Development and Field Deployment of a Compact Dual-Range Infrared Carbon Dioxide Sensor System
Abstract
:1. Introduction
2. Design of the Compact Dual-Range CO2 Sensor System
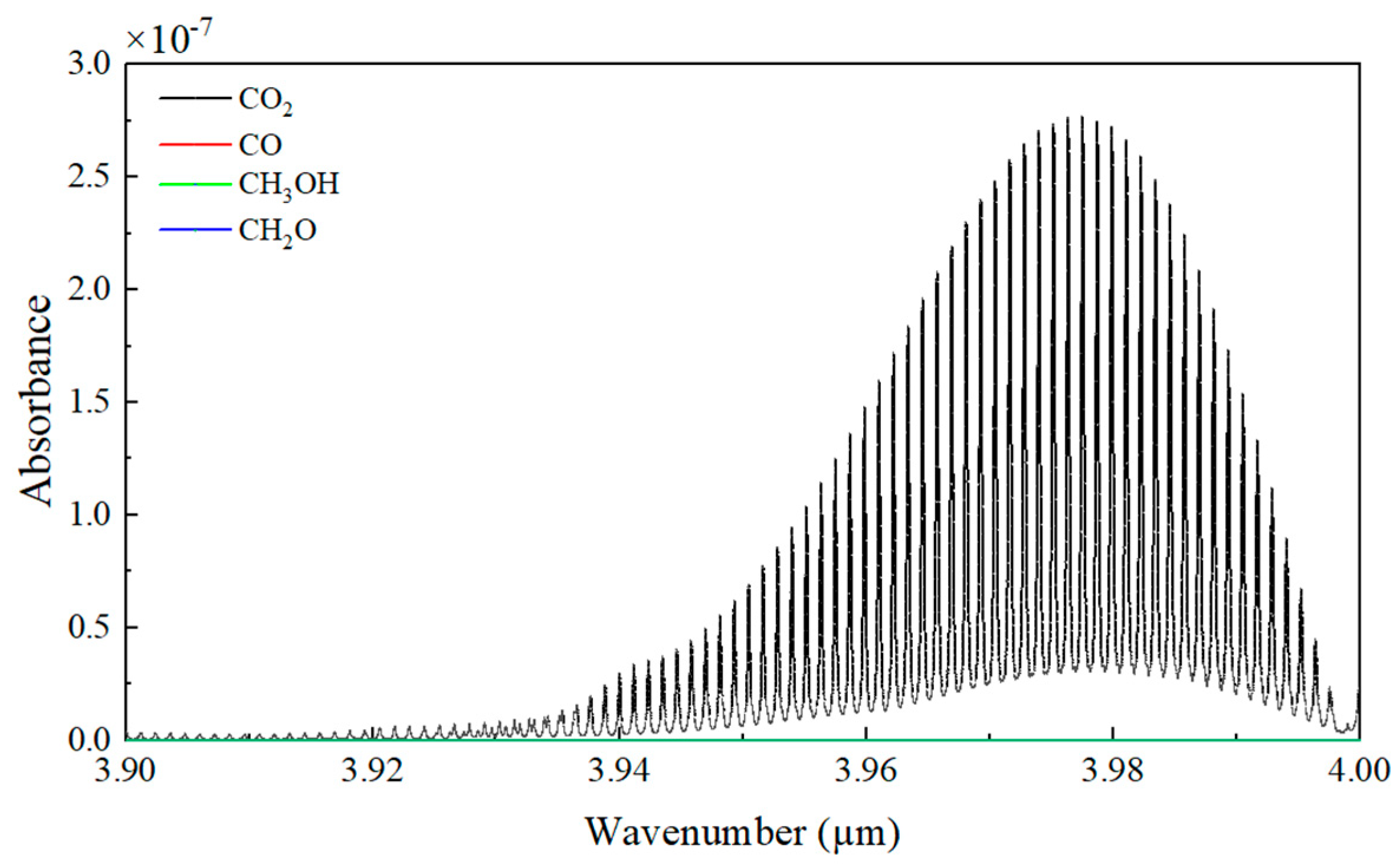
2.1. Detection Principle
2.2. Optical Path Design
2.3. Signal Conditioner
3. Performance Evaluation of the CO2 Sensor System
