lin, C.-W.; Tsao, Y.-C.; Wu, M.-S. Improvement of the sensitivity of the surface plasmon resonance sensors based on multi-layer modulation techniques. Opt. Commun. 2015, 335, 32–36. [Google Scholar] [CrossRef]
- Mostufa, S.; Akib, T.B.A.; Rana, M.M.; Islam, M.R. Highly Sensitive TiO2/Au/Graphene Layer-Based Surface Plasmon Resonance Biosensor for Cancer Detection. Biosensors 2022, 12, 603. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, D.; Deng, S.; Jafarpour, M.; Avaro, J.; Neels, A.; Heier, J.; Zhang, C. Rational Design of Ti3C2Tx MXene Inks for Conductive, Transparent Films. ACS Nano 2023, 17, 3737–3749. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Arif, M.F.H.; Abu Yousuf, M.; Asaduzzaman, S. Highly sensitive open channel based PCF-SPR sensor for analyte refractive index sensing. Results Phys. 2023, 46, 106266. [Google Scholar] [CrossRef]
- Opoku, G.; Danlard, I.; Dede, A.; Kofi Akowuah, E. Design and numerical analysis of a circular SPR based PCF biosensor for aqueous environments. Results Opt. 2023, 12, 100432. [Google Scholar] [CrossRef]
- Hasan, M.R.; Akter, S.; Rifat, A.A.; Rana, S.; Ali, S. A Highly Sensitive Gold-Coated Photonic Crystal Fiber Biosensor Based on Surface Plasmon Resonance. Photonics 2017, 4, 18. [Google Scholar] [CrossRef]
- Praveena, S.; Senthilnathan, K. Performance Enhancement of Gold Coated D-shaped PCF Sensor Using Monolayer MoS2. Plasmonics 2025, 20, 1003–1014. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, X.; Fu, Q.; Yuan, L.; Zhang, Q.; Zhou, S. Femtosecond laser micro-machining for accurate determination of fracture toughness of glass. J. Am. Ceram. Soc. 2025, 108, e20436. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti 3 AlC 2; Jenny Stanford Publishing: Singapore, 2011. [Google Scholar]
