Classifying Storage Temperature for Mandarin (Citrus reticulata L.) Using Bioimpedance and Diameter Measurements with Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Storage Temperature
2.2. Data Acquisition
2.2.1. Bioimpedance Measurements
2.2.2. Diameter Measurements
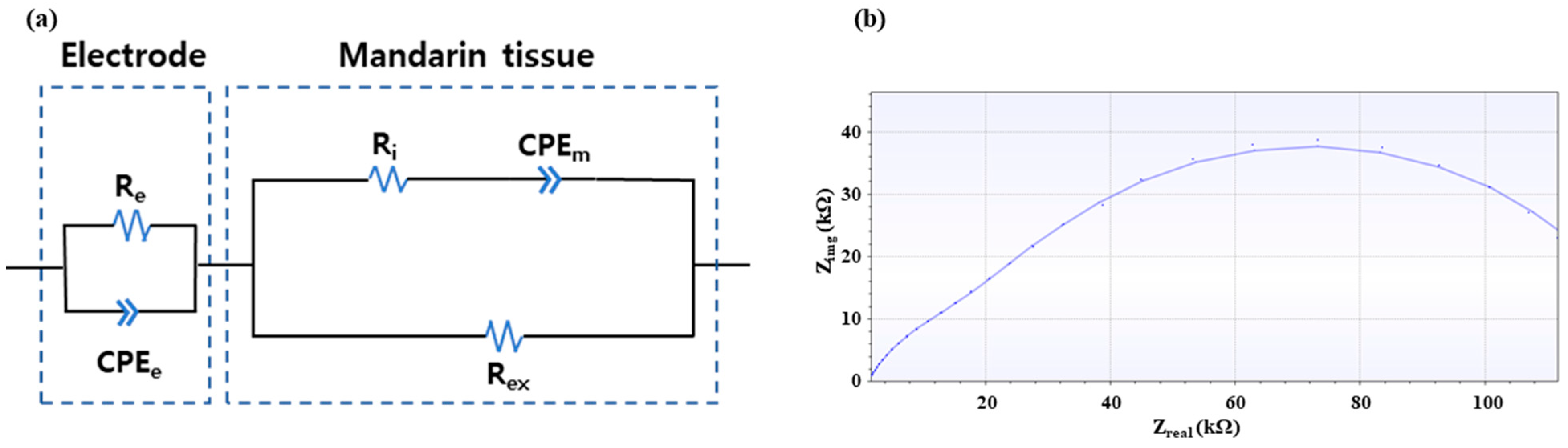
2.2.3. Equivalent Circuit Fitting
2.3. Machine Learning Classifications
2.3.1. Data Preprocessing
2.3.2. Machine Learning Models
2.3.3. Datasets for Machine Learning Model