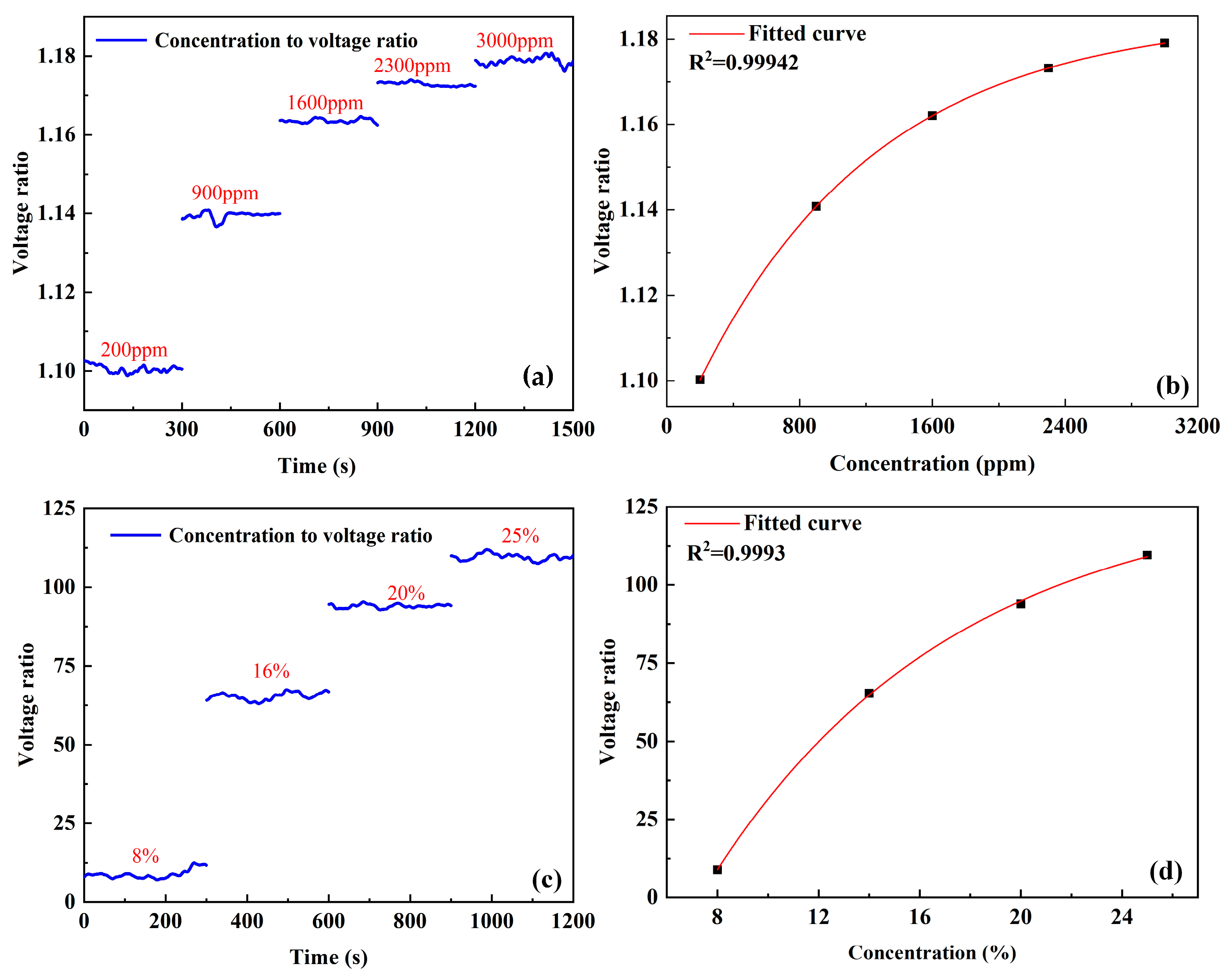
3.1. Calibration Experiment
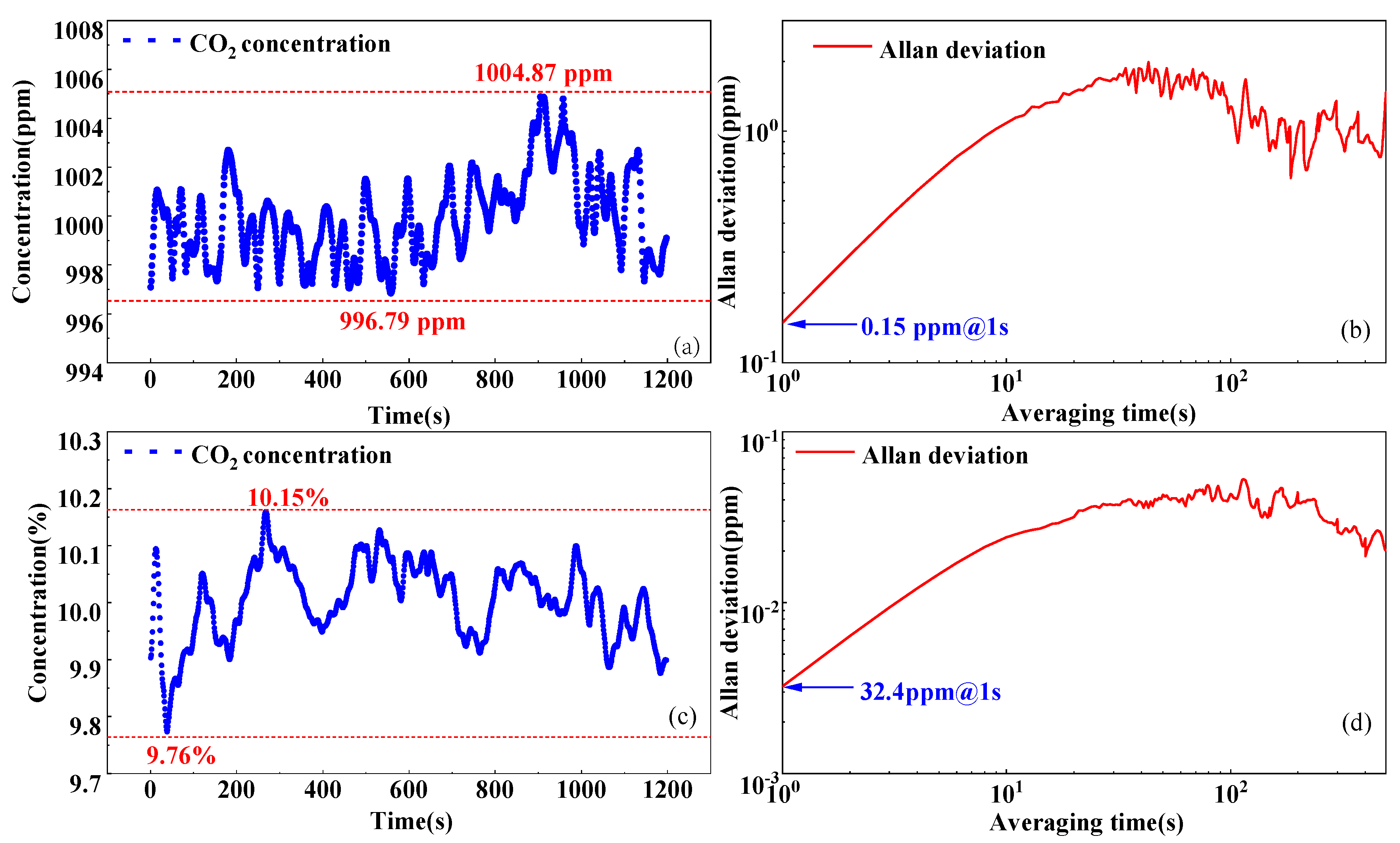
3.2. Stability and Allan Deviation Analysis
3.3. Temperature and Humidity Compensation
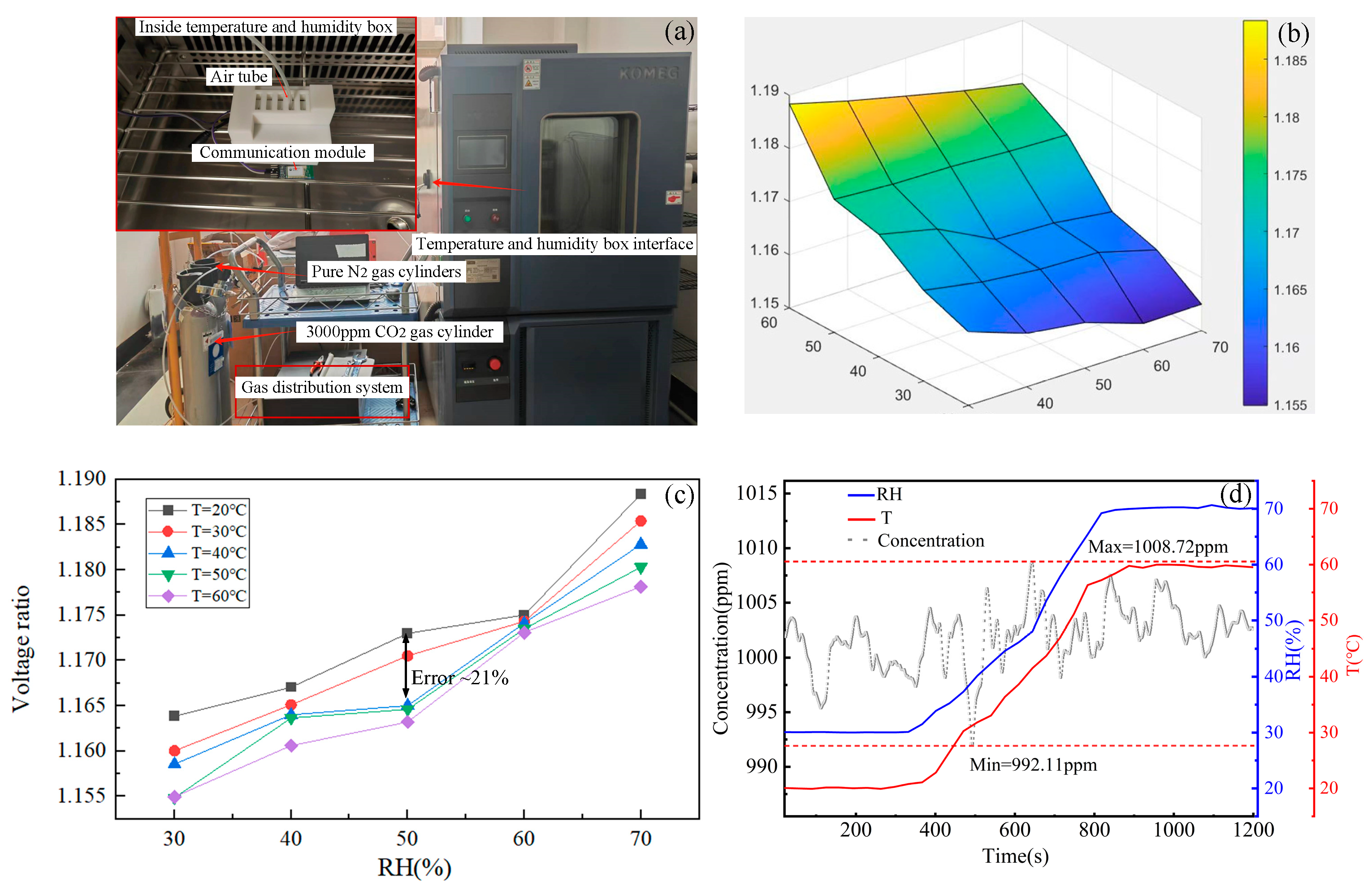
3.3.1. Experimental Environment
3.3.2. Influence of Temperature and Humidity on the Sensor
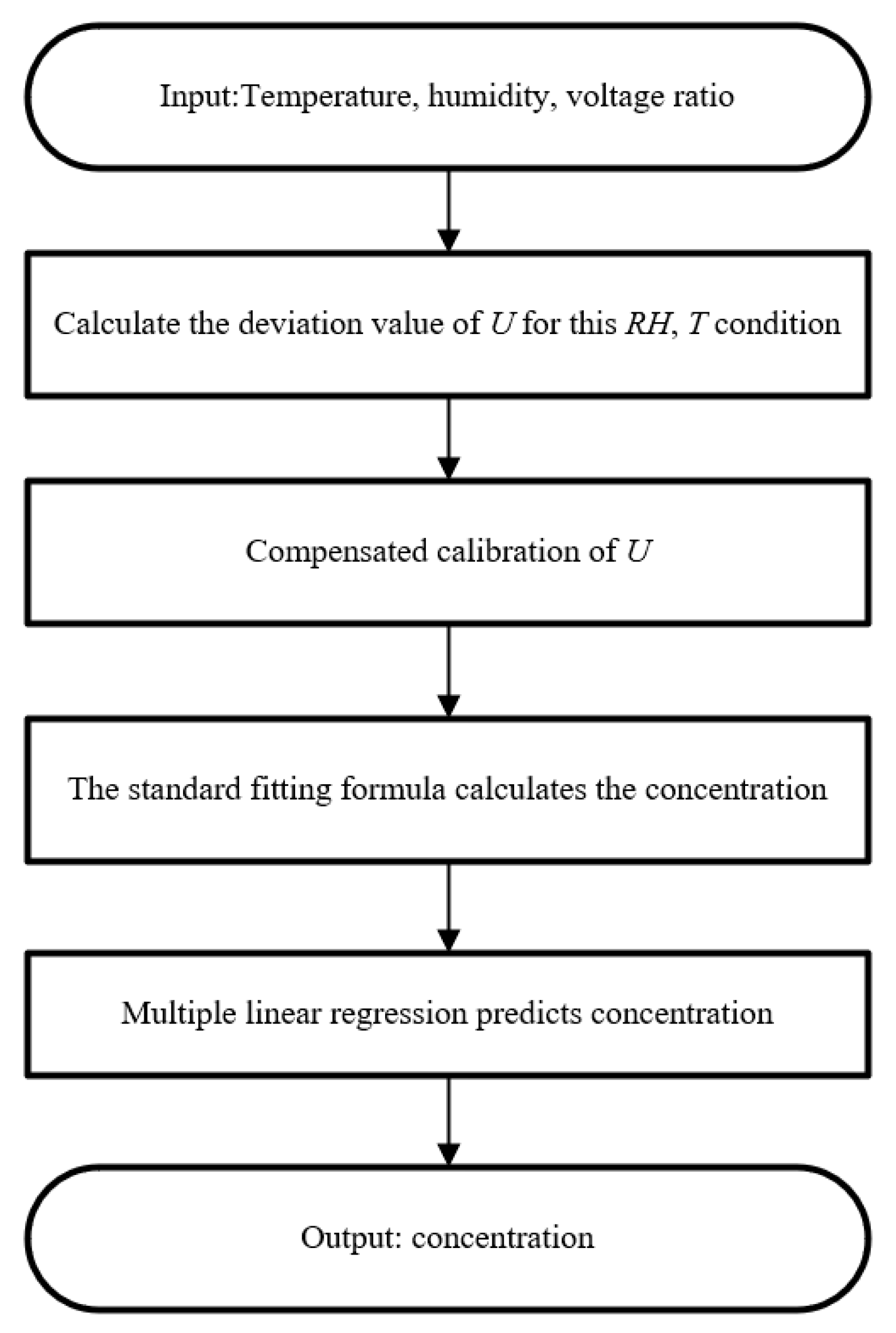
