Molecular Design of Benzothiadiazole-Fused Tetrathiafulvalene Derivatives for OFET Gas Sensors: A Computational Study
Abstract
Highlights
- Theoretical calculations (PBE0/6-311G(d,p)) predict the designed promising organic semiconductors with high mobilities.
- TTF derivatives exhibit sensitivity to gases (NH3, H2S, SO2), suggesting potential for dual-function organic field-effect transistors (OFETs) with integrated sensing capabilities.
Abstract
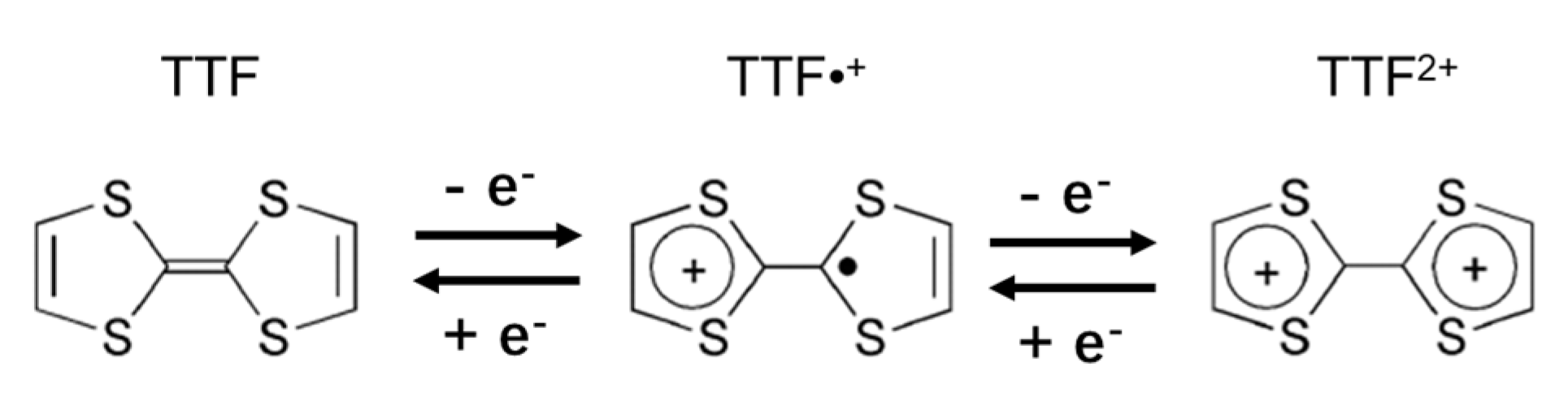
1. Introduction
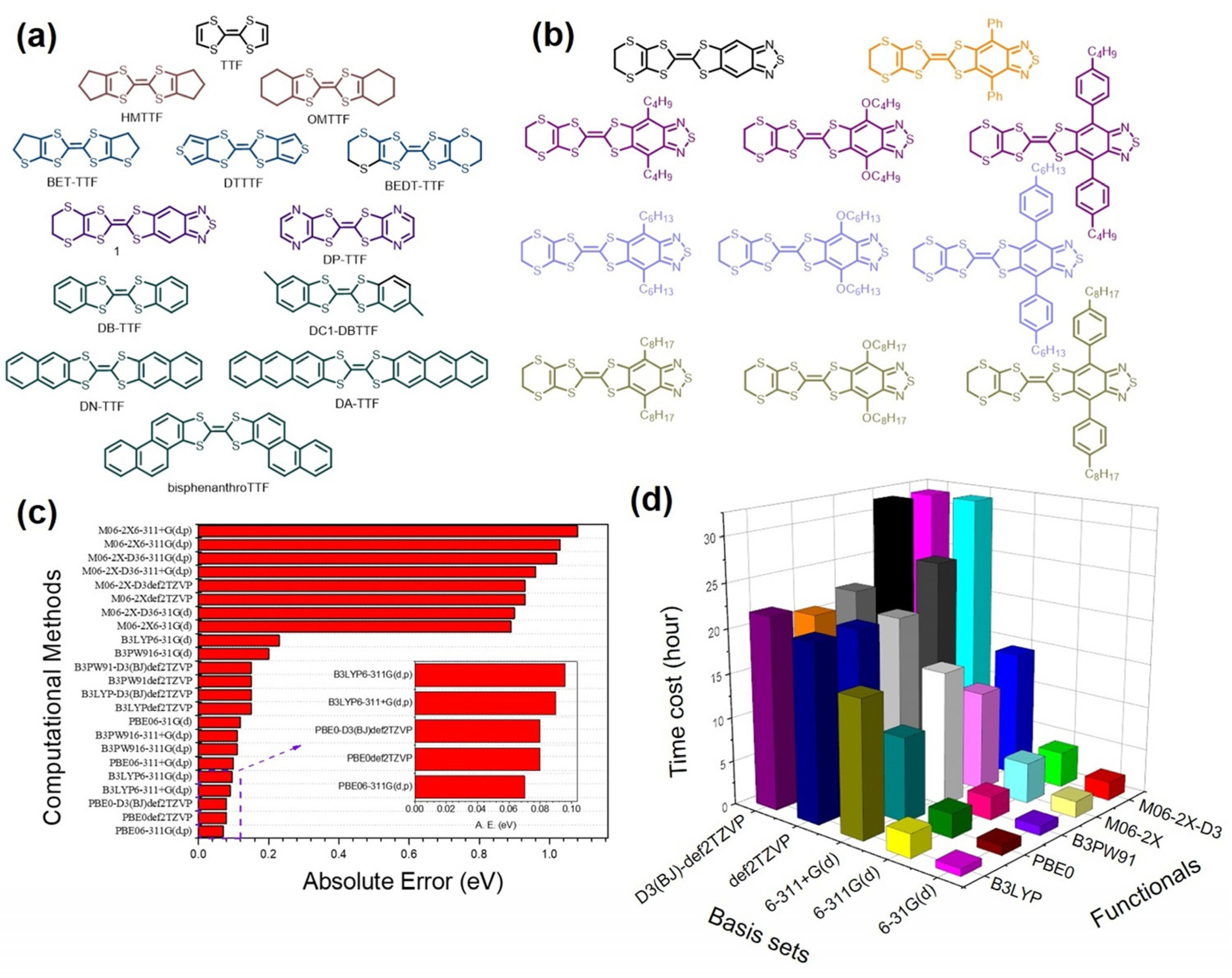
2. Methodology
3. Results and Discussion
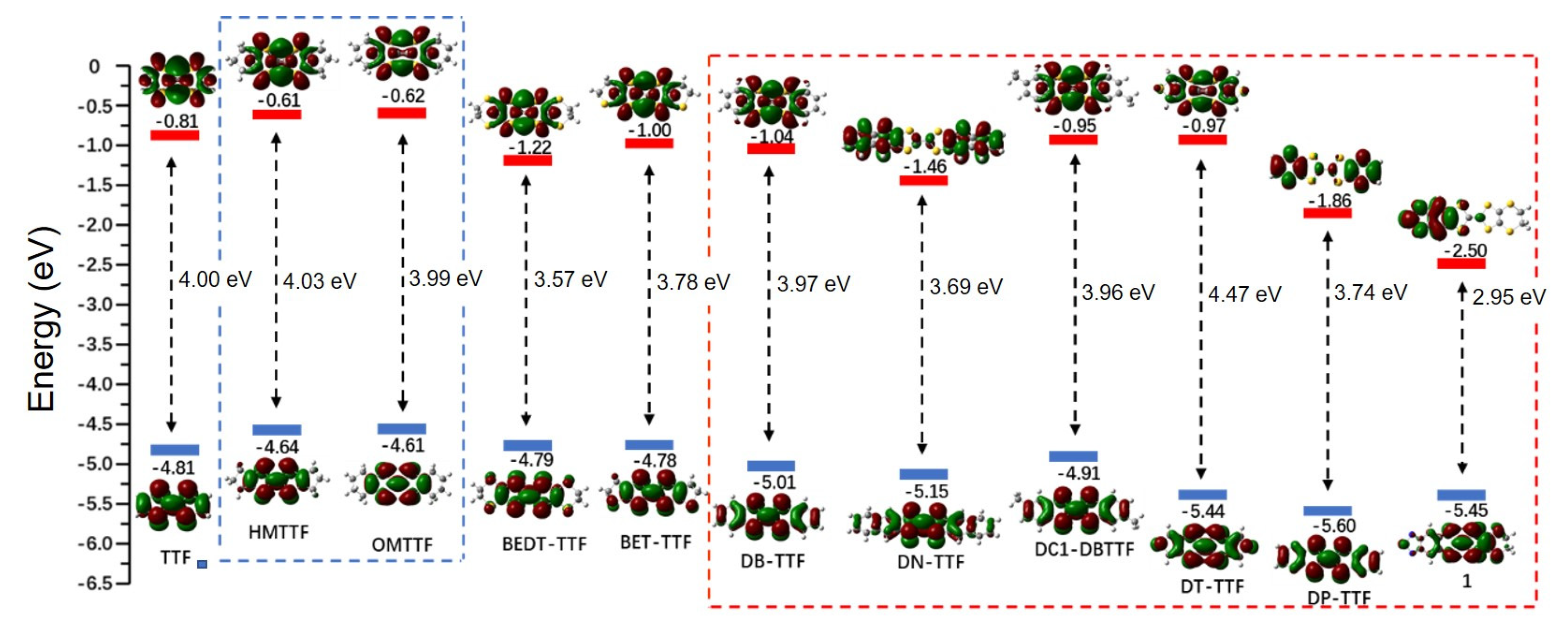
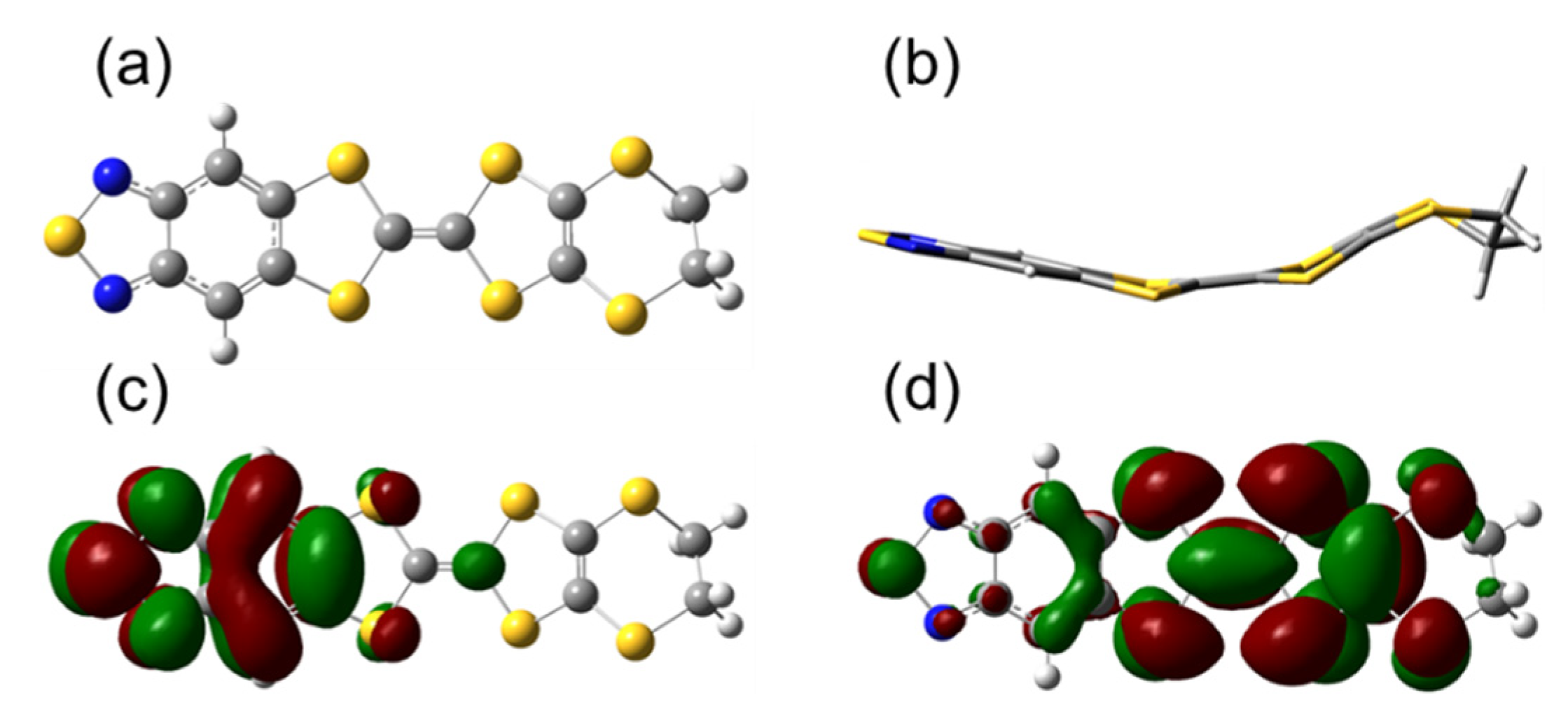
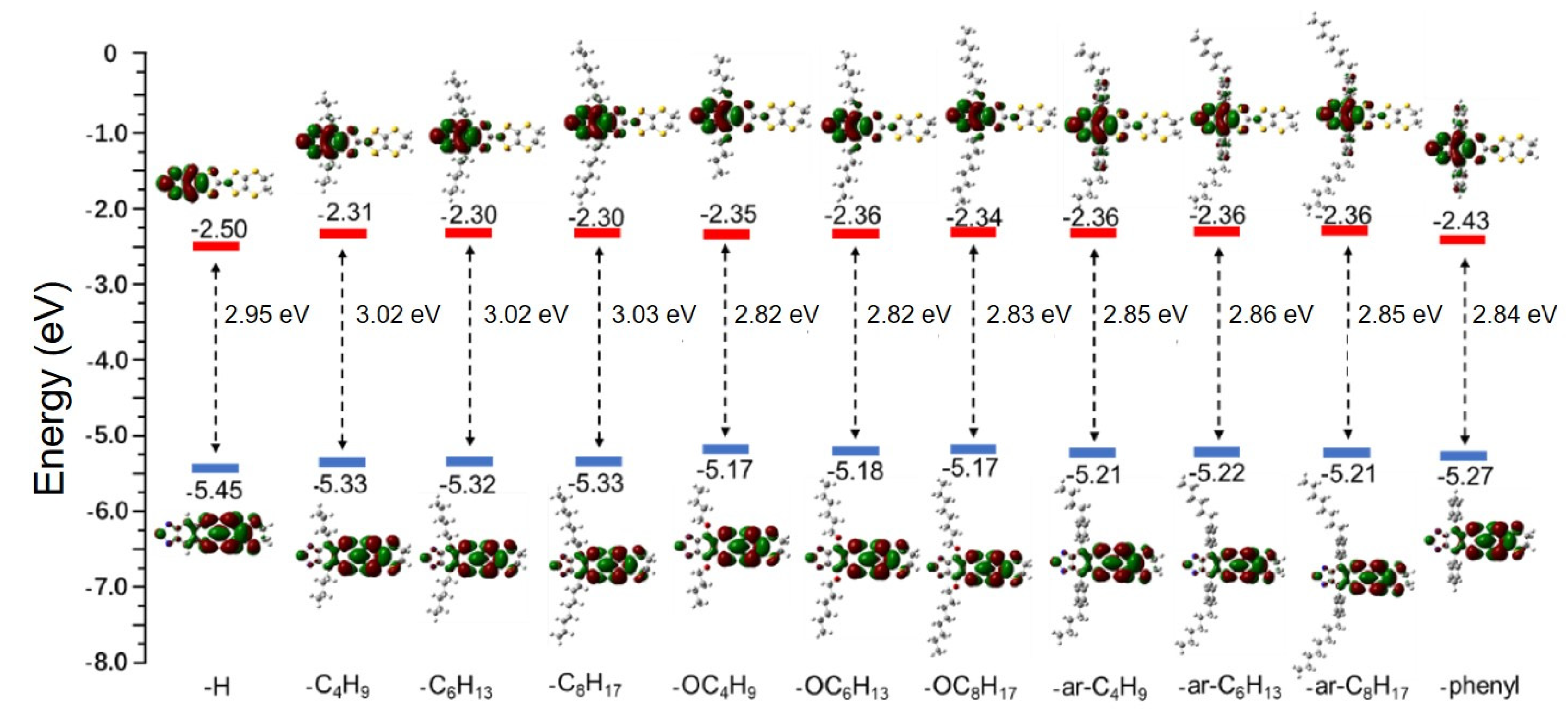
3.1. The Frontier Molecular Orbitals (FMOs)
3.1.1. FMOs Modification of TTF Derivatives’ Core
3.1.2. Modification of FMOs with EDG/EWG Side Chains on TTF Derivatives
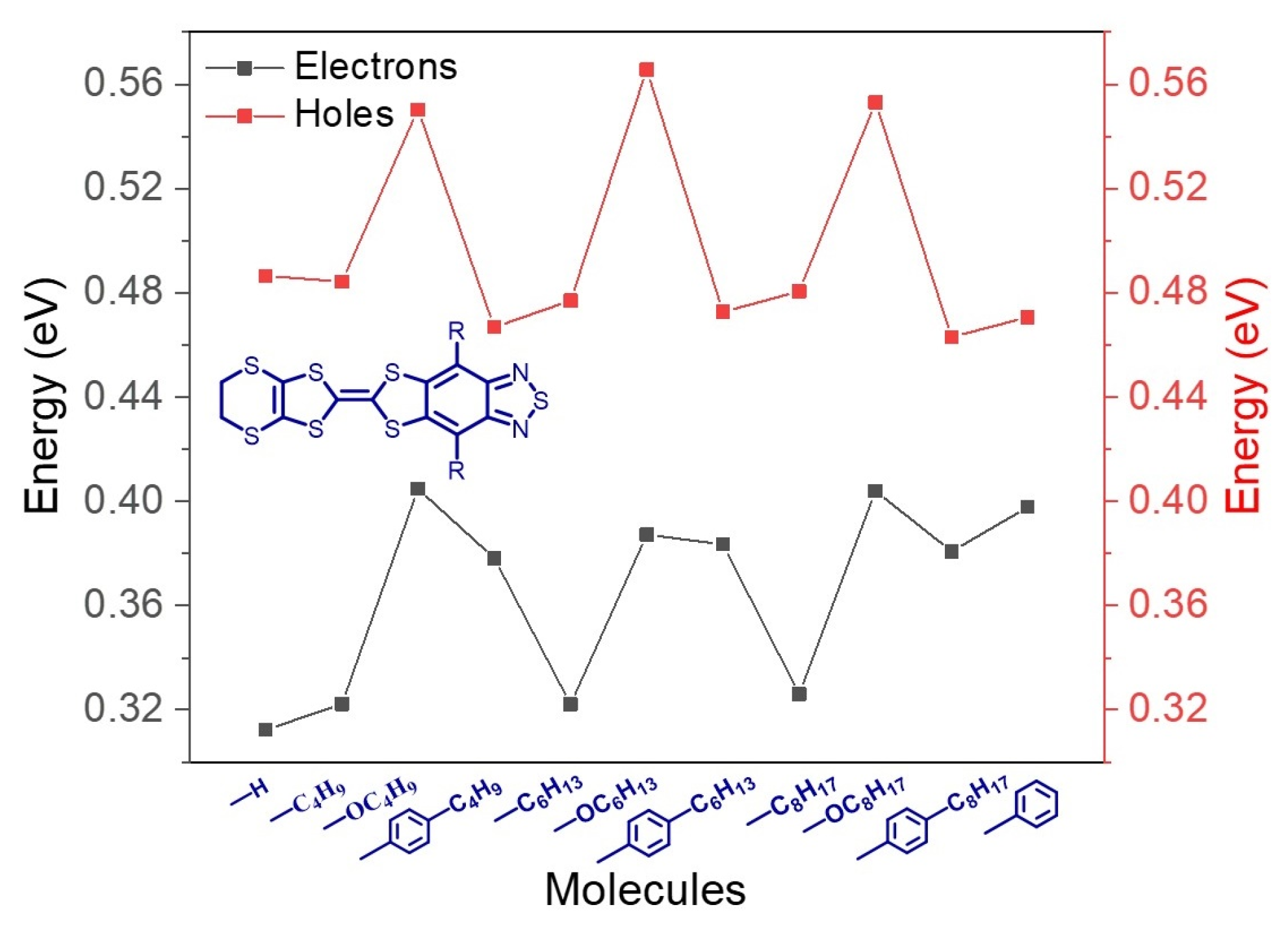
3.2. Reorganization Energy
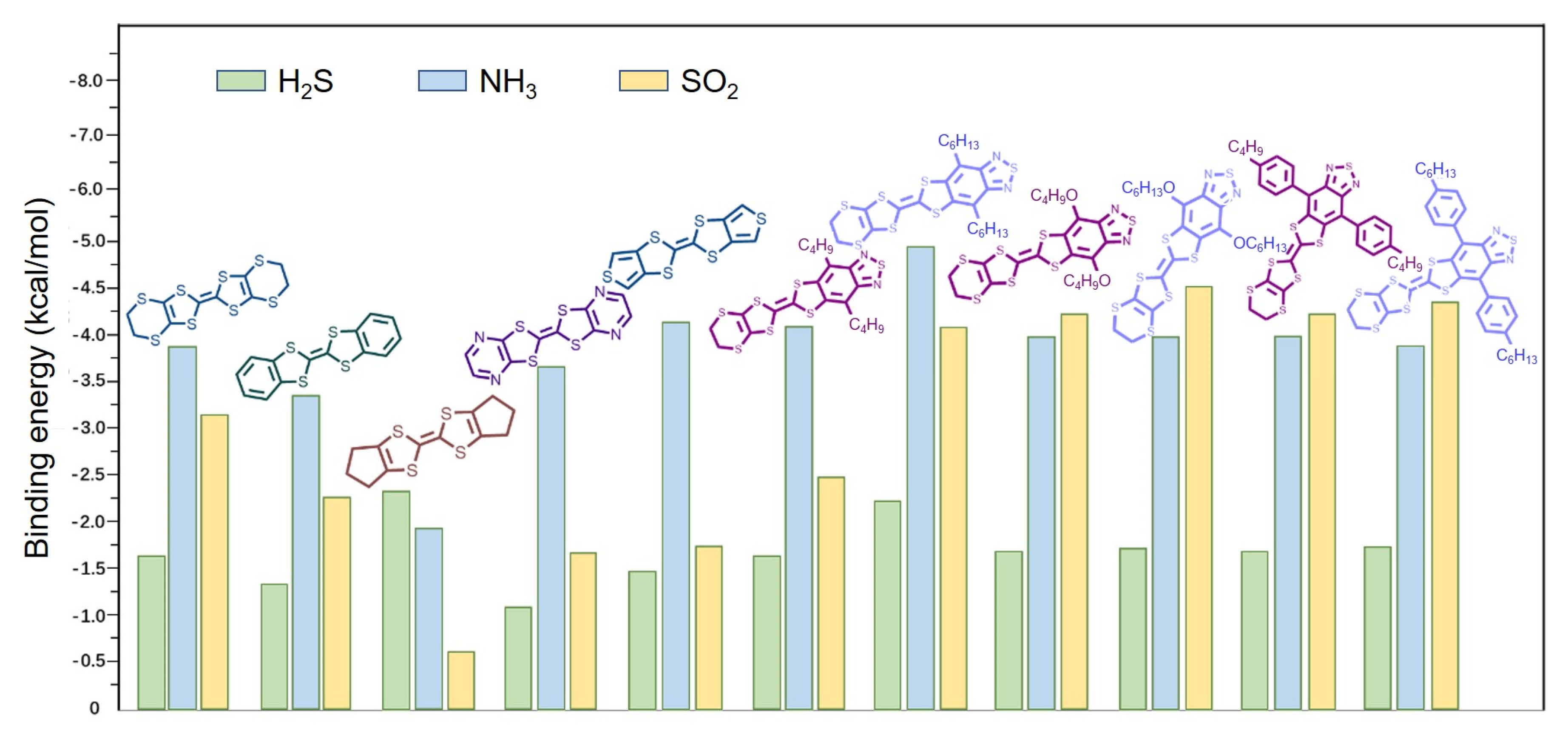
3.3. Study of Binding Energy in Gas Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Shi, S.; Jiang, J.; Lin, D.; Song, J.; Wang, Z.; Huang, W. Bionic olfactory neuron with in-sensor reservoir computing for intelligent gas recognition. Adv. Mater. 2025, 37, e2419159. [Google Scholar] [CrossRef]
- Amna, B.; Ozturk, T. Organic field-effect transistor-based sensors: Recent progress, challenges and future outlook. J. Mater. Chem. C 2025, 13, 8354–8424. [Google Scholar] [CrossRef]
