Fabricating a Three-Dimensional Surface-Enhanced Raman Scattering Substrate Using Hydrogel-Loaded Freeze-Induced Silver Nanoparticle Aggregates for the Highly Sensitive Detection of Organic Pollutants in Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Preparation of AgNAs
2.4. Synthesis of 3D Hydrogel-Loaded Silver Nanoparticle Aggregates
2.5. Spike Detection of MG in Seawater
3. Results
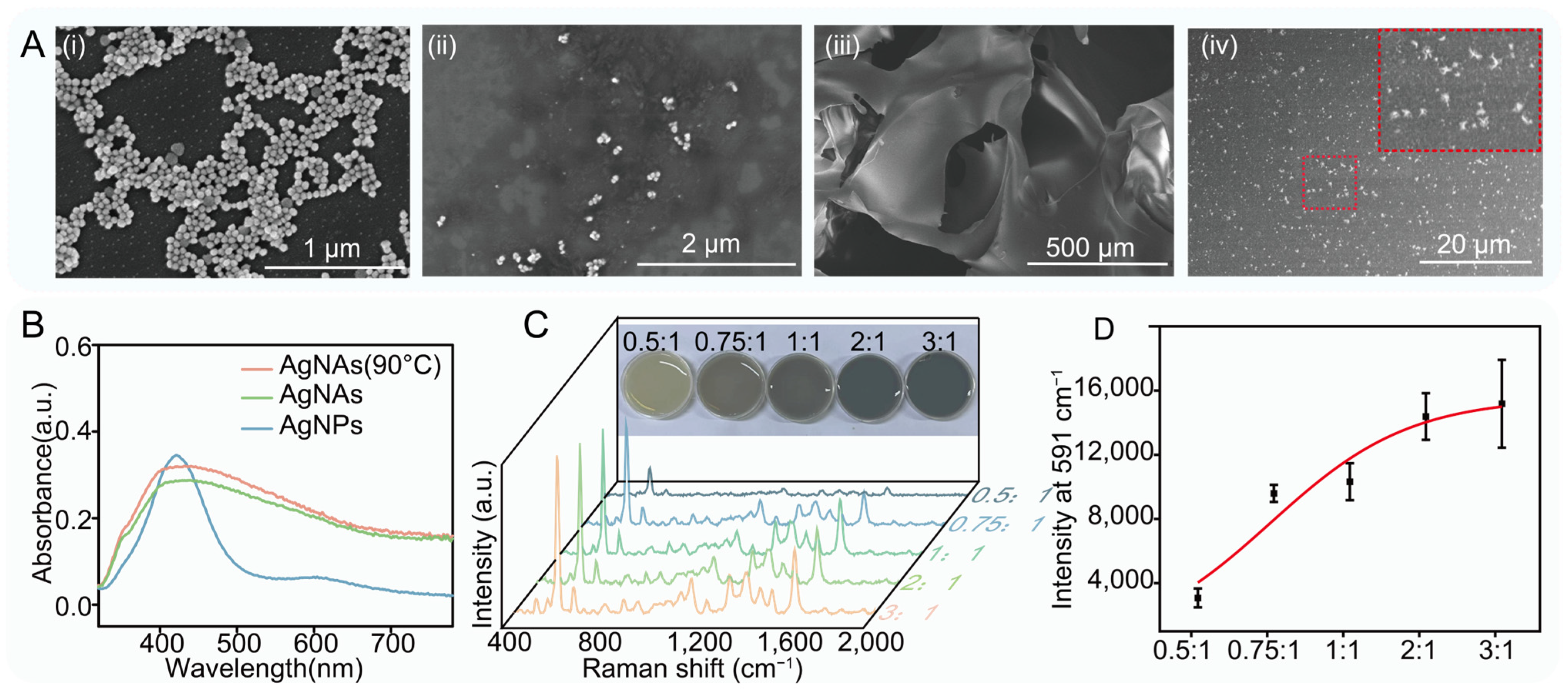
3.1. Preparation and Characterization of Hydrogel-Loaded 3D SERS Substrate
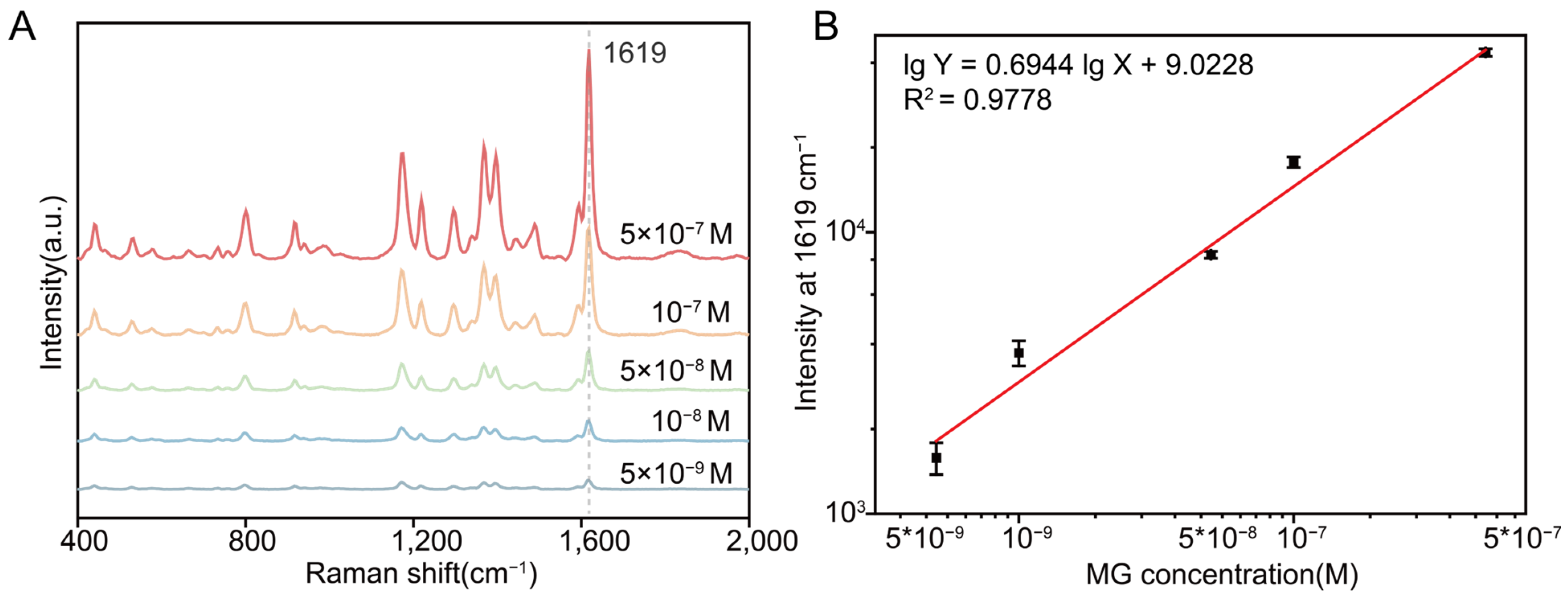
3.2. High Sensitivity of Hydrogel-Loaded 3D SERS Substrate
3.3. Spatial Uniformty of Hydrogel-Loaded 3D SERS Substrate
3.4. Stability of Hydrogel-Loaded 3D SERS Substrate Under High-Salt Conditions
3.5. Detection of Marine Pollutants Using Hydrogel-Loaded 3D SERS Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, C.; Zhao, L.; Zhu, J.; Zhang, S. Simultaneous detection of trace Hg2+ and Ag+ by SERS aptasensor based on a novel cascade amplification in environmental water. Chem. Eng. J. 2022, 435, 133879. [Google Scholar] [CrossRef]
- Yang, C.-W.; Zhang, X.; Yuan, L.; Wang, Y.-K.; Sheng, G.-P. Deciphering the microheterogeneous repartition effect of environmental matrix on surface-enhanced Raman spectroscopy (SERS) analysis for pollutants in natural waters. Water Res. 2023, 232, 119668. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qu, L.; Zhai, W.; Xue, J.; Fossey, J.S.; Long, Y. Facile On-Site Detection of Substituted Aromatic Pollutants in Water Using Thin Layer Chromatography Combined with Surface-Enhanced Raman Spectroscopy. Environ. Sci. Technol. 2011, 45, 4046–4052. [Google Scholar] [CrossRef]
- Han, X.X.; Rodriguez, R.S.; Haynes, C.L.; Ozaki, Y.; Zhao, B. Surface-enhanced Raman spectroscopy. Nat. Rev. Methods Primers 2022, 1, 87. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, R.; Wu, Y.; Zhang, Z.; Chen, Y.; Liu, M.; Zhou, N.; Wang, Y.; Fu, X.; Zhuang, X.; et al. Ultralow-background SERS substrates for reliable identification of organic pollutants and degradation intermediates. J. Hazard. Mater. 2023, 460, 132508. [Google Scholar] [CrossRef]
- Liu, L.; Gao, T.; Zhao, Q.; Xue, Z.; Wu, Y. Self-assembled bimetallic plasmonic nanocavity substrate for supersensitive SERS. Opt. Laser Technol. 2025, 181, 111827. [Google Scholar] [CrossRef]
- Li, H.; Merkl, P.; Sommertune, J.; Thersleff, T.; Sotiriou, G.A. SERS Hotspot Engineering by Aerosol Self-Assembly of Plasmonic Ag Nanoaggregates with Tunable Interparticle Distance. Adv. Sci. 2022, 9, 2201133. [Google Scholar] [CrossRef]