3.3.3. Temperature and Humidity Compensation Algorithm
4. Field Application of the Sensor System
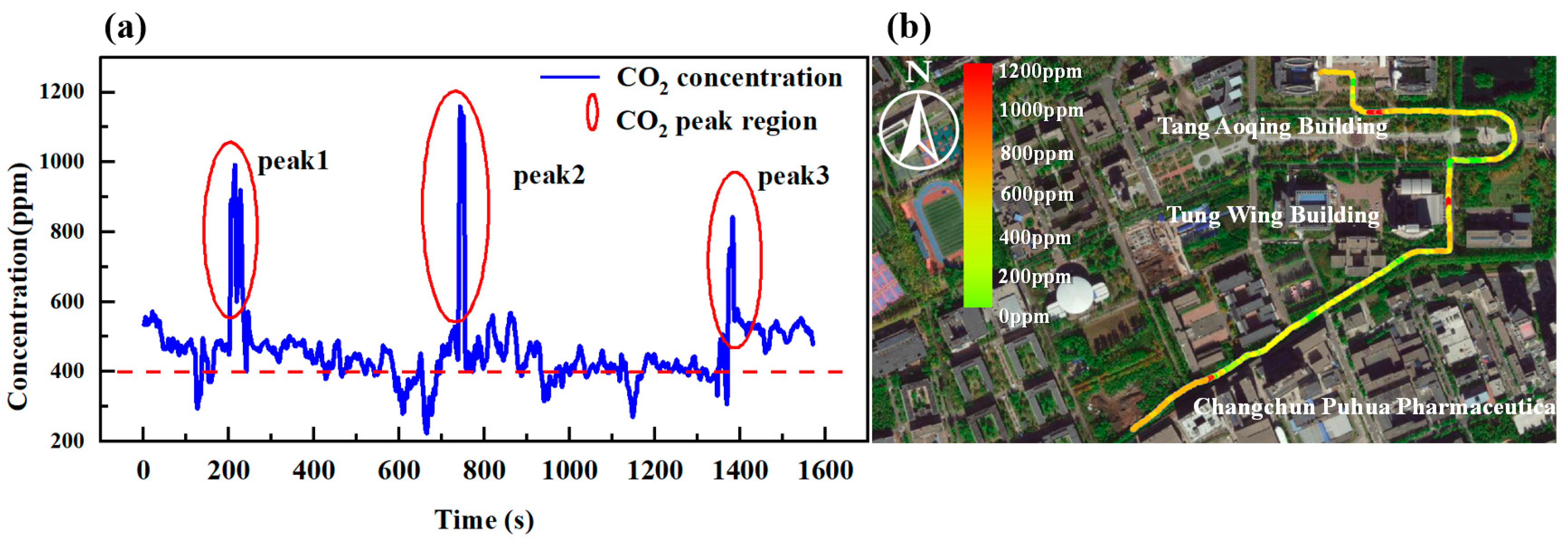
4.1. Inspection Experiments
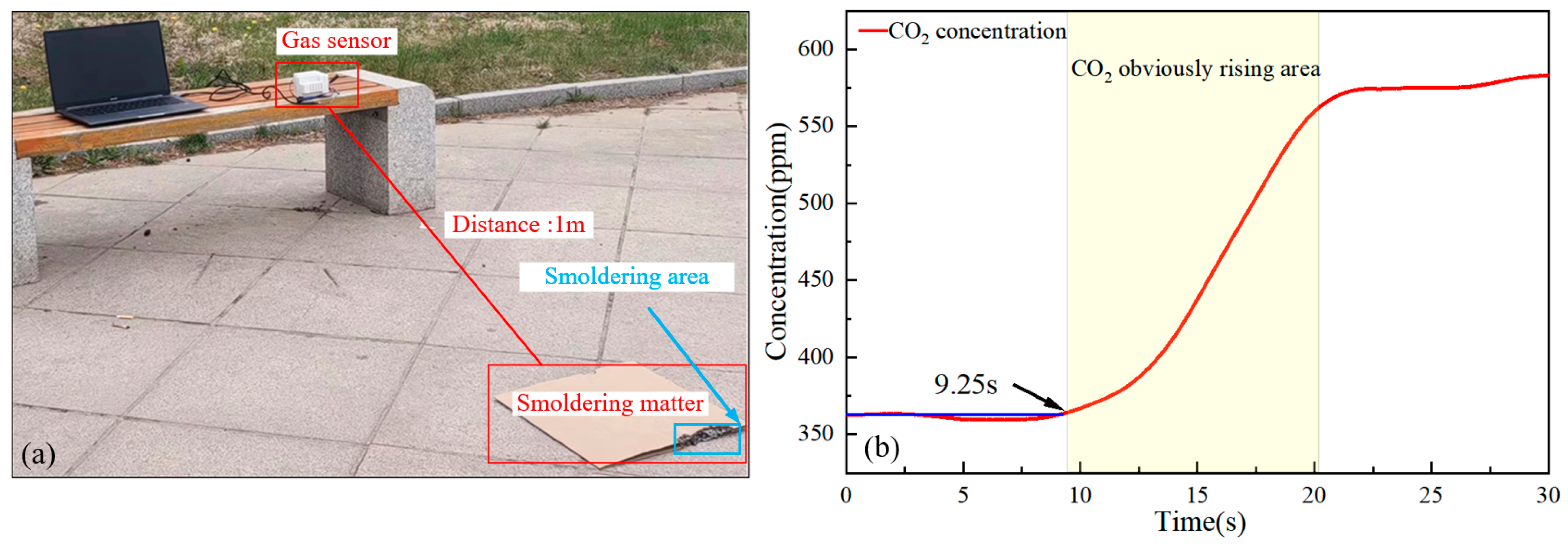
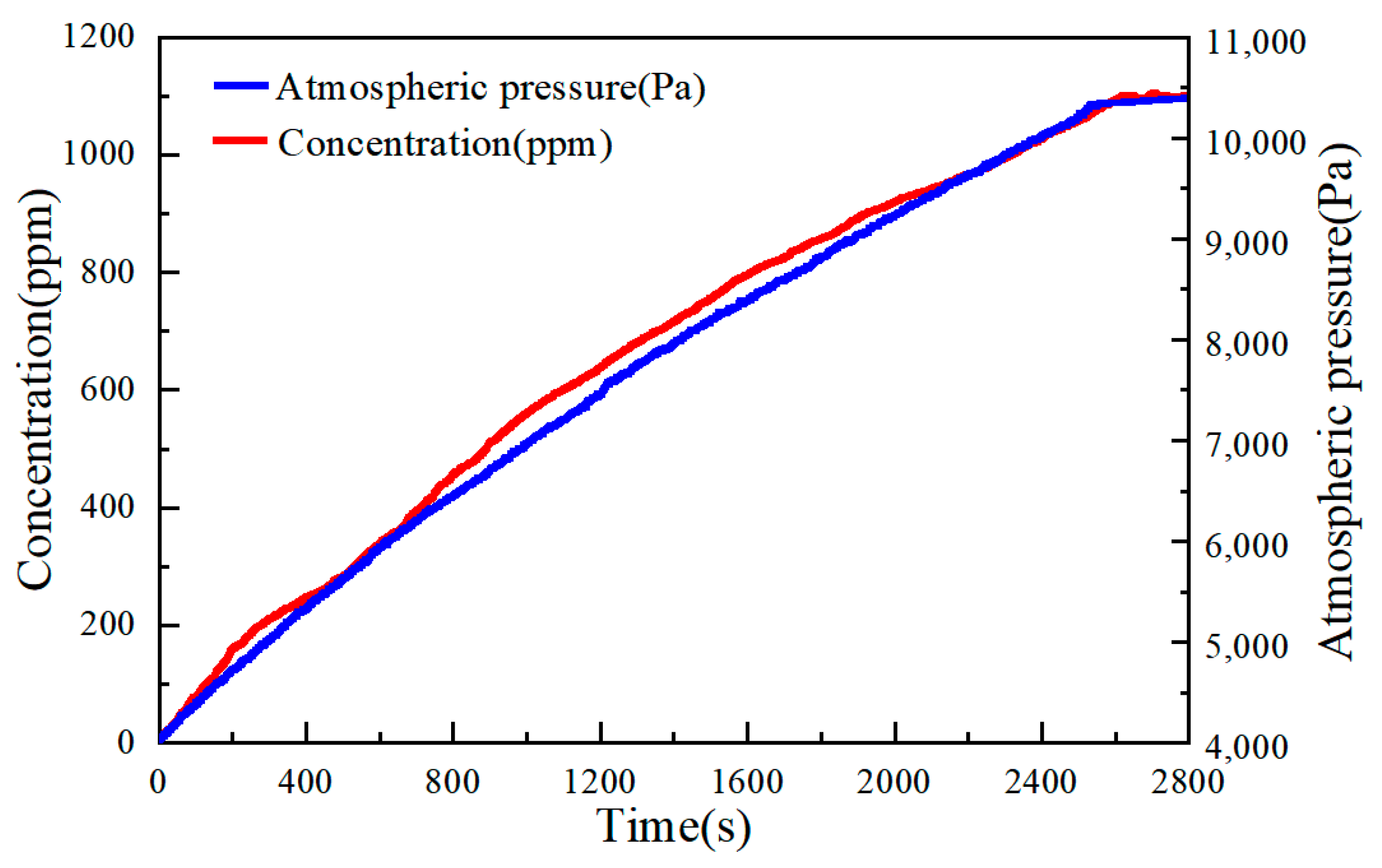
4.2. Fire Detection Experiment
4.3. Continuous Indoor CO2 Monitoring Experiment
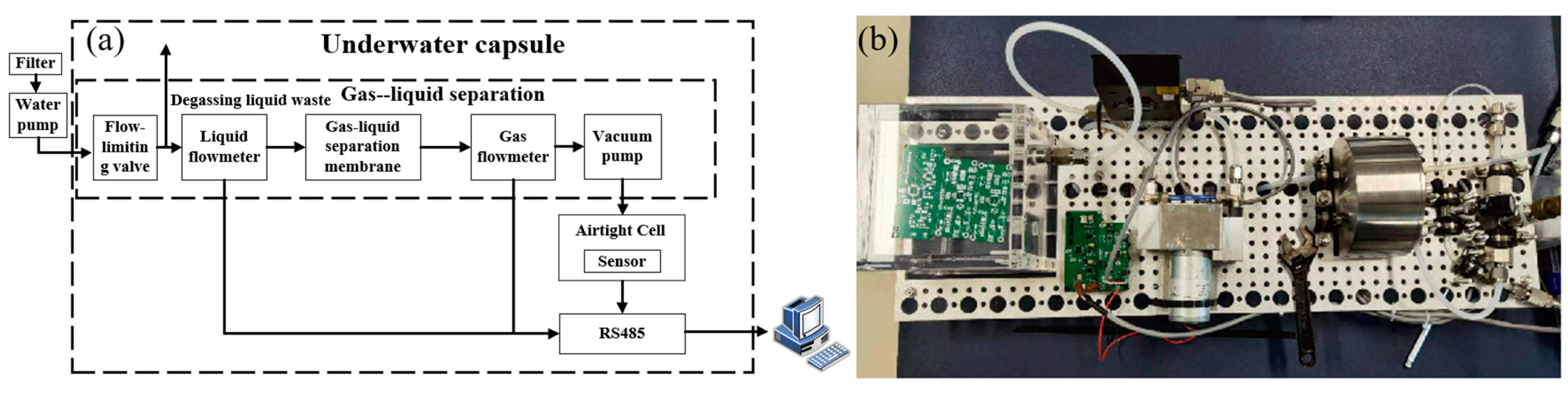