- Ko, I.H.; Park, Y.D. Recent research trends for developing highly sensitive, flexible organic field-effect transistor-based gas sensors. ACS Appl. Polym. Mater. 2025, 7, 2749–2760. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Zhang, J.; Liu, J.; Lei, M.; Jiang, L. Organic semiconductor single crystal arrays: Preparation and applications. Adv. Sci. 2023, 10, 2300483. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Yoshida, Y. Development of conductive organic molecular assemblies: Organic metals, superconductors, and exotic functional materials. Bull. Chem. Soc. Jpn. 2007, 1, 1–137. [Google Scholar] [CrossRef]
- Gao, X.; Qiu, W.; Liu, Y.; Yu, G.; Zhu, D. Organic field-effect transistors based on tetrathiafulvalene derivatives. Pure Appl. Chem. 2008, 80, 2405–2423. [Google Scholar] [CrossRef]
- Zhang, R.; He, M.; Xiang, J.; Cai, S.; Ge, C.; Gao, X. Core-expanded naphthalene diimides-vinylogous tetrathia-fulvalenes toward ambipolar organic semiconductors. Chin. J. Org. Chem. 2024, 44, 2810–2819. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hasegawa, T.; Horiuchi, S.; Kumai, R.; Tokura, Y.; Saito, G. High mobility organic field-effect transistor based on hexamethylenetetrathiafulvalene with organic metal electrodes. Chem. Mater. 2007, 19, 6382–6384. [Google Scholar] [CrossRef]
- Leufgen, M.; Rost, O.; Gould, C.; Schmidt, G.; Geurts, J.; Molenkamp, L.; Oxtoby, N.; Mas-Torrent, M.; Crivillers, N.; Veciana, J.; et al. High-mobility tetrathiafulvalene organic field-effect transistors from solution processing. Org. Electron. 2008, 9, 1101–1106. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, H.; Dong, H.; Fang, Q.; Hu, W. Comparable charge transport property based on S···S interactions with that of π-π stacking in a bis-fused tetrathiafulvalene compound. Sci. China Chem. 2017, 60, 510–515. [Google Scholar] [CrossRef]