2.3.4. Model Tuning and Performance Evaluation for Machine Learning Classifier
3. Results and Discussion
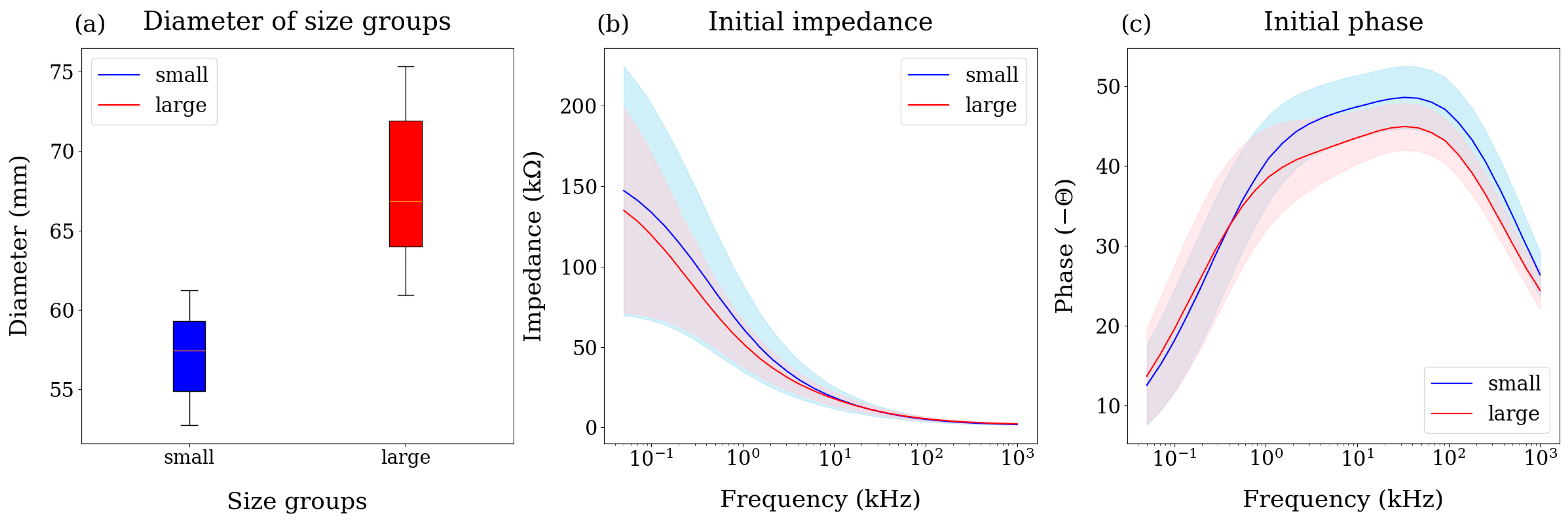
3.1. Bioimpedance and Size Distribution
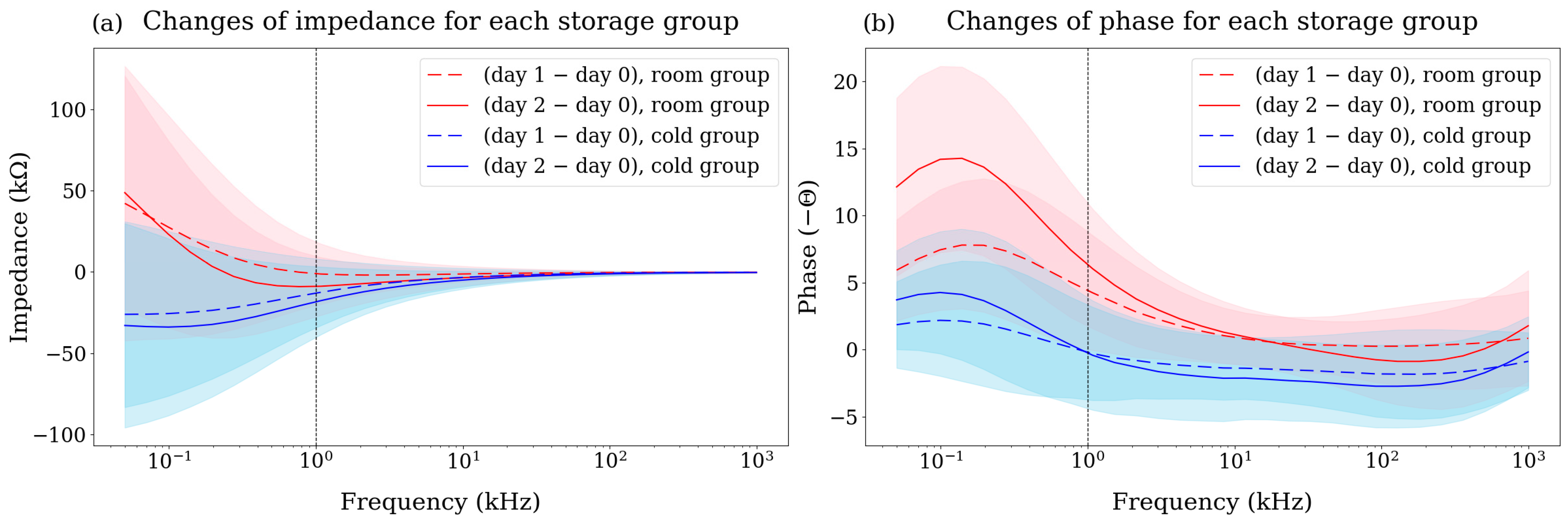
3.2. Changes in Bioimpedance
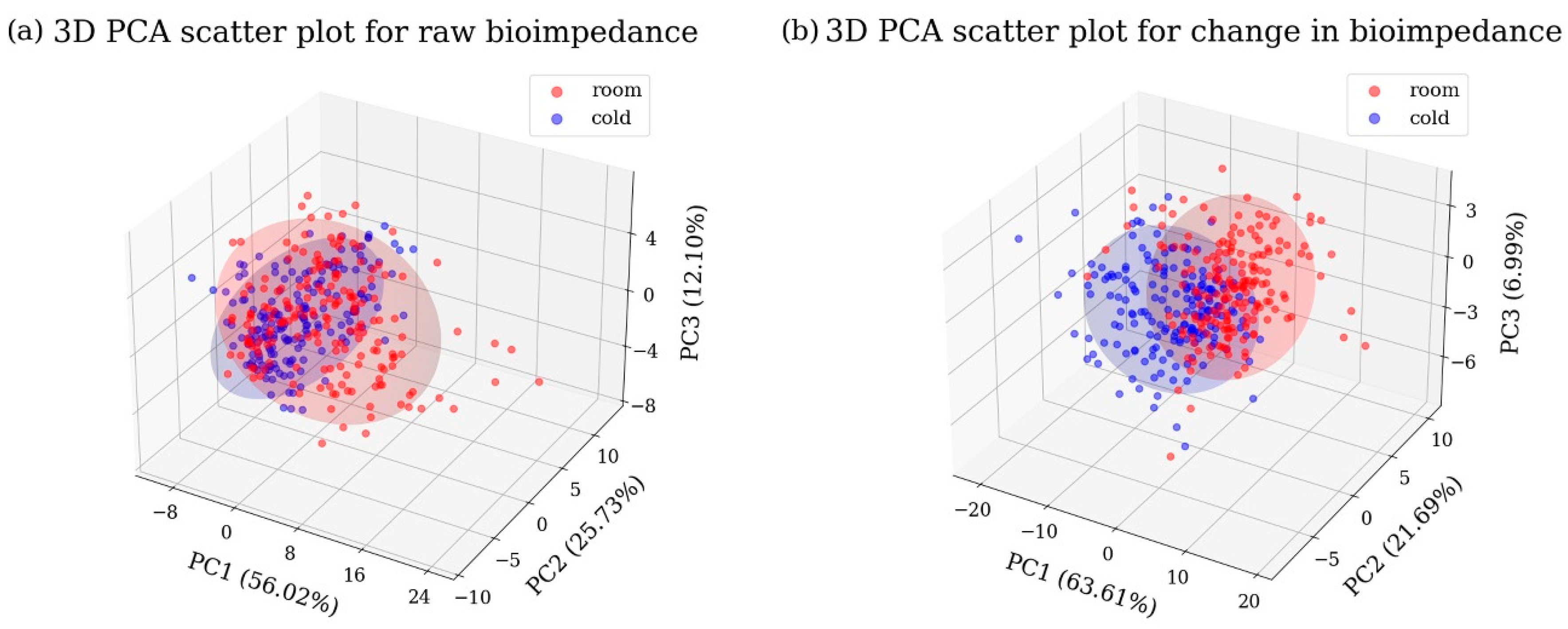
3.3. Storage Temperature Classification with Machine Learning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukhametzyanov, R.R.; Brusenko, S.V.; Khezhev, A.M.; Kelemetov, E.M.; Kirillova, S.S. Changing the Global Production and Trade of Citrus Fruits. In Advances in Science, Technology and Innovation, Part F2358; Springer: Cham, Switzerland, 2024; pp. 19–24. [Google Scholar] [CrossRef]
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Mandarin Fruit Quality: A Review. J. Sci. Food Agric. 2018, 98, 18–26. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Citrus: World Markets and Trade. 2024. Available online: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 21 August 2024).