- Shin, H.-H.; Yeon, G.J.; Choi, H.-K.; Park, S.-M.; Lee, K.S.; Kim, Z.H. Frequency-Domain Proof of the Existence of Atomic-Scale SERS Hot-Spots. Nano Lett. 2018, 18, 262–271. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, Y.; Wang, Z.; Yong, J.; Xiong, W.; Wu, D.; Xu, S. Efficient concentration of trace analyte with ordered hotspot construction for a robust and sensitive SERS platform. Int. J. Extrem. Manuf. 2024, 6, 035505. [Google Scholar] [CrossRef]
- Hong, J.; Jung, D.; Park, S.; Oh, Y.; Oh, K.K.; Lee, S.H. Immobilization of laccase via cross-linked enzyme aggregates prepared using genipin as a natural cross-linker. Int. J. Biol. Macromol. 2021, 169, 541–550. [Google Scholar] [CrossRef]
- Abd Rahman, N.H.; Jaafar, N.R.; Shamsul Annuar, N.A.; Rahman, R.A.; Abdul Murad, A.M.; El-Enshasy, H.A.; Illias, R.M. Efficient substrate accessibility of cross-linked levanase aggregates using dialdehyde starch as a macromolecular cross-linker. Carbohydr. Polym. 2021, 267, 118159. [Google Scholar] [CrossRef]
- Kumari, S.; Nehra, M.; Jain, S.; Dilbaghi, N.; Chaudhary, G.R.; Kim, K.H.; Kumar, S. Metallosurfactant aggregates: Structures, properties, and potentials for multifarious applications. Adv. Colloid Interface Sci. 2024, 323, 103065. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Ding, H.; Xu, S.; Li, M.; Kong, F.; Luo, Y.; Li, G. Self-assembly of noble metallic spherical aggregates from monodisperse nanoparticles: Their synthesis and pronounced SERS and catalytic properties. J. Mater. Chem. A 2013, 1, 3362–3371. [Google Scholar] [CrossRef]
- Bekana, D.; Liu, R.; Amde, M.; Liu, J.-F. Use of Polycrystalline Ice for Assembly of Large Area Au Nanoparticle Superstructures as SERS Substrates. ACS Appl. Mater. Interfaces 2017, 9, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Lao, Z.; Zheng, Y.; Dai, Y.; Hu, Y.; Ni, J.; Ji, S.; Cai, Z.; Smith, Z.J.; Li, J.; Zhang, L.; et al. Nanogap Plasmonic Structures Fabricated by Switchable Capillary-Force Driven Self-Assembly for Localized Sensing of Anticancer Medicines with Microfluidic SERS. Adv. Funct. Mater. 2020, 30, 1909467. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Z.; Zhang, L.; Miao, J.; Chen, Y.; Zhao, R.; Liu, M.; Chen, L.; Wang, X. Size-controllable colloidal Ag nano-aggregates with long-time SERS detection window for on-line high-throughput detection. Talanta 2023, 257, 124358. [Google Scholar] [CrossRef]