- Mas-Torrent, M.; Hadley, P.; Bromley, S.T.; Ribas, X.; Tarre’s, J.; Mas, M.; Molins, E.; Veciana, J.; Rovira, C. Correlation between crystal structure and mobility in organic field-effect transistors based on single crystals of tetrathiafulvalene derivatives. J. Am. Chem. Soc. 2004, 126, 8546–8553. [Google Scholar] [CrossRef]
- Naraso; Nishida, J.-I.; Ando, S.; Yamaguchi, J.; Itaka, K.; Koinuma, H.; Tada, H.; Tokito, S.; Yamashita, Y. High-performance organic field-effect transistors based on π-extended tetrathiafulvalene derivatives. J. Am. Chem. Soc. 2005, 127, 10142–10143. [Google Scholar] [CrossRef] [PubMed]
- Ya-Rui, S.; Hui-Ling, W.; Yu-Fang, L. Research on charge-transport properties of TTF–TTP derivatives and organic interfaces. RSC Adv. 2016, 6, 57057–57068. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, X.; Wang, E.; Fu, Y.; Liu, Y.; Li, H.; Cui, Z.; Liu, Y.; Hu, W. Organic single crystalline micro- and nanowires field-effect transistors of a tetrathiafulvalene (TTF) derivative with strong π–π orbits and S⋯S interactions. Synth. Met. 2011, 161, 136–142. [Google Scholar] [CrossRef]
- Touhami, A.; Ben Chaabane, R.; Allouche, A. Theoretical investigation on electronic, optical, and charge transport properties of new anthracene derivatives. Comput. Theor. Chem. 2015, 1073, 123–130. [Google Scholar] [CrossRef]
- Quinn, J.T.E.; Zhu, J.; Li, X.; Wang, J.; Li, Y. Recent progress in the development of n-type organic semiconductors for organic field effect transistors. J. Mater. Chem. C 2017, 5, 8654–8681. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, X.; Cui, Z.; Liu, Y.; Li, H.; Hu, W.; Kloc, C. Adjusting tetrathiafulvalene (TTF) functionality through molecular design for organic field-effect transistors. CrystEngComm 2014, 16, 5968–5983. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, X.; Zhang, X.; He, Y.; Zeng, X.; Murtaza, I.; Meng, H. Computational screening and molecular design of anthracene-based semiconductors. Org. Electron. 2018, 61, 87–95. [Google Scholar] [CrossRef]
- Gunturkun, D.; Isci, R.; Faraji, S.; Sütay, B.; Majewski, L.A.; Ozturk, T. Synthesis and characterization of naphthalenediimide-thienothiophene-conjugated polymers for OFET and OPT applications. J. Mater. Chem. C 2023, 13, 8354–8424. [Google Scholar] [CrossRef]
- Becke, A.D. Perspective: Fifty years of density-functional theory in chemical physics. J. Chem. Phys. 2014, 140, 18A301. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical hybrid density functional with perturbative second-order correlation. J. Chem. Phys. 2006, 124, 034108. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Revealing the nature of intermolecular interaction and configurational preference of the nonpolar molecular dimers (H2)2, (N2)2, and (H2)(N2). J. Mol. Model. 2013, 19, 5387–5395. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456. [Google Scholar] [CrossRef]
- Liao, T.; Roma, G. First principles defect energetics for simulations of silicon carbide under irradiation: Kinetic mechanisms of silicon di-interstitials. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2014, 327, 52–58. [Google Scholar] [CrossRef]
- Rozas, I.; Alkorta, I.; Elguero, J. Hydrogen bonds and ionic interactions in Guanidine/Guanidinium complexes: A computational case study. Struct. Chem. 2008, 19, 923–933. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef]