- Kashyap, K.; Kashyap, D.; Nitin, M.; Ramchiary, N.; Banu, S. Characterizing the Nutrient Composition, Physiological Maturity, and Effect of Cold Storage in Khasi Mandarin (Citrus Reticulata Blanco). Int. J. Fruit Sci. 2020, 20, 521–540. [Google Scholar] [CrossRef]
- Goldenberg, L.; Yaniv, Y.; Kaplunov, T.; Doron-Faigenboim, A.; Carmi, N.; Porat, R. Diversity in Sensory Quality and Determining Factors Influencing Mandarin Flavor Liking. J. Food Sci. 2015, 80, S418–S425. [Google Scholar] [CrossRef] [PubMed]
- Obenland, D.; Collin, S.; Sievert, J.; Arpaia, M.L. Mandarin Flavor and Aroma Volatile Composition Are Strongly Influenced by Holding Temperature. Postharvest Biol. Technol. 2013, 82, 6–14. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; He, L.; Kaliaperumal, K.; Tan, H.; Jiang, Y.; Zhong, B.; Zhang, J. Effects of Storage Time and Temperature on the Chemical Composition and Organoleptic Quality of Gannan Navel Orange (Citrus Sinensis Osbeck Cv. Newhall). J. Food Meas. Charact. 2022, 16, 935–944. [Google Scholar] [CrossRef]
- Tietel, Z.; Lewinsohn, E.; Fallik, E.; Porat, R. Importance of Storage Temperatures in Maintaining Flavor and Quality of Mandarins. Postharvest Biol. Technol. 2012, 64, 175–182. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Yang, Y.; Mu, B.; Nikitina, M.A.; Xiao, X. Vis/NIR Optical Biosensors Applications for Fruit Monitoring. Biosens. Bioelectron. X 2022, 11, 100197. [Google Scholar] [CrossRef]
- Zakiyyah, A.; Hanif, Z.; Indriani, D.W.; Iqbal, Z.; Damayanti, R.; Al Riza, D.F. Characterization and Classification of Citrus Reticulata Var. Keprok Batu 55 Using Image Processing and Artificial Intelligence. Univers. J. Agric. Res. 2022, 10, 397–404. [Google Scholar] [CrossRef]
- Ringer, T.; Blanke, M. Non-Invasive, Real Time in-Situ Techniques to Determine the Ripening Stage of Banana. J. Food Meas. Charact. 2021, 15, 4426–4437. [Google Scholar] [CrossRef]
- Grandón, S.; Sanchez-Contreras, J.; Torres, C.A. Prediction Models for Sunscald on Apples (Malus Domestica Borkh.) Cv. Granny Smith Using Vis-NIR Reflectance. Postharvest Biol. Technol. 2019, 151, 36–44. [Google Scholar] [CrossRef]
- Nekvapil, F.; Brezestean, I.; Barchewitz, D.; Glamuzina, B.; Chiş, V.; Cintă Pinzaru, S. Citrus Fruits Freshness Assessment Using Raman Spectroscopy. Food Chem. 2018, 242, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, A.; Martínez Gila, D.M.; Gómez Ortega, J.; Gámez García, J. Olive Fly Sting Detection Based on Computer Vision. Postharvest Biol. Technol. 2019, 150, 129–136. [Google Scholar] [CrossRef]
- Mazhar, M.; Joyce, D.; Cowin, G.; Brereton, I.; Hofman, P.; Collins, R.; Gupta, M. Non-Destructive 1H-MRI Assessment of Flesh Bruising in Avocado (Persea Americana M.) Cv. Hass. Postharvest Biol. Technol. 2015, 100, 33–40. [Google Scholar] [CrossRef]