- Ye, Z.; Li, C.; Chen, Q.; Xu, Y.; Bell, S.E.J. Ultra-Stable Plasmonic Colloidal Aggregates for Accurate and Reproducible Quantitative SE(R)RS in Protein-Rich Biomedia. Angew. Chem. Int. Ed. Engl. 2019, 58, 19054–19059. [Google Scholar] [CrossRef]
- Schwartzberg, A.M.; Grant, C.D.; Wolcott, A.; Talley, C.E.; Huser, T.R.; Bogomolni, R.; Zhang, J.Z. Unique Gold Nanoparticle Aggregates as a Highly Active Surface-Enhanced Raman Scattering Substrate. J. Phys. Chem. B 2004, 108, 19191–19197. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Yang, H.; Tong, G.; Qiao, P.; Jing, J.; Fang, J.; Yang, Z.; Li, H. Temperature-Sensitive Porous Hydrogel with High Salt Resistance for Solar Interface Evaporators. ACS Appl. Polym. Mater. 2024, 6, 14660–14667. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, H.; Zhu, H.; Qu, L. Hierarchically Structured Hydrogels for Rapid Solar Vapor Generation with Super Resistance to Salt. Adv. Funct. Mater. 2025, 2500459. [Google Scholar] [CrossRef]
- He, N.; Yang, Y.; Wang, H.; Li, F.; Jiang, B.; Tang, D.; Li, L. Ion-Transfer Engineering via Janus Hydrogels Enables Ultrahigh Performance and Salt-Resistant Solar Desalination. Adv. Mater. 2023, 35, 2300189. [Google Scholar] [CrossRef]
- Yilmaz, D.; Miranda, B.; Lonardo, E.; Rea, I.; De Stefano, L.; De Luca, A.C. SERS-based pH-Dependent detection of sulfites in wine by hydrogel nanocomposites. Biosens. Bioelectron. 2024, 245, 115836. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tian, R.; Liu, G.; Wen, Y.; Bian, X.; Luan, D.; Wang, H.; Lai, K.; Yan, J. Fishing unfunctionalized SERS tags with DNA hydrogel network generated by ligation-rolling circle amplification for simple and ultrasensitive detection of kanamycin. Biosens. Bioelectron. 2022, 207, 114187. [Google Scholar] [CrossRef]
- Wang, H.; Xu, P.; Chen, Y.; Wang, C.; Chen, S.; Ren, J.; Lu, Y.; Chen, J.; Zhang, L.; Liu, Y.; et al. “Partner” cellulose gel with “dialysis” function: Achieve the integration of filtration-enrichment-SERS detection. Biosens. Bioelectron. 2025, 267, 116775. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, R.; Ma, C.; Liu, H.; Zhu, H.; Jin, H.; Wang, F.; Cheng, Z.; Feng, Y.; Yu, L. Dynamically controllable hot spots in DNA-derived hydrogel scaffold SERS substrate for exosome recognition using DNA self-assembly amplification. Chem. Eng. J. 2024, 496, 154270. [Google Scholar] [CrossRef]
