Photoacoustic Imaging for Image-Guided Gastric Tube Placement: Ex Vivo Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. PA Imaging for Evaluating Different Tissue Compositions
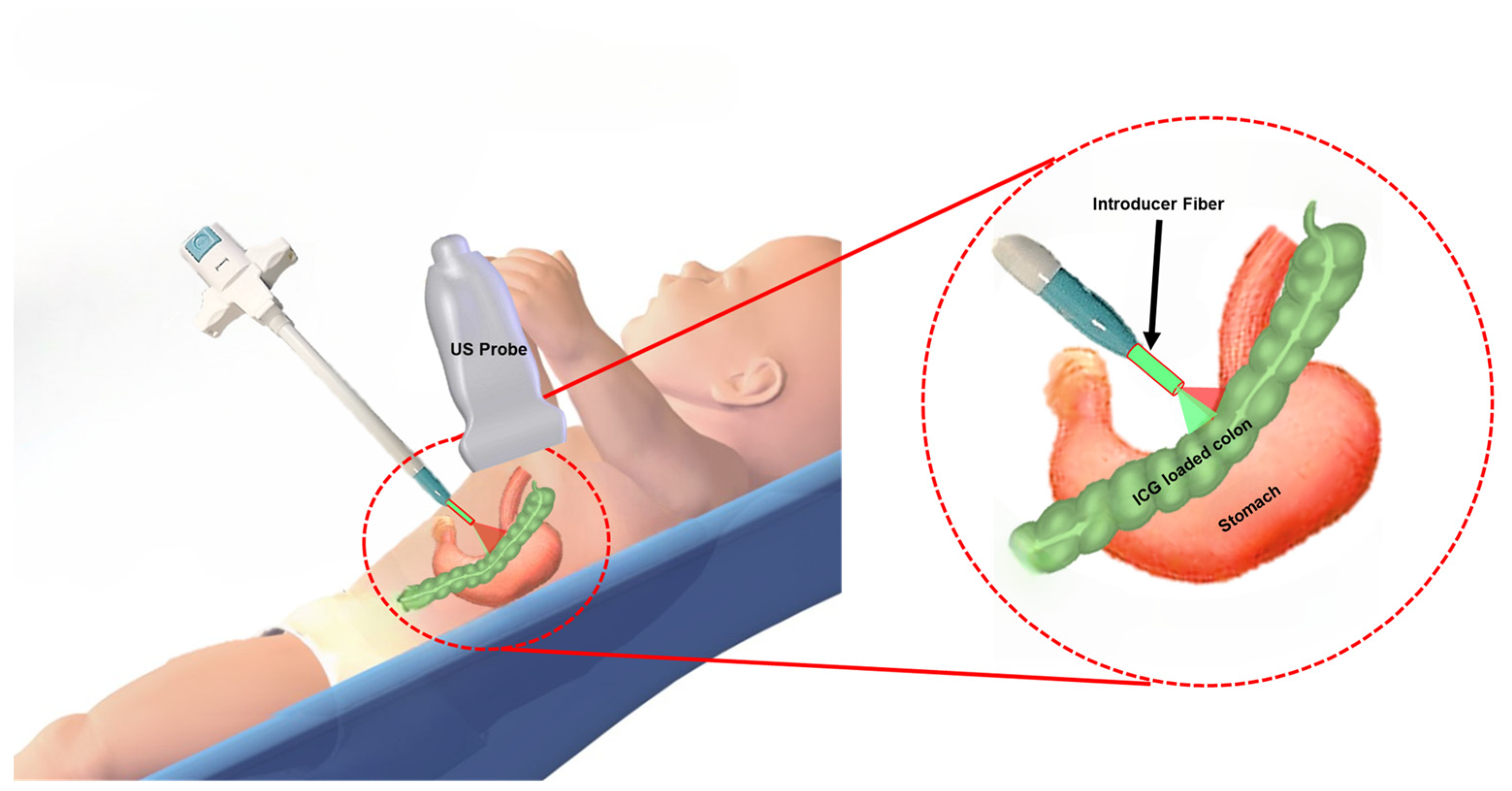
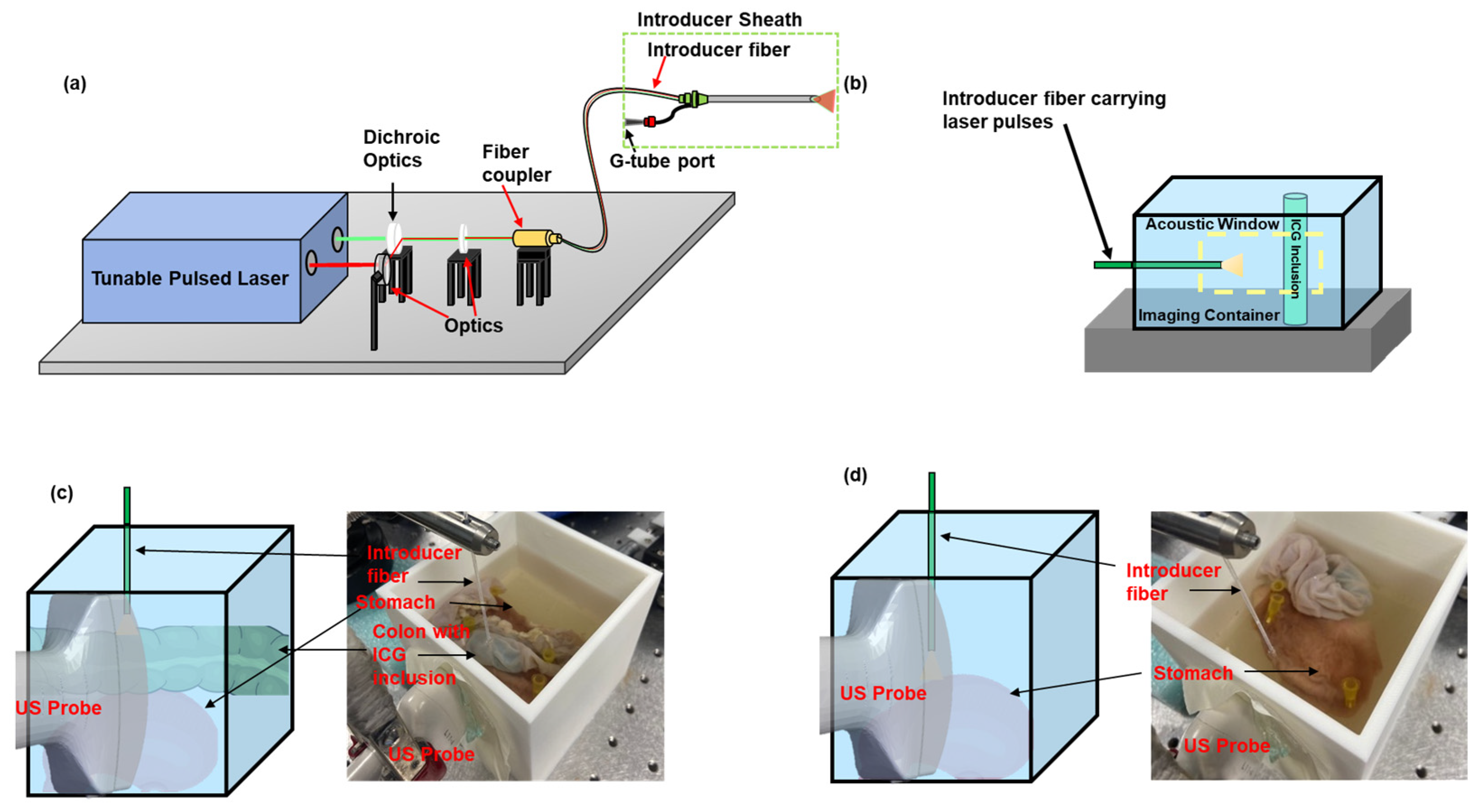
2.2. Integrated US and PA Image-Guided G-Tube Placement System
2.3. Characterization of the Sensitivity of PA Imaging to Detect ICG
2.4. Evaluating the Organ Detection Capability of PA Imaging in an Excised Rabbit Tissue
3. Results
3.1. Sensitivity of PA Imaging to Detect ICG
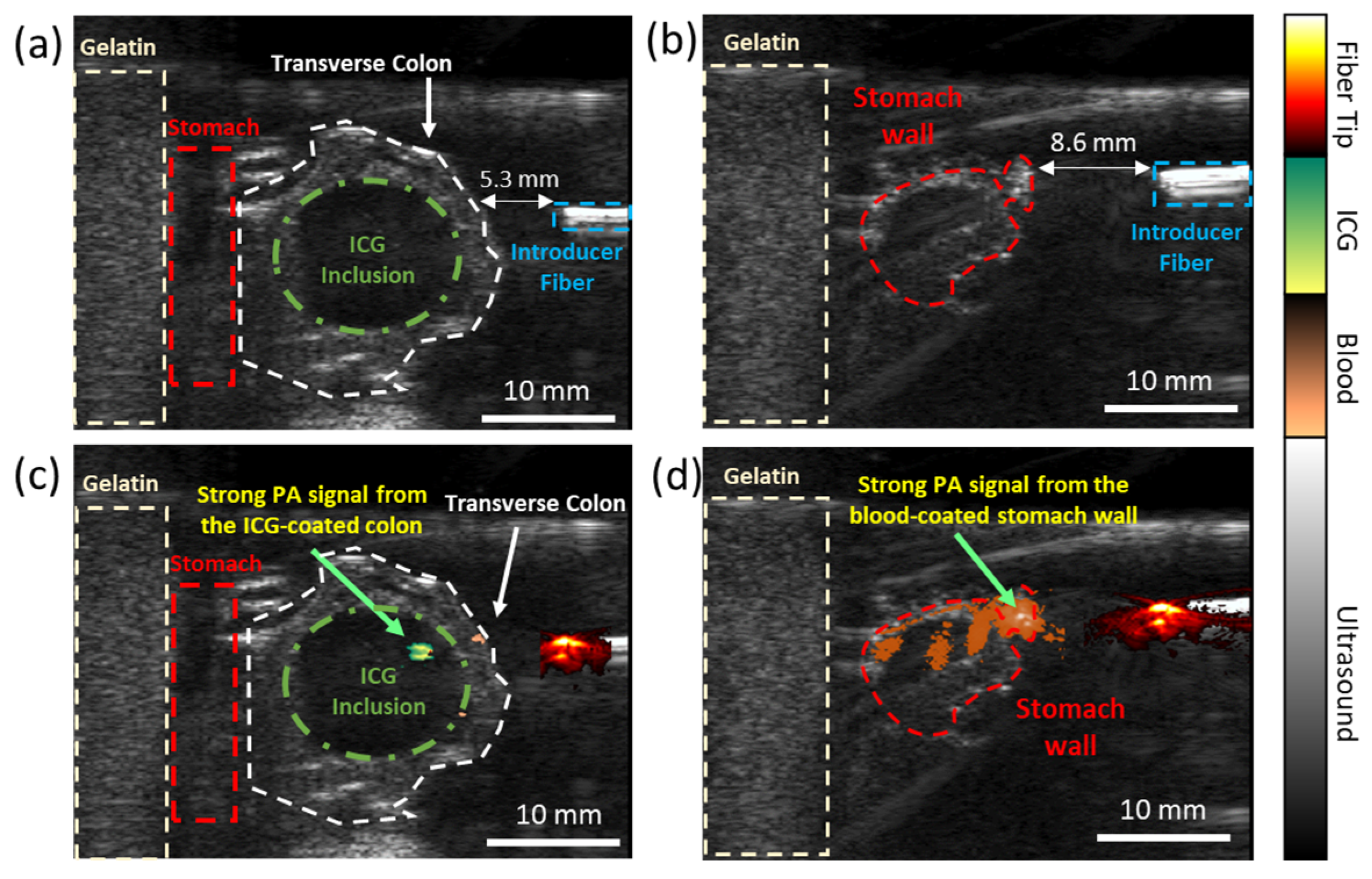
3.2. Evaluating the Dual-Wavelength PA Approach in an Ex Vivo Animal Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, D.; Campagna, E.J.; Friedlander, J.; Partrick, D.A.; Rees, D.I.; Kempe, A. National trends and outcomes of pediatric gastrostomy tube placement. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Velazco, N.K. Gastrostomy tubes: Indications, types, and care. Pediatr. Rev. 2024, 45, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Rahnemai-Azar, A.A.; Rahnemaiazar, A.A.; Naghshizadian, R.; Kurtz, A.; Farkas, D.T. Percutaneous endoscopic gastrostomy: Indications, technique, complications and management. World J. Gastroenterol. WJG 2014, 20, 7739. [Google Scholar] [CrossRef] [PubMed]
- Gauderer, M.W.; Ponsky, J.L.; Izant, R.J. Gastrostomy without laparotomy: A percutaneous endoscopic technique. J. Pediatr. Surg. 1980, 15, 872–875. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Shin, J.H.; Lee, Y.J.; Kim, K.-R.; Kim, J.H.; Song, H.-Y.; Kim, K.M. Fluoroscopically guided nasojejunal enteral tube placement in infants and young children. Am. J. Roentgenol. 2009, 193, 545–548. [Google Scholar] [CrossRef]