- Inoue, J.-I.; Kanno, M.; Ashizawa, M.; Seo, C.; Tanioka, A.; Mori, T. Organic transistors based on octamethylenetetrathiafulvalenes. Chem. Lett. 2010, 39, 538–540. [Google Scholar] [CrossRef]
- Rovira, C. Bis(ethylenethio)tetrathiafulvalene (BET-TTF) and related dissymmetrical electron donors: From the molecule to functional molecular materials and devices (OFETs). Chem. Rev. 2004, 104, 5289–5318. [Google Scholar] [CrossRef] [PubMed]
- Bendikov, M.; Wudl, F.; Perepichka, D.F. Tetrathiafulvalenes, oligoacenenes, and their buckminsterfullerene derivatives: The brick and mortar of organic electronics. Chem. Rev. 2004, 104, 4891–4945. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yamashita, M.; Hayashi, H.; Suzuki, M.; Aratani, N. Semiconducting π-extended tetrathiafulvalene derivatives. Chem. 2018, 24, 18601–18612. [Google Scholar] [CrossRef]
- Sundar, T.S.; Sen, R.; Johari, P. Rationally designed donor–acceptor scheme based molecules for applications in opto-electronic devices. Phys. Chem. Chem. Phys. 2016, 18, 9133–9147. [Google Scholar] [CrossRef]
- Yang, G.; Di, C.-A.; Zhang, G.; Zhang, J.; Xiang, J.; Zhang, D.; Zhu, D. Highly sensitive chemical-vapor sensor based on thin-film organic field-effect transistors with benzothiadiazole-fused-tetrathiafulvalene. Adv. Funct. Mater. 2013, 23, 1671–1676. [Google Scholar] [CrossRef]
- Casado, J.; Zgierski, M.Z.; Ruiz Delgado, M.C.; Lopez Navarrete, J.T.; Mas-Torrent, M.; Rovira, C. Tetrathiafulvalene-based materials for organic field effect transistors. inspection of their semiconductor properties by means of molecular spectroscopy and quantum chemistry. J. Phys. Chem. 2007, 111, 10110–10118. [Google Scholar] [CrossRef]