- Wen, T.; Zheng, L.; Dong, S.; Gong, Z.; Sang, M.; Long, X.; Luo, M.; Peng, H. Rapid Detection and Classification of Citrus Fruits Infestation by Bactrocera Dorsalis (Hendel) Based on Electronic Nose. Postharvest Biol. Technol. 2019, 147, 156–165. [Google Scholar] [CrossRef]
- Ye, H.; Esfahani, E.B.; Chiu, I.; Mohseni, M.; Gao, G.; Yang, T. Quantitative and Rapid Detection of Nanoplastics Labeled by Luminescent Metal Phenolic Networks Using Surface-Enhanced Raman Scattering. J. Hazard. Mater. 2024, 470, 134194. [Google Scholar] [CrossRef]
- Ye, H.; Jiang, S.; Yan, Y.; Zhao, B.; Grant, E.R.; Kitts, D.D.; Yada, R.Y.; Pratap-Singh, A.; Baldelli, A.; Yang, T. Integrating Metal-Phenolic Networks-Mediated Separation and Machine Learning-Aided Surface-Enhanced Raman Spectroscopy for Accurate Nanoplastics Quantification and Classification. ACS Nano 2024, 18, 26281–26296. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, K.; Ogunlade, B.; Herndon, L.; Tadesse, L.F.; Kirane, A.R.; Dionne, J.A. From Genotype to Phenotype: Raman Spectroscopy and Machine Learning for Label-Free Single-Cell Analysis. ACS Nano 2024, 18, 18101–18117. [Google Scholar] [CrossRef]
- Albelda Aparisi, P.; Fortes Sanchez, E.; Contat Rodrigo, L.; Masot Peris, R.; Laguarda-Miro, N. A Rapid Electrochemical Impedance Spectroscopy and Sensor-Based Method for Monitoring Freeze-Damage in Tangerines. IEEE Sens. J. 2021, 21, 12009–12018. [Google Scholar] [CrossRef]
- Chowdhury, A.; Datta, S.; Bera, T.K.; Ghoshal, D.; Chakraborty, B. Design and Development of Microcontroller Based Instrumentation for Studying Complex Bioelectrical Impedance of Fruits Using Electrical Impedance Spectroscopy. J. Food Process Eng. 2018, 41, e12640. [Google Scholar] [CrossRef]
- Hafid, A.; Benouar, S.; Kedir-Talha, M.; Abtahi, F.; Attari, M.; Seoane, F. Full Impedance Cardiography Measurement Device Using Raspberry PI3 and System-on-Chip Biomedical Instrumentation Solutions. IEEE J. Biomed. Health Inform. 2018, 22, 1883–1894. [Google Scholar] [CrossRef]
- Son, D.; Park, J.; Lee, S.; Kim, J.J.; Chung, S. Integrating Non-Invasive VIS-NIR and Bioimpedance Spectroscopies for Stress Classification of Sweet Basil (Ocimum Basilicum L.) with Machine Learning. Biosens. Bioelectron. 2024, 263, 116579. [Google Scholar] [CrossRef]
- Van Haeverbeke, M.; De Baets, B.; Stock, M. Plant Impedance Spectroscopy: A Review of Modeling Approaches and Applications. Front. Plant Sci. 2023, 14, 1187573. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Wahid, K.; Dinh, A. Assessment of Ripening Degree of Avocado by Electrical Impedance Spectroscopy and Support Vector Machine. J. Food Qual. 2018, 2018, 4706147. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Zhu, W.; Wei, X.; Sun, H. Maturity Assessment of Tomato Fruit Based on Electrical Impedance Spectroscopy. Int. J. Agric. Biol. Eng. 2019, 12, 154–161. [Google Scholar] [CrossRef]