- Hoffer, F.; Sandler, R.; Kaplan, L.; Mandell, V.; Haynie, M.; Leichner, A. Fluoroscopic placement of jejunal feeding tubes. Pediatr. Radiol. 1992, 22, 287–289. [Google Scholar] [CrossRef]
- Law, A.C.; Stevens, J.P.; Walkey, A.J. Gastrostomy tube use in the critically ill, 1994–2014. Ann. Am. Thorac. Soc. 2019, 16, 724–730. [Google Scholar] [CrossRef]
- Ambur, V.; Taghavi, S.; Jayarajan, S.; Gaughan, J.; Toyoda, Y.; Dauer, E.; Sjoholm, L.O.; Pathak, A.; Santora, T.; Goldberg, A.J. Comparing open gastrostomy tube to percutaneous endoscopic gastrostomy tube in heart transplant patients. Ann. Med. Surg. 2016, 7, 71–74. [Google Scholar] [CrossRef]
- Mizrahi, I.; Garg, M.; Divino, C.M.; Nguyen, S. Comparison of laparoscopic versus open approach to gastrostomy tubes. JSLS J. Soc. Laparoendosc. Surg. 2014, 18, 28. [Google Scholar] [CrossRef]
- John, S.; Hester, S.; Basij, M.; Paul, A.; Xavierselvan, M.; Mehrmohammadi, M.; Mallidi, S. Niche preclinical and clinical applications of photoacoustic imaging with endogenous contrast. Photoacoustics 2023, 32, 100533. [Google Scholar] [CrossRef]
- Yan, Y.; Gomez-Lopez, N.; Basij, M.; Shahvari, A.V.; Vadillo-Ortega, F.; Hernandez-Andrade, E.; Hassan, S.S.; Romero, R.; MehrMohammadi, M. Photoacoustic imaging of the uterine cervix to assess collagen and water content changes in murine pregnancy. Biomed. Opt. Express 2019, 10, 4643–4655. [Google Scholar] [CrossRef]