4.4. Underwater Carbon Dioxide Detection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, D.; Hubacek, K.; Weber, C.L.; Peters, G.P.; Reiner, D.M. The drivers of Chinese CO2 emissions from 1980 to 2030. Glob. Environ. Change 2008, 18, 626–634. [Google Scholar] [CrossRef]
- Grubb, M.; Sha, F.; Spencer, T.; Hughes, N.; Zhang, Z.; Agnolucci, P. A review of Chinese CO2emission projections to 2030: The role of economic structure and policy. Clim. Policy 2015, 15, S7–S39. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials and hybrid nanocomposites for CO2 capture and utilization: Environmental and energy sustainability. RSC Adv. 2022, 12, 23869–23888. [Google Scholar] [CrossRef] [PubMed]
- Myhre, G.; Alterskjær, K.; Lowe, D. A fast method for updating global fossil fuel carbon dioxide emissions. Environ. Res. Lett. 2009, 4, 034012. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Liu, C.; Li, S.; Li, Q.; Sun, N.; Gao, K.; Jiang, Z.; Chang, K.; Xuan, Y. Air to fuel: Direct capture of CO2 from air and in-situ solar-driven conversion into syngas via Nix/NaA nanomaterials. Nano Res. 2023, 16, 10899–10912. [Google Scholar] [CrossRef]
- Climate Highlights 2023. Available online: https://www.climate.gov/news-features/features/climate-highlights-2023 (accessed on 10 October 2023).
- Global Temperature. Available online: https://climate.nasa.gov/vital-signs/global-temperature (accessed on 10 October 2023).