- Pfattner, R.; Pavlica, E.; Jaggi, M.; Liu, S.-X.; Decurtins, S.; Bratina, G.; Veciana, J.; Mas-Torrent, M.; Rovira, C. Photo-induced intramolecular charge transfer in an ambipolar field-effect transistor based on a π-conjugated donor–acceptor dyad. J. Mater. Chem. C 2013, 1, 3985–3988. [Google Scholar] [CrossRef]
- Miskiewicz, P.; Mas-Torrent, M.; Jung, J.; Kotarba, S.; Glowacki, I.; Gomar-Nadal, E.; Amabilino, D.B.; Veciana, J.; Krause, B.; Carbone, D.; et al. Efficient High Area OFETs by Solution Based Processing of a π-Electron Rich Donor. Chem. Mater. 2006, 18, 4724–4729. [Google Scholar] [CrossRef]
- Zu, X.; Li, J.; Qian, Y.; Duan, W.; Zeng, Q. Progress in self-assembly of TTF derivatives at HOPG interface. New J. Chem. 2018, 43, 1654–1662. [Google Scholar] [CrossRef]
- Doi, I.; Miyazaki, E.; Takimiya, K. Synthesis and Characterization of N-Acyl-substituted PyrroloTTF Derivatives and Improved Air-stability of PyrroloTTF-based OFETs. Chem. Lett. 2008, 37, 1088–1089. [Google Scholar] [CrossRef]
- Shuai, Z.; Wang, L.; Li, Q. Evaluation of Charge Mobility in Organic Materials: From Localized to Delocalized Descriptions at a First----Principles Level. Adv. Mater. 2010, 23, 1145–1153. [Google Scholar] [CrossRef]
- Bromley, S.T.; Mas-Torrent, M.; Hadley, P.; Rovira, C. Importance of intermolecular interactions in assessing hopping mobilities in organic field effect transistors: Pentacene versus dithiophene-tetrathiafulvalene. J. Am. Chem. Soc. 2004, 126, 6544–6545. [Google Scholar] [CrossRef]