- Viator, J.A.; Svaasand, L.O.; Aguilar, G.; Choi, B.; Nelson, J.S. Photoacoustic measurement of epidermal melanin. In Biomedical Optoacoustics IV; SPIE: Bellingham, WA, USA, 2003; Volume 4960, pp. 14–20. [Google Scholar]
- John, S.; Yan, Y.; Kabbani, L.; Kennedy, N.A.; Mehrmohammadi, M. Integration of Endovenous Laser Ablation and Photoacoustic Imaging Systems for Enhanced Treatment of Venous Insufficiency. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–4. [Google Scholar]
- Yan, Y.; John, S.; Ghalehnovi, M.; Kabbani, L.; Kennedy, N.A.; Mehrmohammadi, M. photoacoustic Imaging for Image-guided endovenous Laser Ablation procedures. Sci. Rep. 2019, 9, 2933. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Gavrilova, A.A.; Kapralov, S.V.; Grishaev, V.A.; Tuchin, V.V. Optical properties of human stomach mucosa in the spectral range from 400 to 2000 nm: Prognosis for gastroenterology. Med. Laser Appl. 2007, 22, 95–104. [Google Scholar] [CrossRef]
- Carvalho, S.; Gueiral, N.; Nogueira, E.; Henrique, R.; Oliveira, L.; Tuchin, V.V. Comparative study of the optical properties of colon mucosa and colon precancerous polyps between 400 and 1000 nm. In Dynamics and Fluctuations in Biomedical Photonics XIV; SPIE: Bellingham, WA, USA, 2017; Volume 10063, pp. 218–233. [Google Scholar]