- Kim, D.J.; Jeon, T.Y.; Park, S.-G.; Han, H.J.; Im, S.H.; Kim, D.-H.; Kim, S.-H. Uniform Microgels Containing Agglomerates of Silver Nanocubes for Molecular Size-Selectivity and High SERS Activity. Small 2017, 13, 1604048. [Google Scholar] [CrossRef]
- Yang, K.; Dong, Q.; Liu, H.; Wu, L.; Zong, S.; Wang, Z. A MXene Hydrogel-Based Versatile Microrobot for Controllable Water Pollution Management. Adv. Sci. 2024, 11, 2309257. [Google Scholar] [CrossRef]
- Fathima, H.; Paul, L.; Thirunavukkuarasu, S.; Thomas, K.G. Mesoporous Silica-Capped Silver Nanoparticles for Sieving and Surface-Enhanced Raman Scattering-Based Sensing. ACS Appl. Nano Mater. 2020, 3, 6376–6384. [Google Scholar] [CrossRef]
- Hasi, W.-L.-J.; Lin, X.; Lou, X.-T.; Lin, S.; Yang, F.; Lin, D.-Y.; Lu, Z.-W. Chloride ion-assisted self-assembly of silver nanoparticles on filter paper as SERS substrate. Appl. Phys. A 2015, 118, 799–807. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, J.; Sun, M.; He, J.; Liu, J.; Mi, J.; Zhao, K.; Su, J.; Tu, K.; Peng, J.; et al. Isotropic shrinkage-inspired strategy for plasmonic nanoparticle-loaded hydrogel SERS sensor towards robust and sensitive detection of pesticides. Chem. Eng. J. 2025, 509, 161380. [Google Scholar] [CrossRef]
- Gao, T.; Yachi, T.; Shi, X.; Sato, R.; Sato, C.; Yonamine, Y.; Kanie, K.; Misawa, H.; Ijiro, K.; Mitomo, H. Ultrasensitive Surface-Enhanced Raman Scattering Platform for Protein Detection via Active Delivery to Nanogaps as a Hotspot. ACS Nano 2024, 18, 21593–21606. [Google Scholar] [CrossRef]
- Peng, S.; Yan, L.; You, R.; Lu, Y.; Liu, Y.; Li, L. Cationic cellulose dispersed Ag NCs/C-CNF paper-based SERS substrate with high homogeneity for creatinine and uric acid detection. Int. J. Biol. Macromol. 2024, 282, 136724. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Sun, H.; Xu, S.; He, Y.; Xu, H.; Wang, D. Aggregation, settling characteristics and destabilization mechanisms of nano-particles under different conditions. Sci. Total Environ. 2022, 827, 154228. [Google Scholar] [CrossRef]
- Ding, G.; Xie, S.; Zhu, Y.; Liu, Y.; Wang, L.; Xu, F. Graphene oxide wrapped Fe3O4@Au nanohybrid as SERS substrate for aromatic dye detection. Sens. Actuators B Chem. 2015, 221, 1084–1093. [Google Scholar] [CrossRef]
- Byram, C.; Rathod, J.; Moram, S.S.B.; Mangababu, A.; Soma, V.R. Picosecond Laser-Ablated Nanoparticles Loaded Filter Paper for SERS-Based Trace Detection of Thiram, 1,3,5-Trinitroperhydro-1,3,5-triazine (RDX), and Nile Blue. Nanomaterials 2022, 12, 2150. [Google Scholar] [CrossRef]