- Ramola, A.; Marwaha, A.; Singh, S. Design and investigation of a dedicated PCF SPR biosensor for CANCER exposure employing external sensing. Appl. Phys. A-Mater. Sci. Process. 2021, 127, 643. [Google Scholar] [CrossRef]
- Majeed, M.F.; Ahmad, A.K. Design and analysis of a high sensitivity open microchannel PCF-based surface plasmon resonance refractometric sensor. Opt. Mater. 2024, 147, 114617. [Google Scholar] [CrossRef]
- Meshginqalam, B.; Barvestani, J. High performance surface plasmon resonance-based photonic crystal fiber biosensor for cancer cells detection. Eur. Phys. J. Plus 2022, 137, 417. [Google Scholar] [CrossRef]
- Melwin, G.; Senthilnathan, K. High sensitive D -shaped photonic crystal fiber sensor with V-groove analyte channel. Optik 2020, 213, 164779. [Google Scholar] [CrossRef]
- Hale, G.M.; Querry, M.R. Optical Constants of Water in the 200-nm to 200-μm Wavelength Region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Otupiri, R.; Akowuah, E.K.; Haxha, S.; Ademgil, H.; AbdelMalek, F.; Aggoun, A. A Novel Birefrigent Photonic Crystal Fiber Surface Plasmon Resonance Biosensor. IEEE Photonics J. 2014, 6, 1–11. [Google Scholar] [CrossRef]
- Rogalski, A.; Kopytko, M.; Martyniuk, P. Two-dimensional infrared and terahertz detectors: Outlook and status. Appl. Phys. Rev. 2019, 6, 021316. [Google Scholar] [CrossRef]
- Mittal, S.; Saharia, A.; Ismail, Y.; Petruccione, F.; Bourdine, A.V.; Morozov, O.G.; Demidov, V.V.; Yin, J.; Singh, G.; Tiwari, M. Spiral Shaped Photonic Crystal Fiber-Based Surface Plasmon Resonance Biosensor for Cancer Cell Detection. Photonics 2023, 10, 230. [Google Scholar] [CrossRef]