- Shaymurat, T.; Tang, Q.; Tong, Y.; Dong, L.; Liu, Y. Gas dielectric transistor of CuPc single crystalline nanowire for SO2 detection down to Sub----ppm levels at room temperature. Adv. Mater. 2013, 25, 2269–2273. [Google Scholar] [CrossRef]
- García, G.; Moral, M.; Granadino-Roldán, J.M.; Garzón, A.; Navarro, A.; Fernández-Gómez, M. Theoretical approach to the study of thiophene-based discotic systems as organic semiconductors. J. Phys. Chem. C 2013, 117, 15–22. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Hu, W.; Liu, Y.; Jiang, L. The effect of thickness on the optoelectronic properties of organic field-effect transistors: Towards molecular crystals at monolayer limit. J. Mater. Chem. C 2020, 8, 13154–13168. [Google Scholar] [CrossRef]
- Li, H.; Dailey, J.; Kale, T.; Besar, K.; Koehler, K.; Katz, H.E. Sensitive and selective NO2 sensing based on alkyl- and alkylthio-thiophene polymer conductance and conductance ratio changes from differential chemical doping. ACS Appl. Mater. Interfaces 2017, 9, 20501–20507. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Song, J.; Jang, H.-J.; Dailey, J.; Yu, J.; Katz, H.E. Chemical and biomolecule sensing with organic field-efect transistors. Chem. Rev. 2018, 119, 3–35. [Google Scholar] [CrossRef]
- Canevet, D.; Sallé, M.; Zhang, G.; Zhang, D.; Zhu, D. Tetrathiafulvalene (TTF) derivatives: Key building-blocks for switchable processes. Chem. Commun. 2009, 2245–2269. [Google Scholar] [CrossRef]
- Jiang, M.; Su, J.; Song, X.; Zhang, P.; Zhu, M.; Qin, L.; Tie, Z.; Zuo, J.-L.; Jin, Z. Interfacial reduction nucleation of noble metal nanodots on redox-active metal–organic frameworks for high-efficiency electrocatalytic conversion of nitrate to ammonia. Nano Lett. 2022, 22, 2529–2537. [Google Scholar] [CrossRef]