- Son, G.M.; Kwon, M.S.; Kim, Y.; Kim, J.; Kim, S.H.; Lee, J.W. Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg. Endosc. 2019, 33, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wu, H.; Bao, M.; Luo, S.; Wang, X.; Zhao, C.; Liu, Q.; Wang, X.; Zhou, Z.; Zhou, H. Indocyanine green fluorescence imaging to assess bowel perfusion during totally laparoscopic surgery for colon cancer. BMC Surg. 2020, 20, 102. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Colino, R.; Espin-Basany, E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: A systematic review and meta-analysis. Tech. Coloproctol. 2018, 22, 15–23. [Google Scholar] [CrossRef]
- Wang, X.; Ku, G.; Wegiel, M.A.; Bornhop, D.J.; Stoica, G.; Wang, L.V. Noninvasive photoacoustic angiography of animal brains in vivo with near-infrared light and an optical contrast agent. Opt. Lett. 2004, 29, 730–732. [Google Scholar] [CrossRef]
- Sano, K.; Ohashi, M.; Kanazaki, K.; Makino, A.; Ding, N.; Deguchi, J.; Kanada, Y.; Ono, M.; Saji, H. Indocyanine green-labeled polysarcosine for in vivo photoacoustic tumor imaging. Bioconjug. Chem. 2017, 28, 1024–1030. [Google Scholar] [CrossRef]
- Yan, Y.; John, S.; Shaik, T.; Patel, B.; Lam, M.T.; Kabbani, L.; Mehrmohammadi, M. Photoacoustic-guided endovenous laser ablation: Characterization and in vivo canine study. Photoacoustics 2021, 24, 100298. [Google Scholar] [CrossRef]
- Peltrini, R.; Podda, M.; Castiglioni, S.; Di Nuzzo, M.M.; D’Ambra, M.; Lionetti, R.; Sodo, M.; Luglio, G.; Mucilli, F.; Di Saverio, S.; et al. Intraoperative use of indocyanine green fluorescence imaging in rectal cancer surgery: The state of the art. World J. Gastroenterol. 2021, 27, 6374. [Google Scholar] [CrossRef]