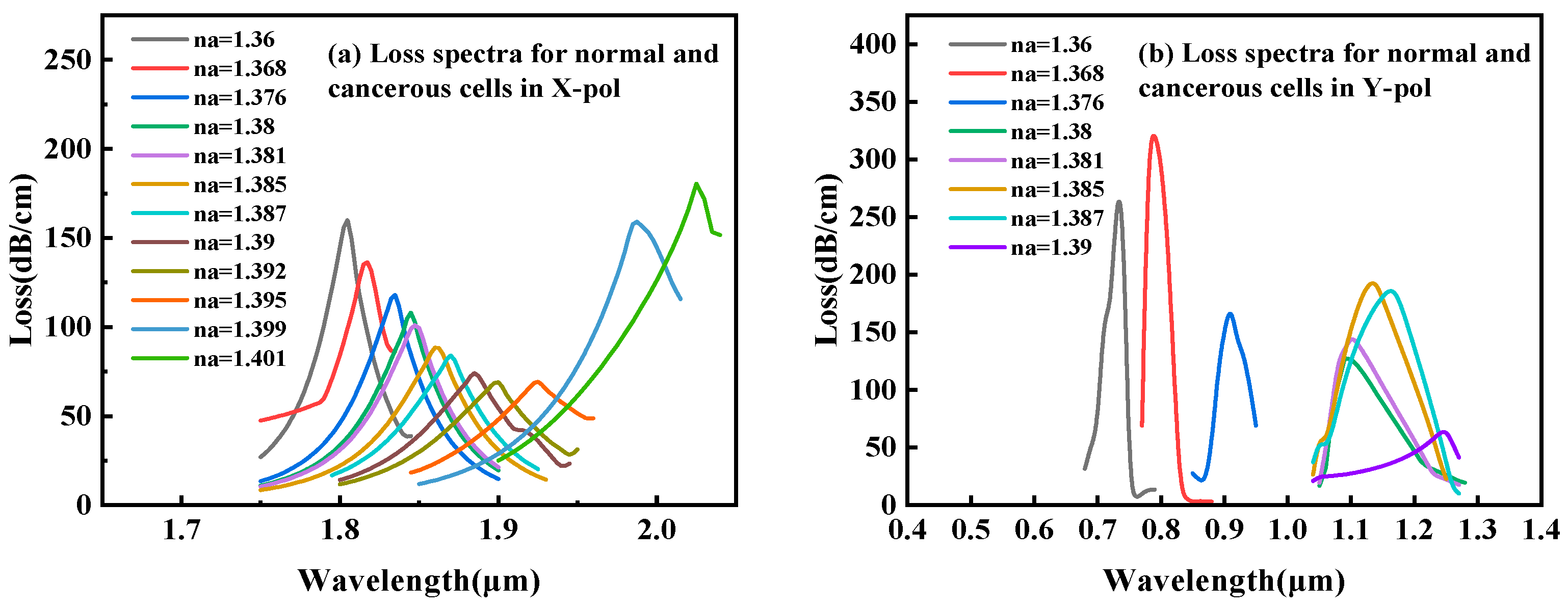
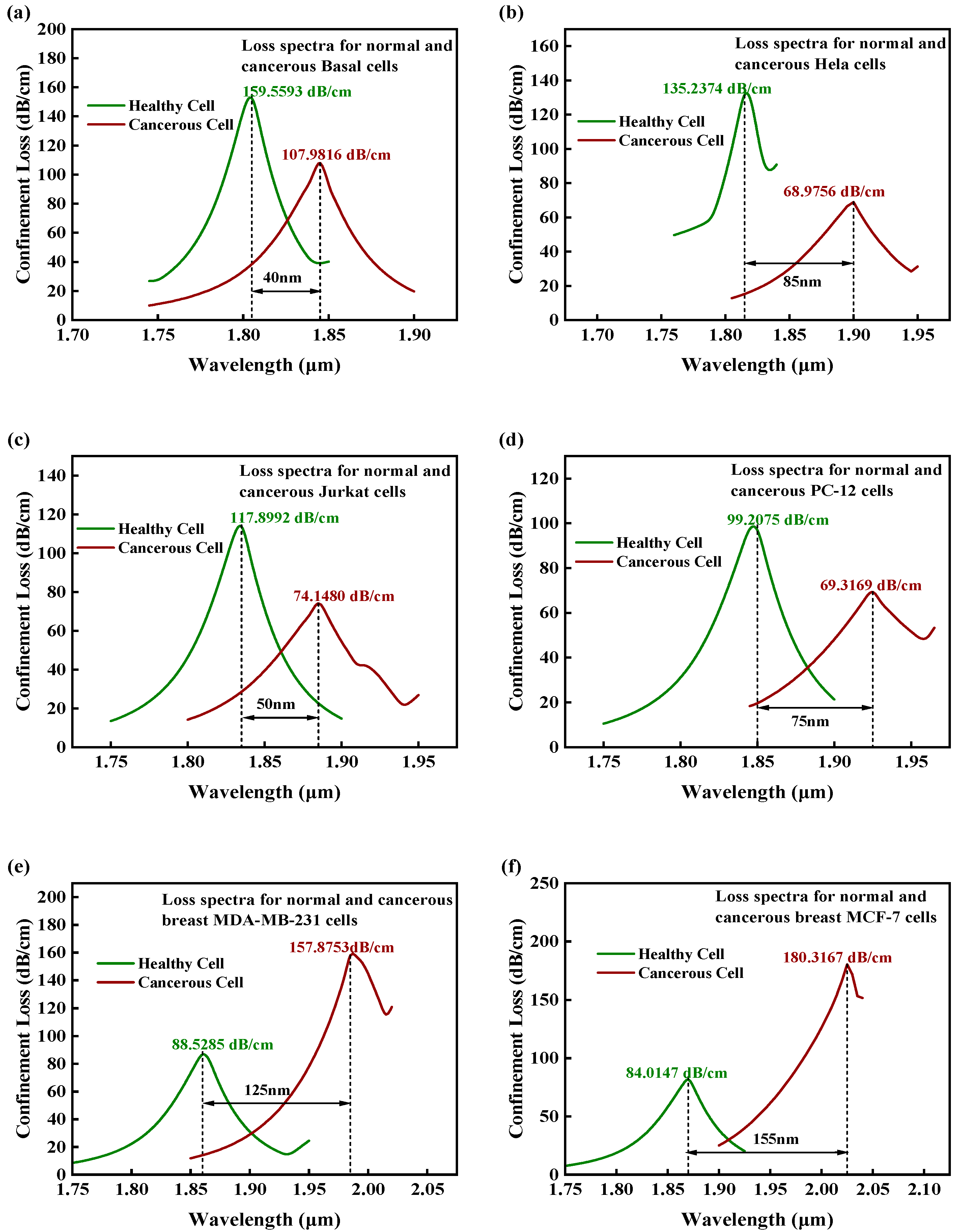


| Cell Name | Cancer Type | RI Normal Cells | RI Cancer Cells | ∆RI |
|---|---|---|---|---|
| Basal | Skin | 1.360 | 1.380 | 0.020 |
| Hela | Cervical | 1.368 | 1.392 | 0.024 |
| Jurkat | Blood | 1.376 | 1.390 | 0.014 |
| PC-12 | Adrenal gland | 1.381 | 1.395 | 0.014 |
| MDA-MB-231 | Breast type-2 | 1.385 | 1.399 | 0.014 |
| MCF-7 | Breast type-1 | 1.387 | 1.401 | 0.014 |
| Cell Name | RW Shift (nm) | (nm/RIU) | RS (RIU) | FWHM (nm) | FOM (RIU−1) |
|---|---|---|---|---|---|
| Basal | 40 | 2000 | 5 × 10−5 | 35 | 57.14 |
| Hela | 85 | 3542 | 2.82 × 10−5 | 30 | 118.06 |
| Jurkat | 50 | 3572 | 2.8 × 10−5 | 40 | 89.30 |
| PC-12 | 75 | 5357 | 1.86 × 10−5 | 50 | 107.14 |
| MDA-MB-231 | 125 | 8929 | 1.12 × 10−5 | 50 | 178.58 |
| MCF-7 | 155 | 11,072 | 9.03 × 10−6 | 55 | 201.30 |
| Sensor | Cell Name | WS (nm/RIU) | Resolution (RIU) | Ref. |
|---|---|---|---|---|
 | Basal | 3000 | 3.33 × 10−5 | [15] |
| Hela | 3333.33 | 3 × 10−5 | ||
| Jurkat | 4285.72 | 2.33 × 10−5 | ||
| PC-12 | 4285.72 | 2.33 × 10−5 | ||
| MDA-MB-231 | 5714.28 | 1.75 × 10−5 | ||
| MCF-7 | 5714.28 | 1.75 × 10−5 | ||
 | Basal | 1500 | 6.7 × 10−5 | [17] |
| Hela | 1666.67 | 6 × 10−5 | ||
| Jurkat | 1428.57 | 7 × 10−5 | ||
| PC-12 | 1428.57 | 7 × 10−5 | ||
| MDA-MB-231 | 2142.86 | 4.67 × 10−5 | ||
| MCF-7 | 2142.86 | 4.67 × 10−5 | ||
 | Basal | 2500 | 4 × 10−5 | [19] |
| Hela | 2916.66 | 3.42 × 10−5 | ||
| Jurkat | 3571.42 | 2.8 × 10−5 | ||
| PC-12 | 3571.42 | 2.8 × 10−5 | ||
| MDA-MB-231 | 4285.71 | 2.33 × 10−5 | ||
| MCF-7 | 4285.71 | 2.33 × 10−5 | ||