- González-Araiza, J.R.; Ortiz-Sánchez, M.C.; Vargas-Luna, F.M.; Cabrera-Sixto, J.M. Application of Electrical Bio-Impedance for the Evaluation of Strawberry Ripeness. Int. J. Food Prop. 2017, 20, 1044–1050. [Google Scholar] [CrossRef]
- Ibba, P.; Tronstad, C.; Moscetti, R.; Mimmo, T.; Cantarella, G.; Petti, L.; Martinsen, Ø.G.; Cesco, S.; Lugli, P. Supervised Binary Classification Methods for Strawberry Ripeness Discrimination from Bioimpedance Data. Sci. Rep. 2021, 11, 11202. [Google Scholar] [CrossRef]
- Chowdhury, A.; Kanti Bera, T.; Ghoshal, D.; Chakraborty, B. Electrical Impedance Variations in Banana Ripening: An Analytical Study with Electrical Impedance Spectroscopy. J. Food Process Eng. 2017, 40, e12387. [Google Scholar] [CrossRef]
- Romero Fogué, D.; Masot Peris, R.; Ibáñez Civera, J.; Contat Rodrigo, L.; Laguarda-Miro, N. Monitoring Freeze-Damage in Grapefruit by Electric Bioimpedance Spectroscopy and Electric Equivalent Models. Horticulturae 2022, 8, 218. [Google Scholar] [CrossRef]
- Fernández, A.O.; Pinatti, C.A.O.; Peris, R.M.; Laguarda-Miró, N. Freeze-Damage Detection in Lemons Using Electrochemical Impedance Spectroscopy. Sensors 2019, 19, 4051. [Google Scholar] [CrossRef]
- Serrano-Pallicer, E.; Muñoz-Albero, M.; Pérez-Fuster, C.; Peris, R.M.; Laguarda-Miró, N. Early Detection of Freeze Damage in Navelate Oranges with Electrochemical Impedance Spectroscopy. Sensors 2018, 18, 4503. [Google Scholar] [CrossRef]
- Ibba, P.; Falco, A.; Abera, B.D.; Cantarella, G.; Petti, L.; Lugli, P. Bio-Impedance and Circuit Parameters: An Analysis for Tracking Fruit Ripening. Postharvest Biol. Technol. 2020, 159, 110978. [Google Scholar] [CrossRef]
- Rehman, M.; Abu Izneid, B.A.J.A.; Abdullah, M.Z.; Arshad, M.R. Assessment of Quality of Fruits Using Impedance Spectroscopy. Int. J. Food Sci. Technol. 2011, 46, 1303–1309. [Google Scholar] [CrossRef]
- Dodde, R.E.; Kruger, G.H.; Shih, A.J. Design of Bioimpedance Spectroscopy Instrument with Compensation Techniques for Soft Tissue Characterization. J. Med. Devices Trans. ASME 2015, 9, 021001. [Google Scholar] [CrossRef] [PubMed]
- Halonen, S.; Kari, J.; Ahonen, P.; Kronström, K.; Hyttinen, J. Real-Time Bioimpedance-Based Biopsy Needle Can Identify Tissue Type with High Spatial Accuracy. Ann. Biomed. Eng. 2019, 47, 836–851. [Google Scholar] [CrossRef]
- Foroni, D.; Lissandrini, M.; Velegrakis, Y. Estimating the Extent of the Effects of Data Quality through Observations. In Proceedings of the 2021 IEEE 37th International Conference on Data Engineering, Chania, Greece, 19–22 April 2021; pp. 1913–1918. [Google Scholar] [CrossRef]
- Neutatz, F.; Chen, B.; Alkhatib, Y.; Ye, J.; Abedjan, Z. Data Cleaning and AutoML: Would an Optimizer Choose to Clean? Datenbank Spektrum 2022, 22, 121–130. [Google Scholar] [CrossRef]
- Serrano-Finetti, E.; Castillo, E.; Alejos, S.; León Hilario, L.M. Toward Noninvasive Monitoring of Plant Leaf Water Content by Electrical Impedance Spectroscopy. Comput. Electron. Agric. 2023, 210, 107907. [Google Scholar] [CrossRef]