- Gandorfer, A.; Haritoglou, C.; Kampik, A. Toxicity of indocyanine green in vitreoretinal surgery. World J. Gastroenterol. 2021, 27, 6374. [Google Scholar]
- Anderson, N.G.; Butler, A.; Scott, N.; Cook, N.; Butzer, J.; Schleich, N.; Firsching, M.; Grasset, R.; De Ruiter, N.; Campbell, M.; et al. Spectroscopic (multi-energy) CT distinguishes iodine and barium contrast material in MICE. Eur. Radiol. 2010, 20, 2126–2134. [Google Scholar] [CrossRef]
- Lin, C.; Chen, F.; Hariri, A.; Chen, C.; Wilder-Smith, P.; Takesh, T.; Jokerst, J. Photoacoustic imaging for noninvasive periodontal probing depth measurements. J. Dent. Res. 2018, 97, 23–30. [Google Scholar] [CrossRef]
- Wang, D.; Lee, D.H.; Huang, H.; Vu, T.; Lim, R.S.A.; Nyayapathi, N.; Chitgupi, U.; Liu, M.; Geng, J.; Xia, J. Ingestible roasted barley for contrast-enhanced photoacoustic imaging in animal and human subjects. Biomaterials 2018, 175, 72–81. [Google Scholar] [CrossRef]
- Zhang, H.; Nagy, A.; Bowman, C.; Peladeau-Pigeon, M.; Hu, A.; Lovell, J.; Steele, C.M.; Xia, J. Food-grade activated charcoal for contrast-enhanced photoacoustic imaging of aspiration: A phantom study. Dysphagia 2022, 37, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Kilian, H.I.; Zhang, H.; Bhurwani, M.M.S.; Nilam, A.M.; Seong, D.; Jeon, M.; Ionita, C.N.; Xia, J.; Lovell, J.F. Barium sulfate and pigment admixture for photoacoustic and x-ray contrast imaging of the gut. J. Biomed. Opt. 2023, 28, 082803. [Google Scholar] [CrossRef] [PubMed]