 | Basal | 2 × 10−5 | [36] | |
| Hela | 1.5 × 10−5 | |||
| Jurkat | 1.4 × 10−4 | |||
| PC-12 | 1.4 × 10−4 | |||
| MDA-MB-231 | 2.33 × 10−4 | |||
| MCF-7 | 2.33 × 10−4 | |||
 | Basal | 2000 | 5 × 10−5 | This Work |
| Hela | 3542 | 2.82 × 10−5 | ||
| Jurkat | 3572 | 2.8 × 10−5 | ||
| PC-12 | 5357 | 1.86 × 10−5 | ||
| MDA-MB-231 | 8929 | 1.12 × 10−5 | ||
| MCF-7 | 11,072 | 9.03 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; He, Y.; Xi, S.; Zhong, P.; Zhang, Y.; Tian, H.; Wang, Y.; Lu, H.; Hu, J.; Tang, J. High-Sensitivity MXene-Functionalized Photonic Crystal Fiber Surface Plasmon Resonance Sensor with Dual Rectangular Grooves for Cancer Detection. Sensors 2025, 25, 5705. https://doi.org/10.3390/s25185705
Lu M, He Y, Xi S, Zhong P, Zhang Y, Tian H, Wang Y, Lu H, Hu J, Tang J. High-Sensitivity MXene-Functionalized Photonic Crystal Fiber Surface Plasmon Resonance Sensor with Dual Rectangular Grooves for Cancer Detection. Sensors. 2025; 25(18):5705. https://doi.org/10.3390/s25185705
Chicago/Turabian StyleLu, Min, Yan He, Shuyu Xi, Pufan Zhong, Yu Zhang, He Tian, Yongmei Wang, Hanglin Lu, Junhui Hu, and Jian Tang. 2025. "High-Sensitivity MXene-Functionalized Photonic Crystal Fiber Surface Plasmon Resonance Sensor with Dual Rectangular Grooves for Cancer Detection" Sensors 25, no. 18: 5705. https://doi.org/10.3390/s25185705
APA StyleLu, M., He, Y., Xi, S., Zhong, P., Zhang, Y., Tian, H., Wang, Y., Lu, H., Hu, J., & Tang, J. (2025). High-Sensitivity MXene-Functionalized Photonic Crystal Fiber Surface Plasmon Resonance Sensor with Dual Rectangular Grooves for Cancer Detection. Sensors, 25(18), 5705. https://doi.org/10.3390/s25185705