- Ando, Y.; Mizutani, K.; Wakatsuki, N. Electrical Impedance Analysis of Potato Tissues During Drying. J. Food Eng. 2024, 121, 24–31. [Google Scholar] [CrossRef]
- Mishra, S.; Jaiswal, K.; Mohanty, A.; Kaetha, K.M.; Dash, D.K.; Varu, D.K.; Mishra, S.; Jaiswal, K.; Mohanty, A.; Kaetha, K.M.; et al. Physiology of Citrus Flowering and Fruiting. In Plant Physiology; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Golowasch, J.; Thomas, G.; Taylor, A.L.; Patel, A.; Pineda, A.; Khalil, C.; Nadim, F. Membrane Capacitance Measurements Revisited: Dependence of Capacitance Value on Measurement Method in Nonisopotential Neurons. J. Neurophysiol. 2009, 102, 2161–2175. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Parihar, P.; Dhanjal, D.S.; Singh, R.; Ramamurthy, P.C.; Prasad, R.; Singh, J. Differential Regulation of Drought Stress by Biological Membrane Transporters and Channels. Plant Cell Rep. 2021, 40, 1565–1583. [Google Scholar] [CrossRef]
- Chowdhury, A.; Singh, P.; Bera, T.K.; Ghoshal, D.; Chakraborty, B. Electrical Impedance Spectroscopic Study of Mandarin Orange during Ripening. Food Meas. 2017, 11, 1654–1664. [Google Scholar] [CrossRef]
- Hua, Q.; Shen, M. Deep Learning-Enhanced Parameter Extraction for Equivalent Circuit Modeling in Electrochemical Impedance Spectroscopy. In Proceedings of the 2023 IEEE Nordic Circuits and Systems Conference (NorCAS), Aalborg, Denmark, 31 October–1 November 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Doonyapisut, D.; Kannan, P.K.; Kim, B.; Kim, J.K.; Lee, E.; Chung, C.H. Analysis of Electrochemical Impedance Data: Use of Deep Neural Networks. Adv. Intell. Syst. 2023, 5, 2300085. [Google Scholar] [CrossRef]
- Barbosa, J.A.; Freitas, V.M.S.; Vidotto, L.H.B.; Schleder, G.R.; De Oliveira, R.A.G.; Da Rocha, J.F.; Kubota, L.T.; Vieira, L.C.S.; Tolentino, H.C.N.; Neckel, I.T.; et al. Biocompatible Wearable Electrodes on Leaves toward the On-Site Monitoring of Water Loss from Plants. ACS Appl. Mater. Interfaces 2022, 14, 22989–23001. Available online: https://pubs.acs.org/doi/10.1021/acsami.2c02943 (accessed on 5 February 2025). [CrossRef] [PubMed]
- Kim, J.J.; Allison, L.K.; Andrew, T.L. Vapor-Printed Polymer Electrodes for Long-Term, on-Demand Health Monitoring. Sci. Adv. 2019, 5, eaaw0463. [Google Scholar] [CrossRef]
- Miyamoto, A.; Lee, S.; Cooray, N.F.; Lee, S.; Mori, M.; Matsuhisa, N.; Jin, H.; Yoda, L.; Yokota, T.; Itoh, A.; et al. Inflammation-Free, Gas-Permeable, Lightweight, Stretchable on-Skin Electronics with Nanomeshes. Nat. Nanotechnol. 2017, 12, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yan, T.; Wang, F.; Yang, J.; Wu, J.; Wang, J.; Yue, T.; Li, Z. Rapid Fabrication of Wearable Carbon Nanotube/Graphite Strain Sensor for Real-Time Monitoring of Plant Growth. Carbon 2019, 147, 295–302. [Google Scholar] [CrossRef]