- Moram, S.S.B.; Byram, C.; Soma, V.R. Femtosecond laser patterned silicon embedded with gold nanostars as a hybrid SERS substrate for pesticide detection. RSC Adv. 2023, 13, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yu, Q.; Kong, X.; Zhang, M. Preparation of Plasmonic Ag@PS Composite via Seed-Mediated In Situ Growth Method and Application in SERS. Front. Chem. 2022, 10, 847203. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, A.; Zhang, X.; Jiao, J.; Yuan, Y.; Huang, Y.; Yan, S. Mxenes–Au NP Hybrid Plasmonic 2D Microplates in Microfluidics for SERS Detection. Biosensors 2022, 12, 505. [Google Scholar] [CrossRef]
- Rathod, J.; Byram, C.; Kanaka, R.K.; Sree Satya Bharati, M.; Banerjee, D.; Akkanaboina, M.; Soma, V.R. Hybrid Surface-Enhanced Raman Scattering Substrates for the Trace Detection of Ammonium Nitrate, Thiram, and Nile Blue. ACS Omega 2022, 7, 15969–15981. [Google Scholar] [CrossRef]




| Sample | Spiked (nM) | Detected (nM) | Recovery (%) |
|---|---|---|---|
| 1 | 50 | 45.2 | 90.4 |
| 2 | 50 | 47.7 | 95.4 |
| 3 | 50 | 54.4 | 108.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Hu, Y.; Zhang, Z. Fabricating a Three-Dimensional Surface-Enhanced Raman Scattering Substrate Using Hydrogel-Loaded Freeze-Induced Silver Nanoparticle Aggregates for the Highly Sensitive Detection of Organic Pollutants in Seawater. Sensors 2025, 25, 2575. https://doi.org/10.3390/s25082575
Liu H, Hu Y, Zhang Z. Fabricating a Three-Dimensional Surface-Enhanced Raman Scattering Substrate Using Hydrogel-Loaded Freeze-Induced Silver Nanoparticle Aggregates for the Highly Sensitive Detection of Organic Pollutants in Seawater. Sensors. 2025; 25(8):2575. https://doi.org/10.3390/s25082575
Chicago/Turabian StyleLiu, Hai, Yufeng Hu, and Zhiyang Zhang. 2025. "Fabricating a Three-Dimensional Surface-Enhanced Raman Scattering Substrate Using Hydrogel-Loaded Freeze-Induced Silver Nanoparticle Aggregates for the Highly Sensitive Detection of Organic Pollutants in Seawater" Sensors 25, no. 8: 2575. https://doi.org/10.3390/s25082575
APA StyleLiu, H., Hu, Y., & Zhang, Z. (2025). Fabricating a Three-Dimensional Surface-Enhanced Raman Scattering Substrate Using Hydrogel-Loaded Freeze-Induced Silver Nanoparticle Aggregates for the Highly Sensitive Detection of Organic Pollutants in Seawater. Sensors, 25(8), 2575. https://doi.org/10.3390/s25082575