- Tian, H.Q.; Lu, C.Q.; Ciais, P.; Michalak, A.M.; Canadell, J.G.; Saikawa, E.; Huntzinger, D.N.; Gurney, K.R.; Sitch, S.; Zhang, B.W.; et al. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature 2016, 531, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, J.M.; Bruns, M.A.; Casadevall, A.; Criddle, C.S.; Eloe-Fadrosh, E.; Karl, D.M.; Nguyen, N.K.; Zhou, J.Z. Microbes and Climate Change: A Research Prospectus for the Future. mBio 2022, 13, 9. [Google Scholar] [CrossRef]
- Liu, L.; Liu, R.; Ma, G.; Feng, S.; Mu, Y.; Meng, D.; Wang, S.; Cai, E. Online Monitoring of Seawater Carbon Dioxide Based on an Infrared Rear Beam Splitter. Sensors 2023, 23, 6273. [Google Scholar] [CrossRef]
- Shen, C.-H.; Wu, J.-J. A New Electro-Optical-Thermal Modelling for Non-Dispersive IR Sensing Technique of Gas Concentration. Appl. Sci. 2022, 12, 7772. [Google Scholar] [CrossRef]
- Behera, K.; Pandey, S.; Kadyan, A.; Pandey, S. Ionic Liquid-Based Optical and Electrochemical Carbon Dioxide Sensors. Sensors 2015, 15, 30487–30503. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Ning, Z.; Ye, S.; Sun, L.; Yang, F.; Wong, K.; Westerdahl, D.; Louie, P. Impact Analysis of Temperature and Humidity Conditions on Electrochemical Sensor Response in Ambient Air Quality Monitoring. Sensors 2018, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Toat, M.; Soekirno, S.; Faisal, F. Monitoring system for carbon dioxide gas concentration using NDIR sensors. AIP Conf. Proc. 2023, 2604, 060006. [Google Scholar]
- Zhou, L.; He, Y.; Zhang, Q.; Zhang, L. Carbon Dioxide Sensor Module Based on NDIR Technology. Micromachines 2021, 12, 845. [Google Scholar] [CrossRef]
- Jha, R.K. Non-Dispersive Infrared Gas Sensing Technology: A Review. IEEE Sens. J. 2022, 22, 6–15. [Google Scholar] [CrossRef]
- Dinh, T.-V.; Choi, I.-Y.; Son, Y.-S.; Kim, J.-C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, T.; Huang, M. High accuracy wide range CO2 detection method based on difference optical path NDIR. Sens. Actuators A Phys. 2023, 36, 114722. [Google Scholar] [CrossRef]
- Xu, M.; Peng, B.; Zhu, X.; Guo, Y. Multi-Gas Detection System Based on Non-Dispersive Infrared (NDIR) Spectral Technology. Sensors 2022, 22, 836. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.; MacGregor, C. A Novel Solid State Non-Dispersive Infrared CO2 Gas Sensor Compatible with Wireless and Portable Deployment. Sensors 2013, 13, 7079–7103. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Wang, X.; Zhang, W. Real-Time Correction of Gas Concentration in Nondispersive Infrared Sensor. IEEE Trans. Instrum. Meas. 2023, 72, 1–10. [Google Scholar] [CrossRef]
- Dinh, T.-V.; Lee, J.-Y.; Ahn, J.-W.; Kim, J.-C. Development of a Wide-Range Non-Dispersive Infrared Analyzer for the Continuous Measurement of CO2 in Indoor Environments. Atmosphere 2020, 11, 1024. [Google Scholar] [CrossRef]
- Xu, M.; Xu, Y.; Tao, J.; Wen, L.; Zheng, C.; Yu, Z.; He, S. Development of a compact NDIR CO2 gas sensor for harsh environments. Infrared Phys. Technol. 2024, 136, 105035. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Y.; Ming, A.; Fang, Y.; Fang, S.; Bi, S.; Chen, J.; Xu, R.; Wei, F.; Mao, C. Application of an NDIR Sensor System Developed for Early Thermal Runaway Warning of Automotive Batteries. Energies 2023, 16, 3620. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.X.; Liu, Y.Y.; Zhang, X.S.; Huan, H.T.; Yin, X.K.; Xi, T.L.; Shao, X.P. Advances in differential photoacoustic spectroscopy for trace gas detection. Microw. Opt. Technol. Lett. 2023, 65, 1506–1515. [Google Scholar] [CrossRef]



| Relative Humidity (%) | 30, 40, 50, 60, 70 |
| Temperature (°C) | 20, 30, 40, 50, 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xiao, X.; Zhang, Z.; Song, F.; Wang, Y.; Zheng, C. Development and Field Deployment of a Compact Dual-Range Infrared Carbon Dioxide Sensor System. Sensors 2025, 25, 1445. https://doi.org/10.3390/s25051445
Liu X, Xiao X, Zhang Z, Song F, Wang Y, Zheng C. Development and Field Deployment of a Compact Dual-Range Infrared Carbon Dioxide Sensor System. Sensors. 2025; 25(5):1445. https://doi.org/10.3390/s25051445
Chicago/Turabian StyleLiu, Xiaoteng, Xuehua Xiao, Zhening Zhang, Fang Song, Yiding Wang, and Chuantao Zheng. 2025. "Development and Field Deployment of a Compact Dual-Range Infrared Carbon Dioxide Sensor System" Sensors 25, no. 5: 1445. https://doi.org/10.3390/s25051445
APA StyleLiu, X., Xiao, X., Zhang, Z., Song, F., Wang, Y., & Zheng, C. (2025). Development and Field Deployment of a Compact Dual-Range Infrared Carbon Dioxide Sensor System. Sensors, 25(5), 1445. https://doi.org/10.3390/s25051445