- Laganà, F.; Bibbò, L.; Calcagno, S.; De Carlo, D.; Pullano, S.A.; Pratticò, D.; Angiulli, G. Smart Electronic Device-Based Monitoring of SAR and Temperature Variations in Indoor Human Tissue Interaction. Appl. Sci. 2025, 15, 2439. [Google Scholar] [CrossRef]



| Dataset | Bioimpedance-Based Dataset | EC Parameter-Based Dataset |
|---|---|---|
| Raw data | Raw Z and θ at 30 frequency points (368 instances, 60 features) | Seven EC parameters (368 instances, 7 features) |
| Changes | Changes of Z and θ at 30 frequency points by Equation (4) (368 instances, 60 features) | Changes of seven EC parameters by Equation (4) (368 instances, 7 features) |
| Changes with diameter | Diameter data column added (368 instances, 61 features) | Diameter data column added (368 instances, 8 features) |
| Dataset | Mean Accuracy (Standard Deviation) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SVM | LR | MLP | kNN | RF | LDA | NB | DT | ||
| Bioimpedance | Raw data | 0.81 a (0.08) | 0.77 a (0.08) | 0.79 a (0.08) | 0.74 a (0.09) | 0.77 a (0.08) | 0.77 a (0.10) | 0.72 a (0.09) | 0.68 a (0.09) |
| Changes in bioimpedance | 0.85 b (0.06) | 0.80 a (0.07) | 0.83 b (0.06) | 0.79 b (0.08) | 0.82 b (0.08) | 0.77 a (0.08) | 0.75 a (0.07) | 0.75 b (0.07) | |
| Changes in bioimpedance with diameter | 0.86 c (0.06) | 0.81 a (0.07) | 0.86 b (0.06) | 0.82 c (0.07) | 0.82 b (0.08) | 0.77 a (0.08) | 0.75 a (0.07) | 0.77 b (0.07) | |
| EC parameter | Raw data | 0.75 a (0.08) | 0.73 a (0.08) | 0.73 a (0.09) | 0.70 a (0.09) | 0.73 a (0.09) | 0.72 a (0.08) | 0.72 a (0.07) | 0.67 a (0.09) |
| Changes in EC parameter | 0.79 b (0.06) | 0.79 b (0.07) | 0.78 b (0.08) | 0.74 b (0.07) | 0.78 b (0.07) | 0.78 b (0.07) | 0.65 b (0.09) | 0.75 b (0.10) | |
| Change in EC parameter with diameter | 0.82 c (0.08) | 0.76 c (0.07) | 0.82 c (0.08) | 0.78 c (0.06) | 0.81 c (0.07) | 0.78 b (0.07) | 0.65 b (0.09) | 0.75 b (0.09) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, D.; Lee, S.; Jeon, S.; Kim, J.J.; Chung, S. Classifying Storage Temperature for Mandarin (Citrus reticulata L.) Using Bioimpedance and Diameter Measurements with Machine Learning. Sensors 2025, 25, 2627. https://doi.org/10.3390/s25082627
Son D, Lee S, Jeon S, Kim JJ, Chung S. Classifying Storage Temperature for Mandarin (Citrus reticulata L.) Using Bioimpedance and Diameter Measurements with Machine Learning. Sensors. 2025; 25(8):2627. https://doi.org/10.3390/s25082627
Chicago/Turabian StyleSon, Daesik, Siun Lee, Sehyeon Jeon, Jae Joon Kim, and Soo Chung. 2025. "Classifying Storage Temperature for Mandarin (Citrus reticulata L.) Using Bioimpedance and Diameter Measurements with Machine Learning" Sensors 25, no. 8: 2627. https://doi.org/10.3390/s25082627
APA StyleSon, D., Lee, S., Jeon, S., Kim, J. J., & Chung, S. (2025). Classifying Storage Temperature for Mandarin (Citrus reticulata L.) Using Bioimpedance and Diameter Measurements with Machine Learning. Sensors, 25(8), 2627. https://doi.org/10.3390/s25082627