- Li, Z.-L.; Zhou, L.-S.; Wei, Y.-H.; Peng, H.-L.; Huang, K. Highly efficient, reversible, and selective absorption of SO2 in 1-Ethyl-3-methylimidazolium chloride plus imidazole deep eutectic solvents. Ind. Eng. Chem. Res. 2020, 59, 13696–13705. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Interaction Region Indicator: A simple real space function clearly revealing both chemical bonds and weak interactions. Chem. 2021, 1, 231–239. [Google Scholar] [CrossRef]
- Yu, Y.-X. Binding Energy and Work Function of organic electrode materials phenanthraquinone, pyromellitic dianhydride and their derivatives adsorbed on graphene. ACS Appl. Mater. Interfaces 2014, 6, 16267–16275. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Huang, C. Molecular Design of Benzothiadiazole-Fused Tetrathiafulvalene Derivatives for OFET Gas Sensors: A Computational Study. Sensors 2025, 25, 6190. https://doi.org/10.3390/s25196190
Xu X, Huang C. Molecular Design of Benzothiadiazole-Fused Tetrathiafulvalene Derivatives for OFET Gas Sensors: A Computational Study. Sensors. 2025; 25(19):6190. https://doi.org/10.3390/s25196190
Chicago/Turabian StyleXu, Xiuru, and Changfa Huang. 2025. "Molecular Design of Benzothiadiazole-Fused Tetrathiafulvalene Derivatives for OFET Gas Sensors: A Computational Study" Sensors 25, no. 19: 6190. https://doi.org/10.3390/s25196190
APA StyleXu, X., & Huang, C. (2025). Molecular Design of Benzothiadiazole-Fused Tetrathiafulvalene Derivatives for OFET Gas Sensors: A Computational Study. Sensors, 25(19), 6190. https://doi.org/10.3390/s25196190