- Van Staveren, H.J.; Moes, C.J.; van Marie, J.; Prahl, S.A.; Van Gemert, M.J. Light scattering in lntralipid-10% in the wavelength range of 400–1100 nm. Appl. Opt. 1991, 30, 4507–4514. [Google Scholar] [CrossRef]
- Homan, K.; Kim, S.; Chen, Y.-S.; Wang, B.; Mallidi, S.; Emelianov, S. Prospects of molecular photoacoustic imaging at 1064 nm wavelength. Opt. Lett. 2010, 35, 2663–2665. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, S.; Yuja Vaquiz, Y.; Nyayapathi, N.; Kabbani, L.; Nilam, A.; Lovell, J.F.; Wilson, N.A.; Yan, Y.; Mehrmohammadi, M. Photoacoustic Imaging for Image-Guided Gastric Tube Placement: Ex Vivo Characterization. Sensors 2025, 25, 1597. https://doi.org/10.3390/s25051597
John S, Yuja Vaquiz Y, Nyayapathi N, Kabbani L, Nilam A, Lovell JF, Wilson NA, Yan Y, Mehrmohammadi M. Photoacoustic Imaging for Image-Guided Gastric Tube Placement: Ex Vivo Characterization. Sensors. 2025; 25(5):1597. https://doi.org/10.3390/s25051597
Chicago/Turabian StyleJohn, Samuel, Yeidi Yuja Vaquiz, Nikhila Nyayapathi, Loay Kabbani, Anoop Nilam, Jonathan F. Lovell, Nicole A. Wilson, Yan Yan, and Mohammad Mehrmohammadi. 2025. "Photoacoustic Imaging for Image-Guided Gastric Tube Placement: Ex Vivo Characterization" Sensors 25, no. 5: 1597. https://doi.org/10.3390/s25051597
APA StyleJohn, S., Yuja Vaquiz, Y., Nyayapathi, N., Kabbani, L., Nilam, A., Lovell, J. F., Wilson, N. A., Yan, Y., & Mehrmohammadi, M. (2025). Photoacoustic Imaging for Image-Guided Gastric Tube Placement: Ex Vivo Characterization. Sensors, 25(5), 1597. https://doi.org/10.3390/s25051597






